Antibodies against Malondialdehyde in Haemodialysis Patients and Its Association with Clinical Outcomes: Differences between Subclasses and Isotypes
Abstract
1. Introduction
2. Materials and Method
2.1. Patient and Experimental Design
2.2. Collection of Clinical and Laboratory Data
2.3. Other Laboratory Analyses
2.4. Body Composition
2.5. Antibody Determination
2.6. Antibody Specificity Assay
2.7. Statistical Analyses
3. Results
3.1. Patient Characteristics
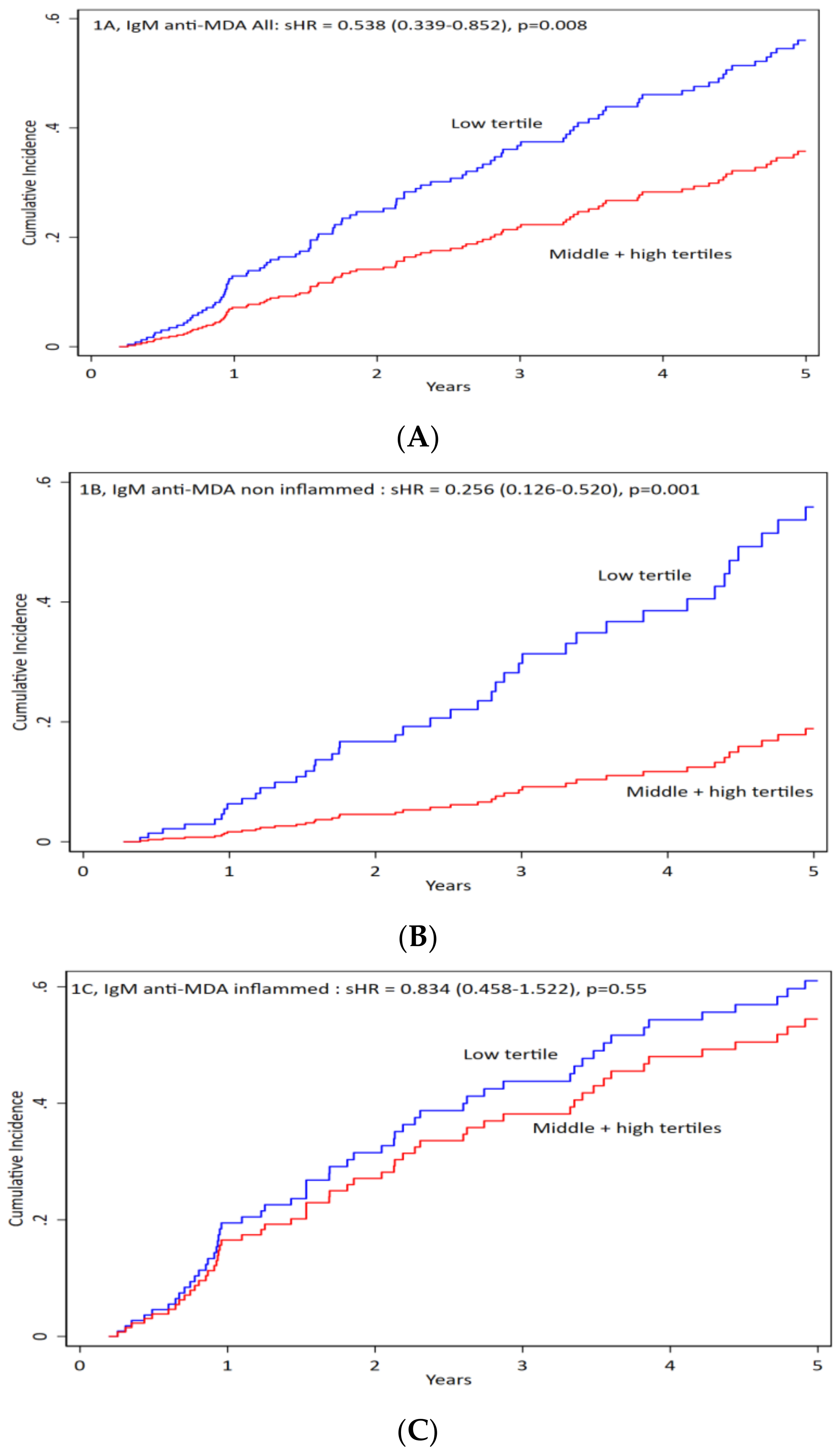
3.2. Correlation of Anti-MDA
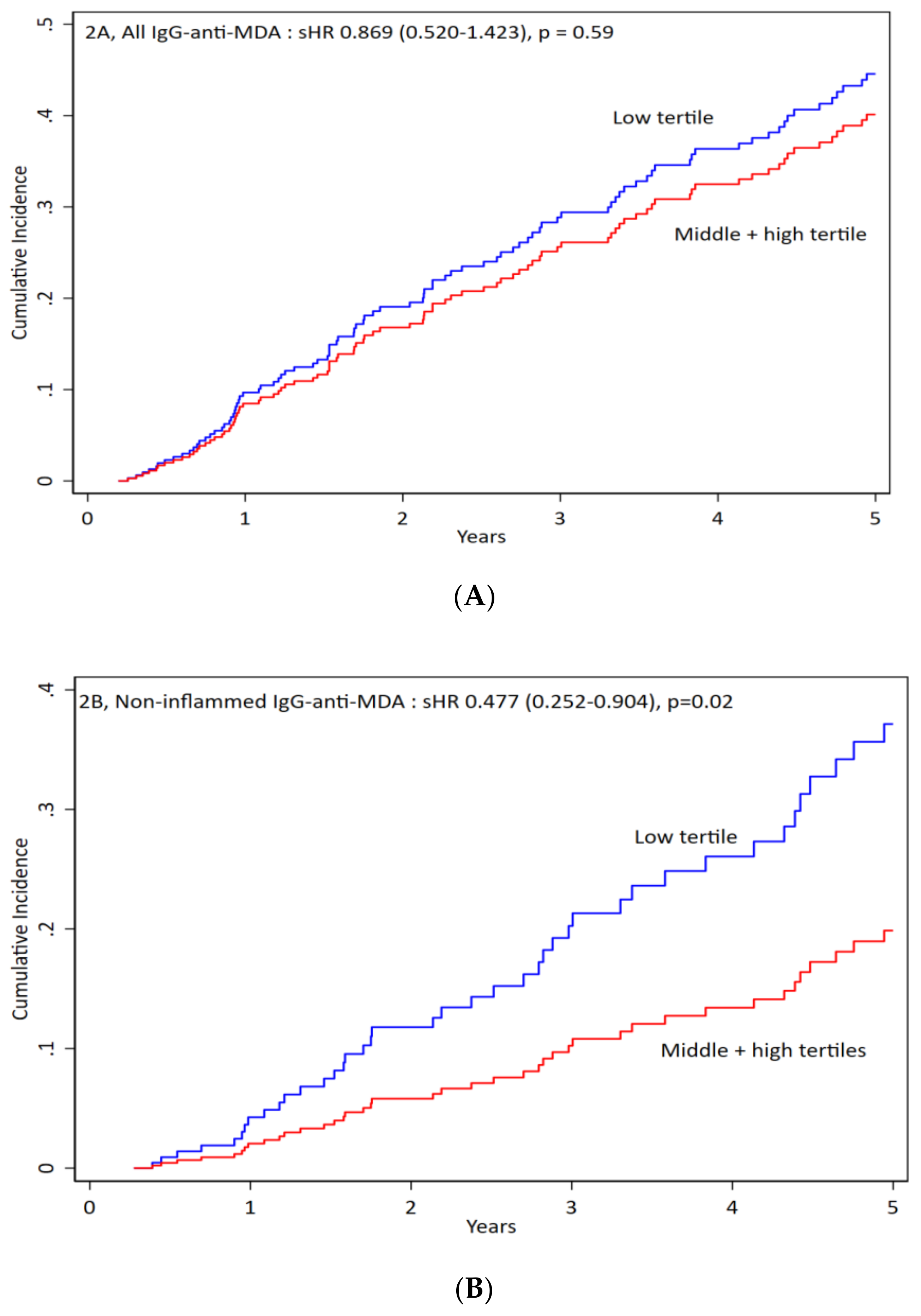
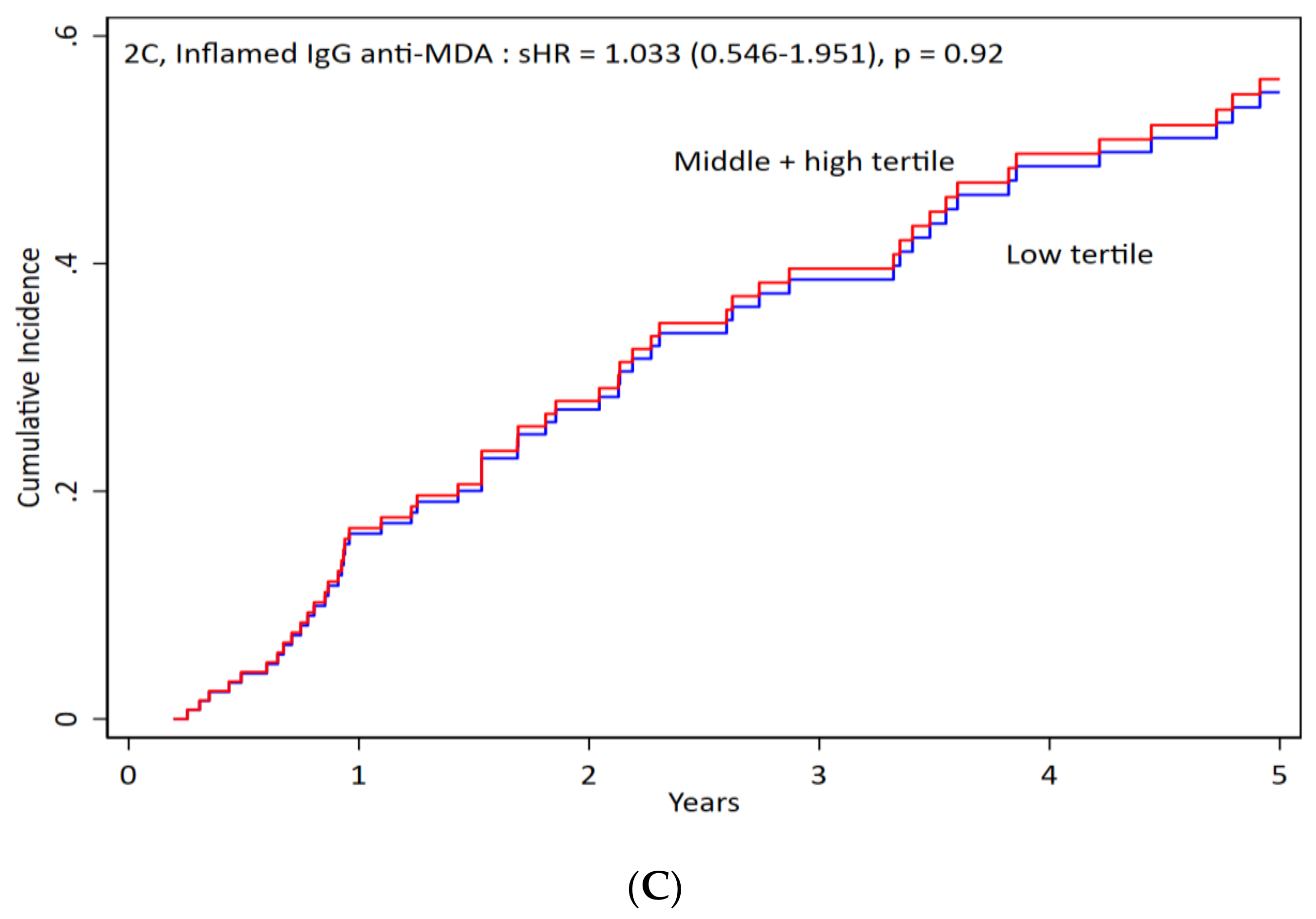
3.3. IgG anti-MDA
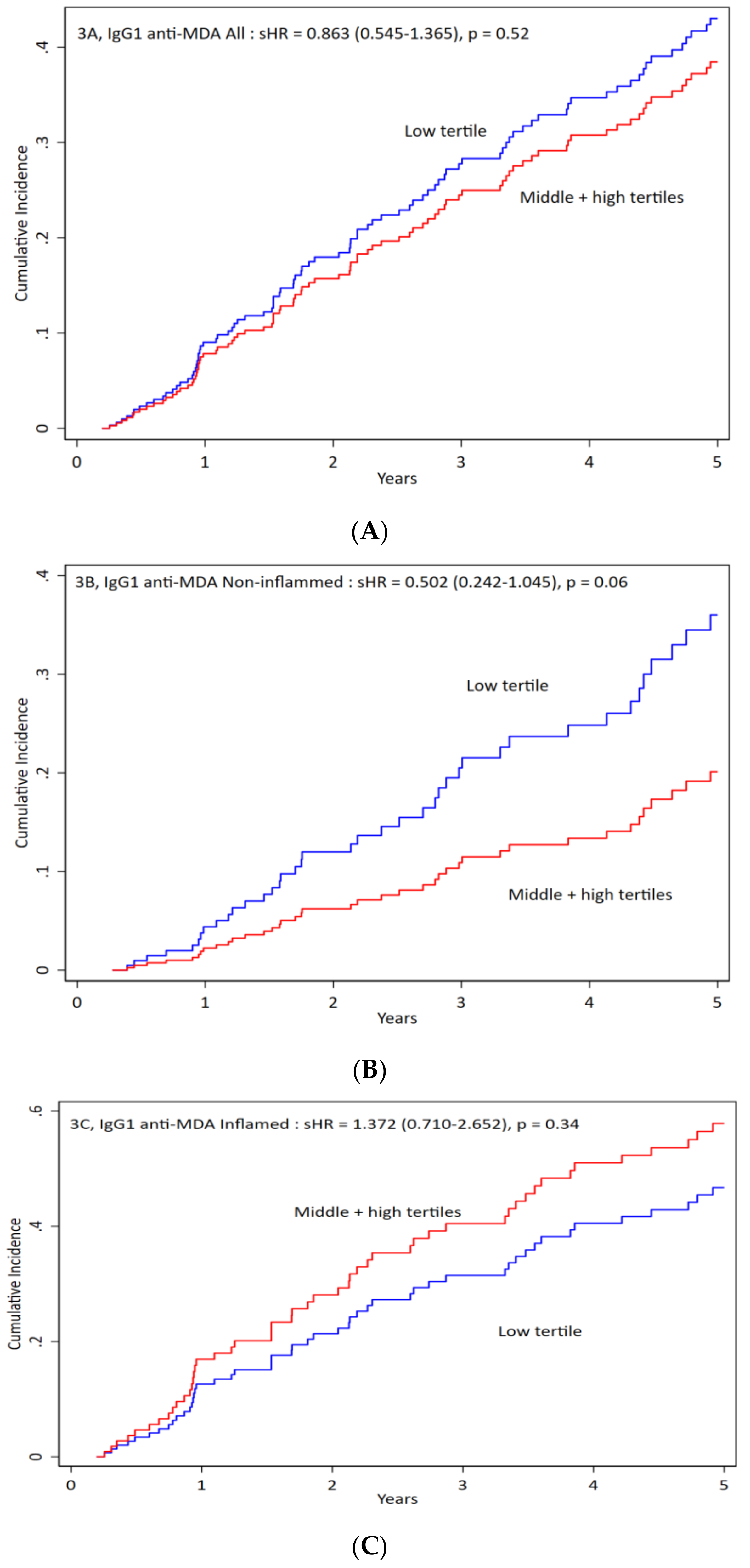
3.4. IgG1 anti-MDA
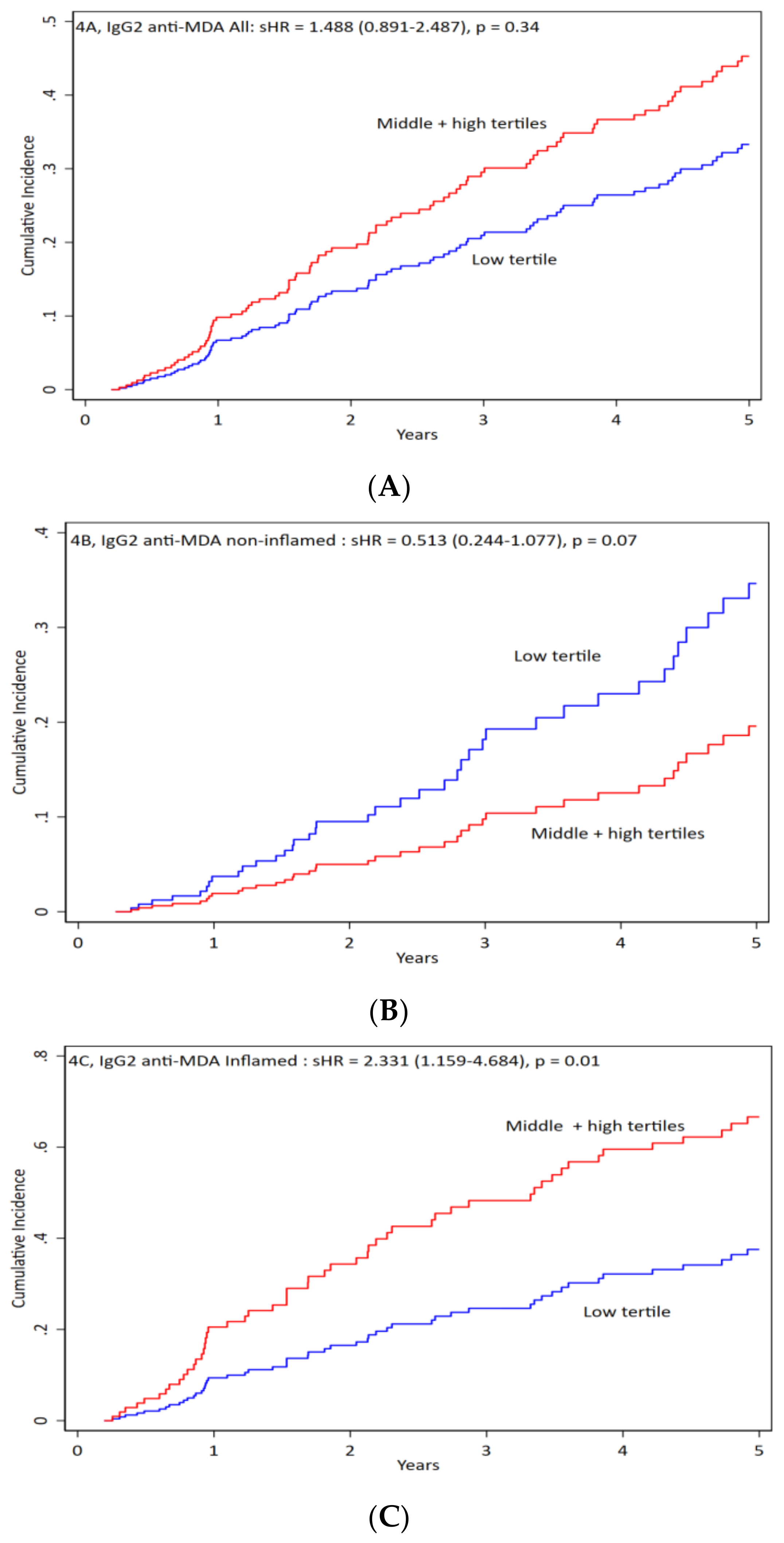
3.5. IgG2 anti-MDA
3.6. IgA anti-MDA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stenvinkel, P. Chronic kidney disease: A public health priority and harbinger of premature cardiovascular disease. J. Intern. Med. 2010, 268, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Carrero, J.J.; Axelsson, J.; Lindholm, B.; Heimburger, O.; Massy, Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin. J. Am. Soc. Nephrol. 2008, 3, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Frostegard, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; de Faire, U.; Lemne, C.; Witztum, J.L.; Frostegard, J. Autoantibodies to OxLDL are decreased in individuals with borderline hypertension. Hypertension 1999, 33, 53–59. [Google Scholar] [CrossRef]
- Vaarala, O.; Alfthan, G.; Jauhiainen, M.; Leirisalo-Repo, M.; Aho, K.; Palosuo, T. Crossreaction between antibodies to oxidised low-density lipoprotein and to cardiolipin in systemic lupus erythematosus. Lancet 1993, 341, 923–925. [Google Scholar] [CrossRef]
- Thiagarajan, D.; Frostegard, A.G.; Singh, S.; Rahman, M.; Liu, A.; Vikstrom, M.; Leander, K.; Gigante, B.; Hellenius, M.L.; Zhang, B.; et al. Human IgM Antibodies to Malondialdehyde Conjugated With Albumin Are Negatively Associated With Cardiovascular Disease Among 60-Year-Olds. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Rahman, M.; Sing, S.; Golabkesh, Z.; Fiskesund, R.; Gustafsson, T.; Jogestrand, T.; Frostegard, A.G.; Hafstrom, I.; Liu, A.; Frostegard, J. IgM antibodies against malondialdehyde and phosphorylcholine are together strong protection markers for atherosclerosis in systemic lupus erythematosus: Regulation and underlying mechanisms. Clin. Immunol. 2016, 166–167, 27–37. [Google Scholar] [CrossRef]
- Snaedal, S.; Heimburger, O.; Qureshi, A.R.; Danielsson, A.; Wikstrom, B.; Fellstrom, B.; Fehrman-Ekholm, I.; Carrero, J.J.; Alvestrand, A.; Stenvinkel, P.; et al. Comorbidity and acute clinical events as determinants of C-reactive protein variation in hemodialysis patients: Implications for patient survival. Am. J. Kidney Dis 2009, 53, 1024–1033. [Google Scholar] [CrossRef]
- Dai, L.; Mukai, H.; Lindholm, B.; Heimburger, O.; Barany, P.; Stenvinkel, P.; Qureshi, A.R. Clinical global assessment of nutritional status as predictor of mortality in chronic kidney disease patients. PLoS ONE 2017, 12, e0186659. [Google Scholar] [CrossRef]
- Su, J.; Georgiades, A.; Wu, R.; Thulin, T.; de Faire, U.; Frostegard, J. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis 2006, 188, 160–166. [Google Scholar] [CrossRef]
- Qureshi, A.R.; Alvestrand, A.; Danielsson, A.; Divino-Filho, J.C.; Gutierrez, A.; Lindholm, B.; Bergström, J. Factors predicting malnutrition in hemodialysis patients: A cross-sectional study. Kidney Int. 1998, 53, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Heimbürger, O.; Qureshi, A.R.; Blaner, W.S.; Berglund, L.; Stenvinkel, P. Hand-grip muscle strength, lean body mass, and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy. Am. J. Kidney Dis. 2000, 36, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Latouche, A. A competing risk analysis should report results on all cause-specific hazards and cumulative incidence functions. J. Clin. Epidemiol. 2013, 66, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Jason, P.F.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Frostegard, A.G.; Hua, X.; Su, J.; Carrero, J.J.; Heimburger, O.; Barany, P.; Stenvinkel, P.; Frostegard, J. Immunoglobulin (Ig)M antibodies against oxidized cardiolipin but not native cardiolipin are novel biomarkers in haemodialysis patients, associated negatively with mortality. Clin. Exp. Immunol. 2013, 174, 441–448. [Google Scholar] [CrossRef]
- Carrero, J.J.; Hua, X.; Stenvinkel, P.; Qureshi, A.R.; Heimburger, O.; Barany, P.; Lindholm, B.; Frostegard, J. Low levels of IgM antibodies against phosphorylcholine-A increase mortality risk in patients undergoing haemodialysis. Nephrol. Dial. Transpl. 2009, 24, 3454–3460. [Google Scholar] [CrossRef]
- Tabas, I.; Bornfeldt, K.E. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ. Res. 2016, 118, 653–667. [Google Scholar] [CrossRef]
- Febbraio, M.; Podrez, E.A.; Smith, J.D.; Hajjar, D.P.; Hazen, S.L.; Hoff, H.F.; Sharma, K.; Silverstein, R.L. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 2000, 105, 1049–1056. [Google Scholar] [CrossRef]
- Niemann, B.; Rohrbach, S.; Miller, M.R.; Newby, D.E.; Fuster, V.; Kovacic, J.C. Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution: Part 3 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 230–251. [Google Scholar] [CrossRef]
- Pawlak, K.; Mysliwiec, M.; Pawlak, D. oxLDL—the molecule linking hypercoagulability with the presence of cardiovascular disease in hemodialyzed uraemic patients. Thromb. Res. 2014, 134, 711–716. [Google Scholar] [CrossRef]
- Pawlak, K.; Mysliwiec, M.; Pawlak, D. Oxidized low-density lipoprotein (oxLDL) plasma levels and oxLDL to LDL ratio- are they real oxidative stress markers in dialyzed patients? Life Sci. 2013, 92, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, A.; Pacanis, A.; Kortas-Stempak, B.; Cwiklinska, A.; Zietkiewicz, M.; Renke, M.; Rutkowski, B. Estimation of oxidative stress markers in chronic kidney disease. Kidney Blood Press Res. 2011, 34, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Hanayama, R.; Kawane, K. Autoimmunity and the clearance of dead cells. Cell 2010, 140, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Frostegard, J. Low level natural antibodies against phosphorylcholine: A novel risk marker and potential mechanism in atherosclerosis and cardiovascular disease. Clin. Immunol. 2010, 134, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ajeganova, S.; Fiskesund, R.; de Faire, U.; Hafstrom, I.; Frostegard, J. Effect of biological therapy on levels of atheroprotective antibodies against phosphorylcholine and apolipoproteins in rheumatoid arthritis—A one year study. Clin. Exp. Rheumatol. 2011, 29, 942–950. [Google Scholar]
- Anania, C.; Gustafsson, T.; Hua, X.; Su, J.; Vikstrom, M.; de Faire, U.; Heimburger, M.; Jogestrand, T.; Frostegard, J. Increased prevalence of vulnerable atherosclerotic plaques and low levels of natural IgM antibodies against phosphorylcholine in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2010, 12, R214. [Google Scholar] [CrossRef]
- Su, J.; Hua, X.; Concha, H.; Svenungsson, E.; Cederholm, A.; Frostegard, J. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology 2008, 47, 1144–1150. [Google Scholar] [CrossRef]
- Rahman, M.; Steuer, J.; Gillgren, P.; Vegvari, A.; Liu, A.; Frostegard, J. Malondialdehyde Conjugated With Albumin Induces Pro-Inflammatory Activation of T Cells Isolated From Human Atherosclerotic Plaques Both Directly and Via Dendritic Cell-Mediated Mechanism. JACC Basic Transl. Sci. 2019, 4, 480–494. [Google Scholar] [CrossRef]
| Demography and Clinical Characteristics | |||
|---|---|---|---|
| Survivor (n = 105) | Non-survivor (n = 105) | ||
| Age (years) | 56.0 (45.0–69.0) | 71.0 (63.0–78.0) | 0.001 |
| Males, n (%) | 55 (52.4 | 62 (59.0) | 0.33 |
| Diabetes mellitus, n (%) | 16 (15.2) | 36 (34.3) | 0.001 |
| Cardiovascular disease, n (%) | 55 (52.4) | 78 (74.3) | <0.001 |
| Nutritional status | |||
| Malnutrition (SGA >1), n (%) | 35 (34.0) | 62 (60.2) | <0.001 |
| Body mass index (kg/m2) | 24.0 (21.3–28.1) | 23.8 (20.7–26.2) | 0.096 |
| Handgrip strength (%) | 66.7 (55.6–85.2) | 55.1 (42.9–63.3) | <0.001 |
| Biochemicals | |||
| Hemoglobin (g/L) | 116.0 (108.0–122.0) | 115.0(107.0–126.0) | 0.88 |
| Albumin (g/L) | 36.0 (34.0–39.0) | 34.0 (31.0–36.0) | <0.001 |
| hsCRP (mg/L) | 4.2 (1.9–9.9) | 8.4 (3.3–20.5) | 0.001 |
| Triglyceride (mmol/L) | 1.7 (1.2–2.5) | 1.6 (1.1–2.1) | 0.15 |
| Total cholesterol (mmol/L) | 4.3 (3.6–5.1) | 4.4 (3.7–5.0) | 0.56 |
| Ferritin (μg/L) | 412.0(264.0–654.0) | 488.0(266.0–657.0) | 0.46 |
| Fibrinogen (g/L) | 3.7 (3.0–4.4) | 4.3 (3.3–4.8) | 0.010 |
| IL-10 (pg/mL) | 1.2 (0.9–2.7) | 1.4 (0.9–2.1) | 0.34 |
| IL-6 (pg/mL) | 6.4 (3.5–9.6) | 11.0 (6.5–20.5) | <0.001 |
| TNF-α (pg/mL) | 12.8 (10.5–16.1) | 14.0 (12.0–17.9) | 0.025 |
| Leucocytes count (109/L) | 7.0 (6.0–8.7) | 8.1 (6.5–9.6) | 0.035 |
| T3 (nmol/mL) | 0.9 (0.8–1.1) | 0.8 (0.6–1.0) | 0.002 |
| T4 (nmol/mL) | 68.2 (55.3–88.8) | 68.2 (51.5–82.4) | 0.35 |
| TSH (mIU/mL) | 1.3 (0.9–2.3) | 1.8 (0.9–2.8) | 0.15 |
| Pro-BNP (ng/mL) | 6474.0 (2102.0–16827.0) | 15488.0 (6954.0–35001.0) | <0.001 |
| Medications | |||
| β-blockers, n (%) | 51 (48.6) | 54 (51.4) | 0.68 |
| ACEi/ARB, n (%) | 36 (34.3) | 34 (32.7) | 0.81 |
| Statins, n (%) | 37 (35.2) | 32 (30.5) | 0.46 |
| Anti-MDA | |||
| IgM anti-MDA | 88.8 (72.3–104.0) | 78.0 (55.1–95.6) | 0.007 |
| IgG anti-MDA | 80.5 (66.7–93.2) | 81.2 (68.4–97.3) | 0.62 |
| IgG1 anti-MDA | 109.4 (79.4–137.3) | 98.7 (67.9–147.7) | 0.25 |
| IgG2 anti-MDA | 138.7 (118.2–173.9) | 140.8 (115.1–174.5) | 0.78 |
| IgA anti-MDA | 93.1 (57.5–137.5) | 104.0 (73.9–152.5) | 0.21 |
| Variables | IgM anti-MDA | IgG anti-MDA | IgG1 anti-MDA | IgG2 anti-MDA | IgA anti-MDA |
|---|---|---|---|---|---|
| Age (years) | −0.156 * | 0.103 | −0.048 | −0.089 | 0.106 |
| Males, n (%) | −0.029 | 0.084 | 0.044 | 0.064 | 0.080 |
| Smoking | 0.128 * | −0.070 | −0.080 | 0.007 | −0.037 |
| Malnutrition (SGA >1), | 0.007 | 0.079 | 0.071 | 0.044 | 0.074 |
| Cardiovascular disease | −0.073 | 0.067 | 0.031 | 0.021 | 0.082 |
| Diabetes mellitus | −0.070 | −0.054 | −0.050 | 0.004 | 0.079 |
| Davies score | −0.075 | 0.006 | 0.017 | −0.066 | 0.073 |
| β-blockers | −0.036 | 0.137 | 0.112 | 0.026 | 0.053 |
| ACEi/ARB | −0.010 | −0.024 | −0.058 | 0.045 | 0.035 |
| Statins therapy | −0.050 | −0.014 | 0.003 | −0.075 | 0.079 |
| hsCRP (mg/L) | 0.062 | 0.131 * | 0.112 | 0.219 * | 0.057 |
| Albumin (g/L) | −0.154 * | −0.192 * | −0.156 * | −0.162 * | −0.265 * |
| Body mass index (kg/m2) | −0.040 | 0.007 | −0.044 | −0.008 | −0.017 |
| Handgrip strength (%) | 0.038 | −0.045 | −0.011 | −0.079 | 0.053 |
| ferritin_1 | −0.004 | 0.112 | 0.053 | 0.075 | 0.105 |
| Fibrinogen (g/L) | 0.003 | 0.089 | 0.050 | 0.095 | 0.146 * |
| Hemoglobin (g/L) | 0.015 | 0.002 | 0.028 | −0.101 | −0.048 |
| IL-10 (pg/mL) | 0.122 | 0.040 | 0.101 | 0.012 | −0.058 |
| IL-6 (pg/mL) | 0.080 | 0.191 * | 0.100 | 0.109 | 0.192 * |
| Leucocytes count (109/L) | −0.190 * | −0.025 | −0.080 | 0.099 | 0.108 |
| T3 | 0.037 | −0.057 | −0.034 | −0.057 | −0.047 |
| TNF-α (pg/mL) | 0.124 * | 0.432 * | 0.328 * | 0.299 * | 0.056 |
| T4 (nmol/mL) | 0.007 | −0.017 | 0.008 | −0.000 | 0.029 |
| TSH (mIU/mL) | −0.062 | −0.094 | −0.107 | −0.116 | −0.111 |
| Pro-BNP (ng/mL) | −0.032 | 0.130 * | 0.144 * | 0.023 | 0.060 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samal, S.K.; Qureshi, A.R.; Rahman, M.; Stenvinkel, P.; Frostegård, J. Antibodies against Malondialdehyde in Haemodialysis Patients and Its Association with Clinical Outcomes: Differences between Subclasses and Isotypes. J. Clin. Med. 2020, 9, 753. https://doi.org/10.3390/jcm9030753
Samal SK, Qureshi AR, Rahman M, Stenvinkel P, Frostegård J. Antibodies against Malondialdehyde in Haemodialysis Patients and Its Association with Clinical Outcomes: Differences between Subclasses and Isotypes. Journal of Clinical Medicine. 2020; 9(3):753. https://doi.org/10.3390/jcm9030753
Chicago/Turabian StyleSamal, Shailesh Kumar, Abdul Rashid Qureshi, Mizanur Rahman, Peter Stenvinkel, and Johan Frostegård. 2020. "Antibodies against Malondialdehyde in Haemodialysis Patients and Its Association with Clinical Outcomes: Differences between Subclasses and Isotypes" Journal of Clinical Medicine 9, no. 3: 753. https://doi.org/10.3390/jcm9030753
APA StyleSamal, S. K., Qureshi, A. R., Rahman, M., Stenvinkel, P., & Frostegård, J. (2020). Antibodies against Malondialdehyde in Haemodialysis Patients and Its Association with Clinical Outcomes: Differences between Subclasses and Isotypes. Journal of Clinical Medicine, 9(3), 753. https://doi.org/10.3390/jcm9030753






