Improvement in Subjective Symptoms and Tolerability in Response to Nintedanib Treatment in Elderly Patients with Idiopathic Pulmonary Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Subjective Assessments
2.3. Physiological Assessments
2.4. Gender, Age and 2 Lung Physiology Variables (GAP) Stage
2.5. Japanese Severity Stage
2.6. Adverse Events
2.7. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. Therapeutic Effects of Nintedanib on Subjective Symptoms
3.3. Therapeutic Effects of Nintedanib on Forced Vital Capacity
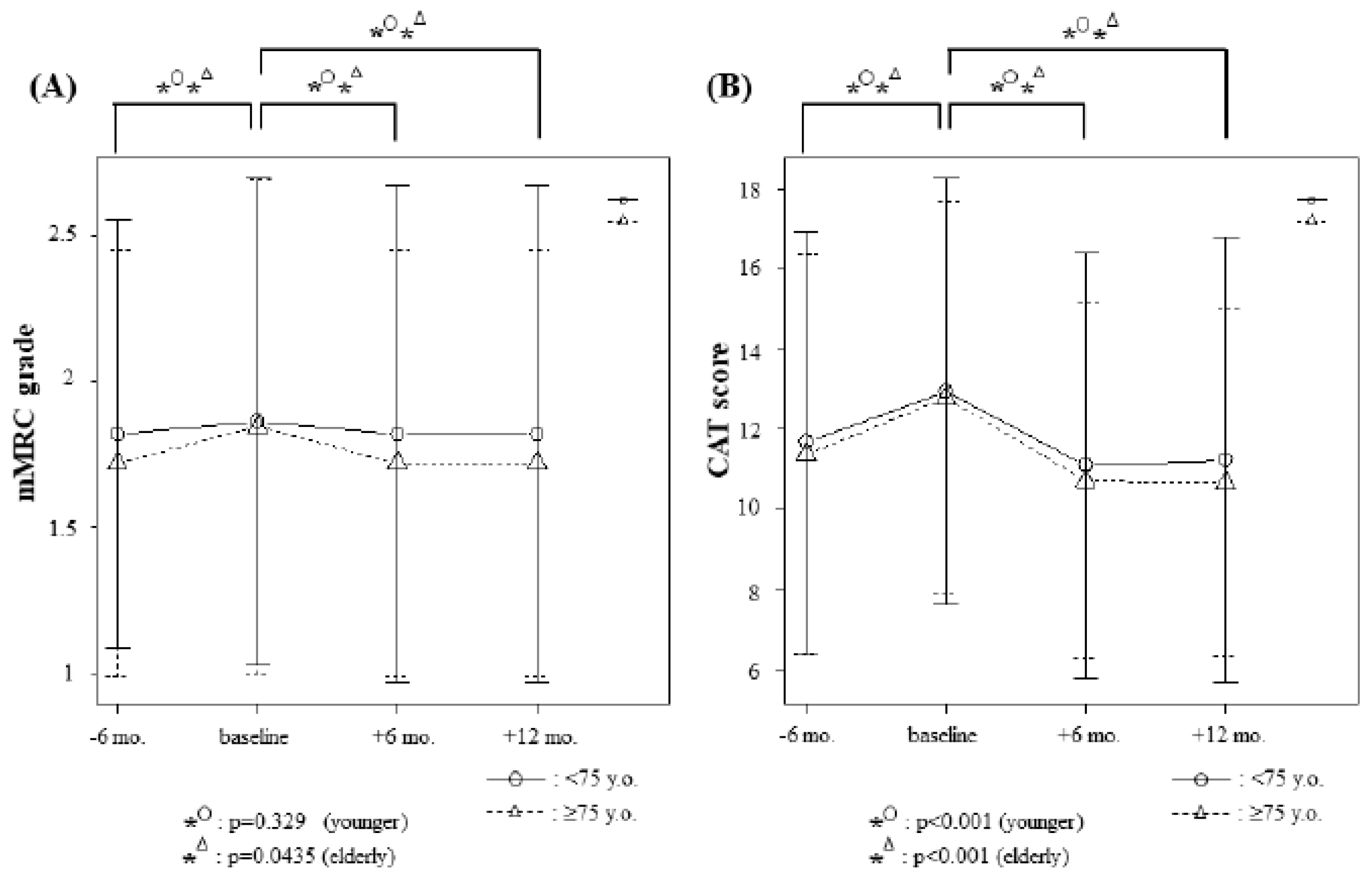
3.4. Changes in Modified Medical Research Council (mMRC) Grades and Chronic Obstructive Pulmonary Disease Assessment Test (CAT) Scores
3.5. Relationship between Subjective and Objective Improvement
3.6. Idiopathic Pulmonary Fibrosis with Acute Exacerbation (IPF-AE)
3.7. Adverse Events
3.8. Logistic Regression Analysis and Multiple Linear Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, R.M. An earlier and more confident diagnosis of idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2012, 21, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Natsuizaka, M.; Chiba, H.; Kuronuma, K.; Otsuka, M.; Kudo, K.; Mori, M.; Bando, M.; Sugiyama, Y.; Takahashi, H. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am. J. Respir. Crit. Care Med. 2014, 190, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Garcia, C.A.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015, 192, e3–e19. [Google Scholar] [PubMed]
- Kim, D.S.; Collard, H.R.; King, T.E., Jr. Classification and natural history of the idiopathic interstitial pneumonias. Proc. Am. Thorac. Soc. 2006, 3, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kärkkäinen, M.; Kettunen, H.P.; Nurmi, H.; Selander, T.; Purokivi, M.; Kaarteenaho, R. Comparison of disease progression subgroups in idiopathic pulmonary fibrosis. BMC Pulm. Med. 2019, 19, 228. [Google Scholar] [CrossRef]
- Collard, H.R.; Moore, B.B.; Flaherty, K.R.; Brown, K.K.; Kaner, R.J.; King, T.E., Jr.; Lasky, J.A.; Loyd, J.E.; Noth, I.; Olman, M.A.; et al. Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 636–643. [Google Scholar] [CrossRef]
- Collard, H.R.; Ryerson, C.J.; Corte, T.J.; Jenkins, G.; Kondoh, Y.; Lederer, D.J.; Lee, J.S.; Maher, T.M.; Wells, A.U.; Antoniou, K.M.; et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am. J. Respir. Crit. Care Med. 2016, 194, 265–275. [Google Scholar] [CrossRef]
- Kondoh, S.; Chiba, H.; Nishikiori, H.; Umeda, Y.; Kuronuma, K.; Otsuka, M.; Yamada, G.; Ohnishi, H.; Mori, M.; Kondoh, Y.; et al. Validation of the Japanese disease severity classification and the GAP model in Japanese patients with idiopathic pulmonary fibrosis. Respir. Investig. 2016, 54, 327–333. [Google Scholar] [CrossRef]
- Suzuki, A.; Kondoh, Y. The clinical impact of major comorbidities on idiopathic pulmonary fibrosis. Respir. Investig. 2017, 55, 94–103. [Google Scholar] [CrossRef]
- Ley, B.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.H.; Tomassetti, S.; Lee, J.S.; Poletti, V.; Buccioli, M.; Elicker, B.M.; Jones, K.D.; et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 2012, 156, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E., Jr.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Albera, C.; Costabel, U.; Fagan, E.A.; Glassberg, M.K.; Gorina, E.; Lancaster, L.; Lederer, D.J.; Nathan, S.D.; Spirig, D.; Swigris, J.J. Efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis with more preserved lung function. Eur. Respir. J. 2016, 48, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Kolb, M.; Richeldi, L.; Behr, J.; Maher, T.M.; Tang, W.; Stowasser, S.; Hallmann, C.; du Bois, R.M. Nintedanib in patients with idiopathic pulmonary fibrosis and preserved lung volume. Thorax 2017, 72, 340–346. [Google Scholar] [CrossRef]
- Guenther, A.; Krauss, E.; Tello, S.; Wagner, J.; Paul, B.; Kuhn, S.; Maurer, O.; Heinemann, S.; Costabel, U.; Barbero, M.A.N.; et al. The European IPF registry (eurIPFreg): Baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 141. [Google Scholar] [CrossRef]
- Gribbin, J.; Hubbard, R.B.; Le Jeune, I.; Smith, C.J.; West, J.; Tata, L.J. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax 2006, 61, 980–985. [Google Scholar] [CrossRef]
- Pérez, E.R.F.; Daniels, C.E.; Schroeder, D.R.; Sauver, J.S.; Hartman, T.E.; Bartholmai, B.J.; Yi, E.S.; Ryu, J.H. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: A population-based study. Chest 2010, 137, 129–137. [Google Scholar] [CrossRef]
- Sakayori, M.; Terada, J.; Abe, M.; Hirasawa, Y.; Suzuki, K.; Yoshioka, K.; Tsushima, K.; Tatsumi, K. Differences in tolerability of pirfenidone between elderly and younger patients with idiopathic pulmonary fibrosis. Drug Des. Dev. Ther. 2019, 13, 2295–2303. [Google Scholar] [CrossRef]
- Corte, T.; Bonella, F.; Crestani, B.; Demedts, M.G.; Richeldi, L.; Coeck, C.; Pelling, K.; Quaresma, M.; Lasky, J.A. Safety, tolerability and appropriate use of nintedanib in idiopathic pulmonary fibrosis. Respir. Res. 2015, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Swigris, J.J.; Fairclough, D. Patient-reported outcomes in idiopathic pulmonary fibrosis research. Chest 2012, 142, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Taniguchi, H.; Ando, M.; Kondoh, Y.; Kimura, T.; Kataoka, K.; Sakamoto, K.; Suzuki, A.; Furukawa, T.; Hasegawa, Y. COPD Assessment Test for measurement of health status in patients with idiopathic pulmonary fibrosis: A cross-sectional study. Respirology 2017, 22, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Gruden, J.F.; Panse, P.M.; Leslie, K.O.; Tazelaar, H.D.; Colby, T.V. UIP diagnosed at surgical lung biopsy, 2000–2009: HRCT patterns and proposed classification system. AJR Am. J. Roentgenol. 2013, 200, W458–W467. [Google Scholar] [CrossRef]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Leidy, N.K. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Homma, S.; Sugino, K.; Sakamoto, S. Usefulness of a disease severity staging classification system for IPF in Japan: 20 years of experience from empirical evidence to randomized control trial enrollment. Respir. Investig. 2015, 53, 7–12. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Kolb, M.; Vancheri, C.; Tang, W.; Conoscenti, C.S.; Richeldi, L. Stability or improvement in forced vital capacity with nintedanib in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2018, 52, 1702593. [Google Scholar] [CrossRef]
- Idiopathic Pulmonary Fibrosis Clinical Research Network; Raghu, G.; Anstrom, K.J.; King, T.E., Jr.; Lasky, J.A.; Martinez, F.J. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 2012, 366, 1968–1977. [Google Scholar]
- Ahluwalia, N.; Shea, B.S.; Tager, A.M. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am. J. Respir. Crit. Care Med. 2014, 190, 867–878. [Google Scholar] [CrossRef]
- Crestani, B.; Huggins, J.T.; Kaye, M.; Costabel, U.; Glaspole, I.; Ogura, T.; Song, J.W.; Stansen, W.; Quaresma, M.; Stowasser, S.; et al. Long-term safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: Results from the open-label extension study, INPULSIS-ON. Lancet Respir. Med. 2019, 7, 60–68. [Google Scholar] [CrossRef]
- Jo, H.E.; Glaspole, I.; Moodley, Y.; Chapman, S.; Ellis, S.; Goh, N.; Hopkins, P.; Keir, G.; Mahar, A.; Cooper, W.; et al. Disease progression in idiopathic pulmonary fibrosis with mild physiological impairment: Analysis from the Australian IPF registry. BMC Pulm. Med. 2018, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Wollin, L.; Wex, E.; Pautsch, A.; Schnapp, G.; Hostettler, K.E.; Stowasser, S.; Kolb, M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Hostettler, K.E.; Zhong, J.; Papakonstantinou, E.; Karakiulakis, G.; Tamm, M.; Seidel, P.; Sun, Q.; Mandal, J.; Lardinois, D.; Lambers, C.; et al. Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Respir. Res. 2014, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.; Bendstrup, E.; Russell, A.M.; Bajwah, S.; Lindell, K.; Adir, Y.; Brown, C.E.; Calligaro, G.; Cassidy, N.; Corte, T.J.; et al. Palliative care in interstitial lung disease: Living well. Lancet Respir. Med. 2017, 5, 968–980. [Google Scholar] [CrossRef]
- Takeda, T.; Takeuchi, M.; Saitoh, M.; Takeda, S. Improvement in Patient-Reported Outcomes and Forced Vital Capacity during Nintedanib Treatment of Idiopathic Pulmonary Fibrosis. Tohoku J. Exp. Med. 2018, 245, 107–114. [Google Scholar] [CrossRef]
- Swigris, J.J.; Brown, K.K.; Behr, J.; du Bois, R.M.; King, T.E.; Raghu, G.; Wamboldt, F.S. The SF-36 and SGRQ: Validity and first look at minimum important differences in IPF. Respir. Med. 2010, 104, 296–304. [Google Scholar] [CrossRef]
- Swigris, J.J.; Esser, D.; Conoscenti, C.S.; Brown, K.K. The psychometric properties of the St George’s Respiratory Questionnaire (SGRQ) in patients with idiopathic pulmonary fibrosis: A literature review. Health Qual. Life Outcomes 2014, 12, 124. [Google Scholar] [CrossRef]
- Swigris, J.J.; Esser, D.; Wilson, H.; Conoscenti, C.S.; Schmidt, H.; Stansen, W.; Leidy, N.K.; Brown, K.K. Psychometric properties of the St George’s Respiratory Questionnaire in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2017, 49, 1601788. [Google Scholar] [CrossRef]
- Swigris, J.J.; Wilson, H.; Esser, D.; Conoscenti, C.S.; Stansen, W.; Leidy, N.K.; Brown, K.K. Psychometric properties of the St George’s Respiratory Questionnaire in patients with idiopathic pulmonary fibrosis: Insights from the INPULSIS trials. BMJ Open Respir. Res. 2018, 5, e000278. [Google Scholar] [CrossRef]
- Collard, H.R.; Richeldi, L.; Kim, D.S.; Taniguchi, H.; Tschoepe, I.; Luisetti, M.; Roman, J.; Tino, G.; Schlenker-Herceg, R.; Hallmann, C.; et al. Acute exacerbations in the INPULSIS trials of nintedanib in idiopathic pulmonary fibrosis. Eur. Respir. J. 2017, 49, 1601339. [Google Scholar] [CrossRef]
- Kondoh, Y.; Taniguchi, H.; Katsuta, T.; Kataoka, K.; Kimura, T.; Nishiyama, O.; Sakamoto, K.; Johkoh, T.; Nishimura, M.; Ono, K.; et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010, 27, 103–110. [Google Scholar] [PubMed]
- Song, J.W.; Hong, S.B.; Lim, C.M.; Koh, Y.; Kim, D.S. Acute exacerbation of idiopathic pulmonary fibrosis: Incidence, risk factors and outcome. Eur. Respir. J. 2011, 37, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Collard, H.R.; Yow, E.; Richeldi, L.; Anstrom, K.J.; Glazer, C. IPFnet investigators. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir. Res. 2013, 14, 73. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 54) | ≥75 years (n = 32) | <75 years (n = 22) | p-value | |
|---|---|---|---|---|
| Mean age (years, SD) | 74.50 (4.90) | 77.75 (2.68) | 69.86 (3.43) | <0.001 |
| Sex (women / men; n, %) | 7 (13.0%) / 47 (87.0%) | 5 (15.6%) / 27 (84.4%) | 2 (9.1%) / 20 (90.9%) | 0.687 |
| Never smoker (n, %) | 20 (37.0%) | 12 (37.5%) | 8 (36.4%) | 1.000 |
| mMRC grade 0 / 1 / 2 / 3 / 4 (n, %) | 3 (5.6%) / 12 (22.2%) / 31 (57.4%) / 6 (11.1%) / 2 (3.7%) | 2 (6.2%) / 7 (21.9%) / 18 (56.3%) / 4 (12.5%) / 1 (3.1%) | 1 (4.6%) / 5 (22.7%) / 13 (59.0%) / 2 (9.1%) / 1 (4.6%) | 1.000 |
| CAT score (mean, SD) | 12.85 (5.04) | 12.78 (4.91) | 12.95 (5.33) | 0.852 |
| GAP stage I / II / III (n, %) | 12 (22.2%) / 32 (59.3%) / 10 (18.5%) | 3 (9.4%) / 22 (68.7%) / 7 (21.9%) | 9 (40.9%) / 10 (45.5%) / 3 (13.6%) | 0.026 |
| Japanese severity stage I / II / III / IV (n, %) | 9 (16.7%) / 25 (46.3%) / 18 (33.3%) / 2 (3.7%) | 7 (21.9%) / 14 (43.8%) / 10 (31.2%) / 1 (3.1%) | 2 (9.1%) / 11 (50.0%) / 8 (36.4%) / 1 (4.5%) | 0.357 |
| Mean FVC (ml, range) | 2286.3 (770.0–3860.0) | 2252.2 (770.0–3710.0) | 2335.9 (1750.0–3860.0) | 0.846 |
| Mean % FVC (%, range) | 72.2 (27.1–106.3) | 72.8 (27.1–106.3) | 71.8 (39.5–98.2) | 0.470 |
| Honeycombing + / − (n, %) | 44 (81.5%) / 10 (18.5%) | 26 (81.2%) / 6 (18.8%) | 18 (81.8%) / 4 (18.2%) | 1.000 |
| Emphysema + / − (n, %) | 26 (48.1%) / 28 (51.9%) | 14 (43.8%) / 18 (56.2%) | 12 (54.5%) / 10 (45.5%) | 0.580 |
| Previous pirfenidone (n, %) | 12 (22.2%) | 7 (21.9%) | 5 (22.7%) | 1.000 |
| Nintedanib starting dose 300 / 200mg (n, %) | 35 (64.8%) / 19 (35.2%) | 18 (56.2%) / 14 (43.8%) | 17 (77.3%) / 5 (22.7%) | 0.151 |
| Total (n = 54) | ≥75 years (n = 32) | <75 years (n = 22) | p-value | |
|---|---|---|---|---|
| Subjective Improvement after 6 Months (n, %) | ||||
| Elderly vs. Younger | 25 / 54 (46.3%) | 16 / 32 (50.0%) | 9 / 22 (40.9%) | 0.585 |
| mMRC grade ≥ 1 point | 5 / 54 (9.26%) | 4 / 32 (12.5%) | 1 / 22 (4.55%) | 0.683 |
| CAT score ≥ 3 points | 25 / 54 (46.3%) | 16 / 32 (50.0%) | 9 / 22 (40.9%) | 0.585 |
| Honeycombing +/− ≥75y: n = 26 / 6, <75y: n = 18 / 4 | (+); 21 / 44 (47.7%) (−); 4 / 10 (40.0%) | (+); 13 / 26 (50.0%) (−); 3 / 6 (50.0%) | (+); 8 / 18 (44.4%) (−); 1 / 4 (25.0%) | 0.767 0.571 |
| Emphysema +/− ≥75y: n = 14 / 18, <75y: n = 12 / 10 | (+); 14 / 26 (53.8%) (−); 11 / 28 (39.3%) | (+); 7 / 14 (50.0%) (−); 9 / 18 (50.0%) | (+); 7 / 12 (58.3%) (−); 2 / 10 (20.0%) | 0.713 0.226 |
| Objective Improvement; Median ΔFVC%+6M (%, range) | ||||
| Elderly vs. Younger | −1.42 (−11.4 – +12.2) | −1.18 (-11.1 – +12.1) | −1.64 (−11.4 – +12.2) | 0.398 |
| Honeycombing +/− ≥75y: n = 26 / 6, <75y: n = 18 / 4 | −1.36 (−11.4 – +10.8), −2.21 (−11.1 – +12.2) | −0.67 (−11.1 – +10.8) / −6.63 (−11.0 – +12.1) | −1.68 (−11.4 – +6.40) / −2.04 (−11.1 – +12.2) | 1.000 |
| Emphysema +/− ≥75y: n = 14/18, <75y: n = 12/10 | −1.42 (−11.4 – +12.2), −1.59 (−11.1 – +10.8) | −1.10 (−11.4 – +12.1) / −1.17 (−11.1 – +10.8) | −1.10 (−11.4 – +12.2) / −2.74 (−11.7 – +9.76) | 0.580 |
| Subjective Improvement after 12 Months (n, %) | ||||
| Elderly vs. Younger | 25 / 54 (46.3%) | 16 / 32 (50.0%) | 9 / 22 (40.9%) | 0.585 |
| mMRC grade ≥ 1 point | 5 / 54 (9.26%) | 4 / 32 (12.5%) | 1 / 22 (4.55%) | 0.683 |
| CAT score ≥ 3 points | 25 / 54 (46.3%) | 16 / 32 (50.0%) | 9 / 22 (40.9%) | 0.585 |
| Honeycombing +/− ≥75y: n = 26 / 6, <75y: n = 18 / 4 | (+); 21 / 44 (47.7%) (−); 4 / 10 (40.0%) | (+); 13 / 26 (50.0%) (−); 3 / 6 (50.0%) | (+); 8 / 18 (44.4%) (−); 1 / 4 (25.0%) | 0.767 0.571 |
| Emphysema +/− ≥75y: n = 14 / 18, <75y: n = 12 / 10 | (+); 14 / 26 (53.8%) (−); 11 / 28 (39.3%) | (+); 7 / 14 (50.0%) (−); 9 / 18 (50.0%) | (+); 7 / 12 (58.3%) (−); 2 / 10 (20.0%) | 0.713 0.226 |
| Objective Improvement; Median ΔFVC%+12M (%, range) | ||||
| Elderly vs. Younger | −2.66 (−11.7 – +9.76) | −1.36 (−11.6 – +9.55) | −2.89 (−11.7 – +9.76) | 0.360 |
| Honeycombing +/− ≥75y: n = 26 / 6, <75y: n = 18 / 4 | −2.63 (−11.7 – +7.55), −2.66 (−6.61 – +9.76) | −1.94 (−11.6 – +7.84) / −4.08 (−6.61 – +9.55) | −2.51 (-11.7 – +5.91) / −1.63 (-5.75 – +9.76) | 1.000 |
| Emphysema +/− ≥75y: n = 14 / 18,<75y: n = 12 / 10 | −2.67 (−11.7 – +9.76), −2.79 (−9.71 – +7.84) | −1.72 (−11.6 – +9.55) / −2.38 (−9.71 – +7.84) | −2.74 (-11.7 – +9.76) / −3.40 (-9.71 – −1.41) | 0.151 |
| 6-Month Evaluation | Stable or Improved ΔFVC+6M (n = 17) | Declined ΔFVC+6M (n = 37) | p-value |
| mMRC grade ≥ 1 point mMRC grade < 1 point | 1 (5.9%) 16 (94.1%) | 4 (10.8%) 33 (89.2%) | 1.000 |
| CAT score ≥ 3 points CAT score < 3 points | 14 (82.4%) 3 (17.6%) | 11 (29.7%) 26 (70.3%) | <0.001 |
| 12-Month Evaluation | Stable or Improved ΔFVC+12M (n = 13) | Declined ΔFVC+12M (n = 41) | |
| mMRC grade ≥ 1 point mMRC grade < 1 point | 0 (0%) 13 (100%) | 5 (12.2% )36 (87.8%) | 0.321 |
| CAT score ≥ 3 point sCAT score < 3 points | 10 (76.9%) 3 (23.1%) | 15 (36.6%) 26 (63.4%) | 0.023 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, T.; Kunimatsu, Y.; Tani, N.; Hashimoto, I.; Kurono, Y.; Hirose, K. Improvement in Subjective Symptoms and Tolerability in Response to Nintedanib Treatment in Elderly Patients with Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2020, 9, 755. https://doi.org/10.3390/jcm9030755
Takeda T, Kunimatsu Y, Tani N, Hashimoto I, Kurono Y, Hirose K. Improvement in Subjective Symptoms and Tolerability in Response to Nintedanib Treatment in Elderly Patients with Idiopathic Pulmonary Fibrosis. Journal of Clinical Medicine. 2020; 9(3):755. https://doi.org/10.3390/jcm9030755
Chicago/Turabian StyleTakeda, Takayuki, Yusuke Kunimatsu, Nozomi Tani, Izumi Hashimoto, Yuri Kurono, and Kazuki Hirose. 2020. "Improvement in Subjective Symptoms and Tolerability in Response to Nintedanib Treatment in Elderly Patients with Idiopathic Pulmonary Fibrosis" Journal of Clinical Medicine 9, no. 3: 755. https://doi.org/10.3390/jcm9030755
APA StyleTakeda, T., Kunimatsu, Y., Tani, N., Hashimoto, I., Kurono, Y., & Hirose, K. (2020). Improvement in Subjective Symptoms and Tolerability in Response to Nintedanib Treatment in Elderly Patients with Idiopathic Pulmonary Fibrosis. Journal of Clinical Medicine, 9(3), 755. https://doi.org/10.3390/jcm9030755





