A Multiplex Test Assessing MiR663ame and VIMme in Urine Accurately Discriminates Bladder Cancer from Inflammatory Conditions
Abstract
1. Introduction
2. Experimental Section
2.1. Patients and Tumour Sample Collection
2.2. Urine Sample Collection and Processing
2.3. Nucleic Acids Isolation, Bisulfite Modification and Multiplex qMSP Analysis
2.4. Statistical Analysis
3. Results
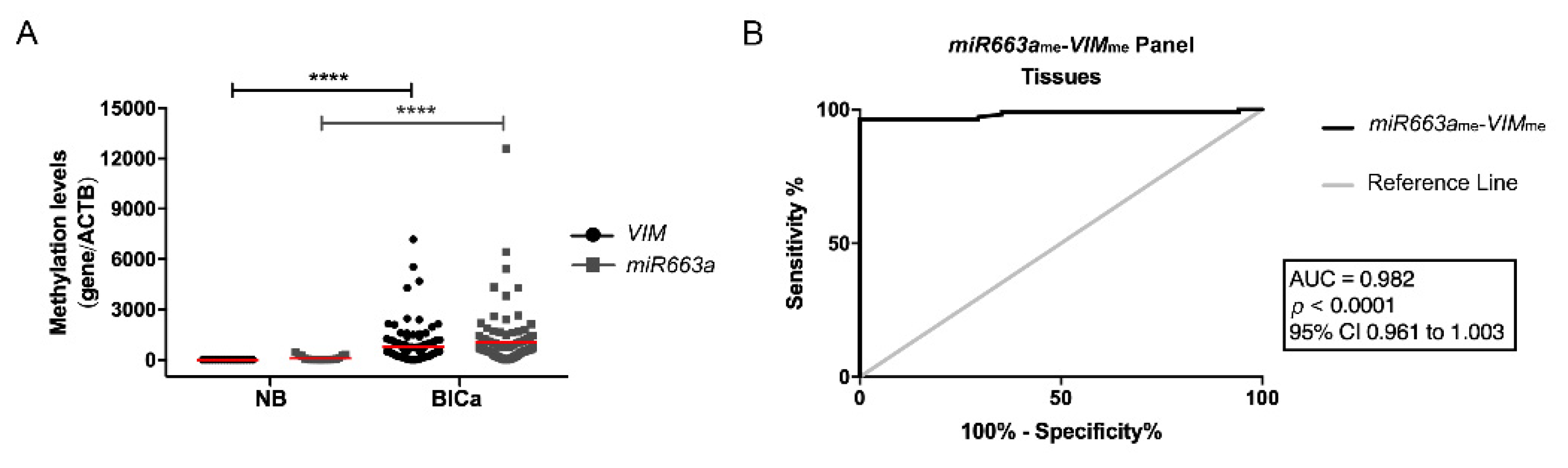
3.1. Methylation Analysis and Performance of the Multiplex Panel in BlCa Tissue Series
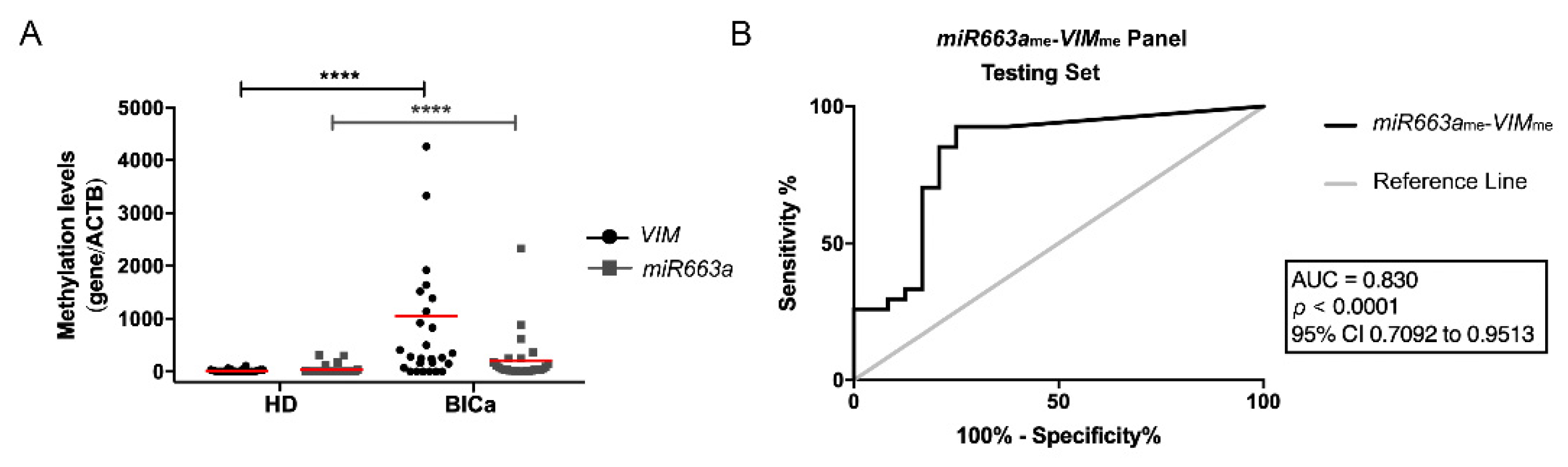
3.2. Methylation Analysis and Performance of Multiplex Panel in BlCa Testing Set
3.3. Methylation Analysis and Performance of VIMme and miR663me Multiplex Panel for BlCa vs. HD
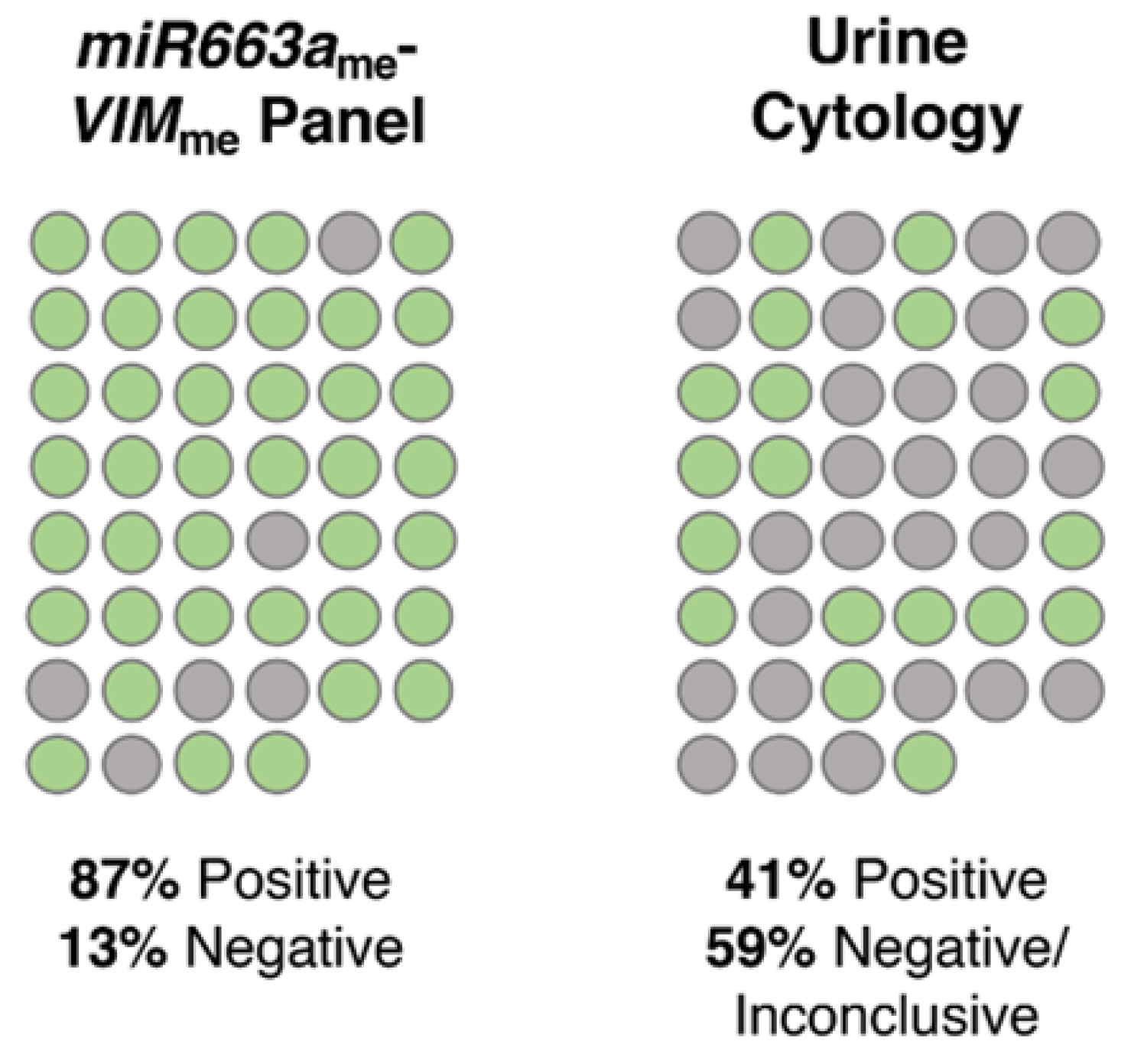
3.4. Methylation Analysis and Performance of VIMme and miR663me Multiplex Panel for BlCa vs. IC
3.5. Clinicopathologic Correlations and Survival Analyses
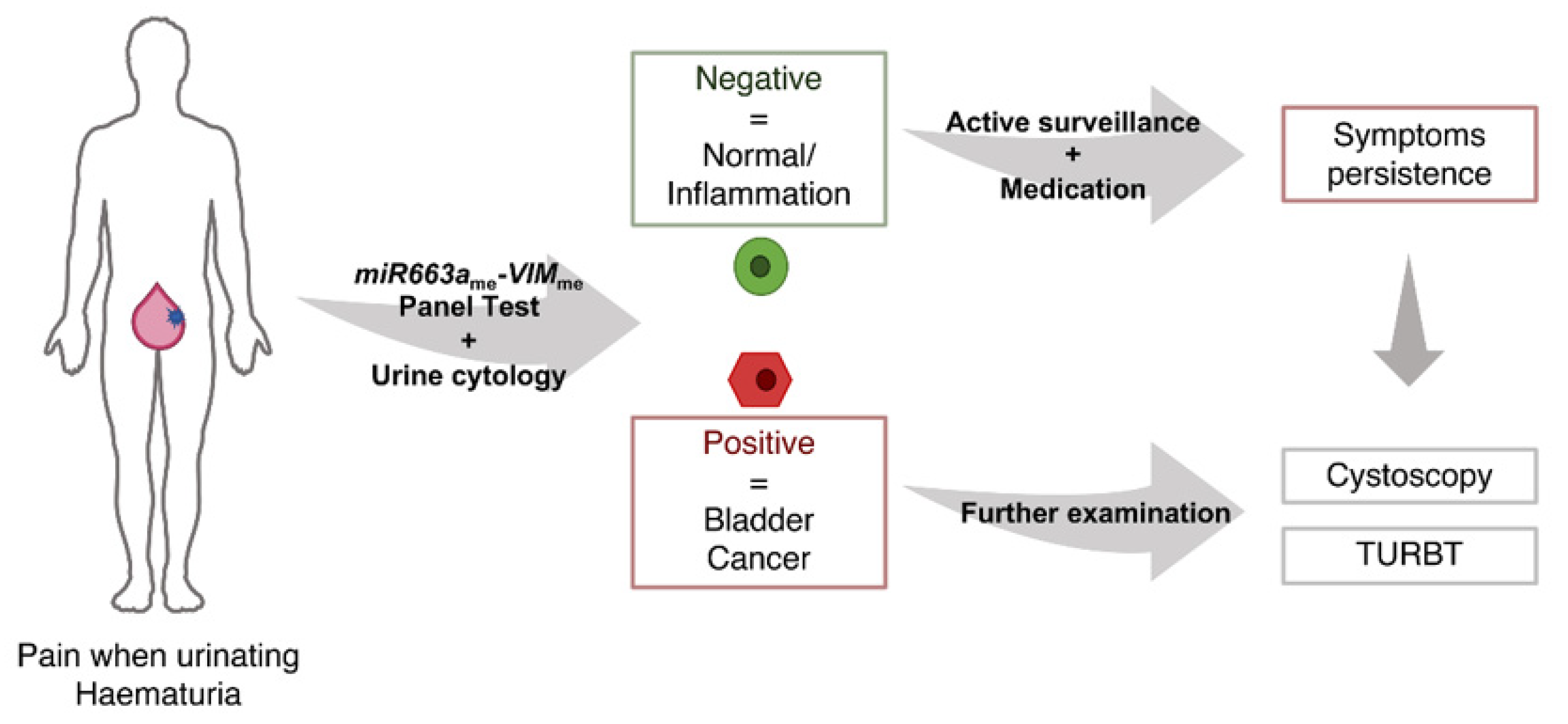
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/home (accessed on 26 October 2019).
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Primers 2017, 3, 17022. [Google Scholar] [CrossRef]
- International Agency for Cancer Research. WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed.; Moch, H., Ulbright, T., Humphrey, P., Reuter, V., Eds.; IARC: Lyon, France, 2016. [Google Scholar]
- Kaufman, D.S.; Shipley, W.U.; Feldman, A.S. Bladder cancer. Lancet (London, England) 2009, 374, 239–249. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Comperat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Alfred Witjes, J.; Lebret, T.; Comperat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernandez, V.; Espinos, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef]
- Leal, J.; Luengo-Fernandez, R.; Sullivan, R.; Witjes, J.A. Economic Burden of Bladder Cancer Across the European Union. Eur. Urol. 2016, 69, 438–447. [Google Scholar] [CrossRef]
- Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinheiro, P.; Montezuma, D.; Henrique, R.; Jeronimo, C. Diagnostic and prognostic epigenetic biomarkers in cancer. Epigenomics 2015, 7, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.L.; Henrique, R.; Danielsen, S.A.; Duarte-Pereira, S.; Eknaes, M.; Skotheim, R.I.; Rodrigues, A.; Magalhaes, J.S.; Oliveira, J.; Lothe, R.A.; et al. Three epigenetic biomarkers, GDF15, TMEFF2, and VIM, accurately predict bladder cancer from DNA-based analyses of urine samples. Clin. Cancer Res. 2010, 16, 5842–5851. [Google Scholar] [CrossRef] [PubMed]
- Padrao, N.A.; Monteiro-Reis, S.; Torres-Ferreira, J.; Antunes, L.; Leca, L.; Montezuma, D.; Ramalho-Carvalho, J.; Dias, P.C.; Monteiro, P.; Oliveira, J.; et al. MicroRNA promoter methylation: A new tool for accurate detection of urothelial carcinoma. Br. J. Cancer 2017, 116, 634–639. [Google Scholar] [CrossRef]
- Heller, M.T.; Tublin, M.E. In search of a consensus: Evaluation of the patient with hematuria in an era of cost containment. Am. J. Roentgenol. 2014, 202, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Srivastava, A.; Lee, R.; Tewari, A.K.; Te, A.E. Role of inflammation in bladder function and interstitial cystitis. Ther. Adv. Urol. 2011, 3, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.; Stirling, D. DNA extraction from tissue. In PCR Protocols, 2nd ed.; Bartlett, J.M.S., Stirling, D., Eds.; Humana Press: Totowa, NJ, USA, 2003. [Google Scholar]
- McGee, S. Simplifying likelihood ratios. J. Gen. Intern. Med. 2002, 17, 646–649. [Google Scholar] [CrossRef]
- Guest, P.C. Multiplex Biomarker Approaches to Enable Point-of-Care Testing and Personalized Medicine. Methods Mol. Biol. 2017, 1546, 311–315. [Google Scholar] [CrossRef]
- Larsen, L.K.; Lind, G.E.; Guldberg, P.; Dahl, C. DNA-Methylation-Based Detection of Urological Cancer in Urine: Overview of Biomarkers and Considerations on Biomarker Design, Source of DNA, and Detection Technologies. Int. J. Mol. Sci. 2019, 20, 2657. [Google Scholar] [CrossRef]
- Anna K Füzéry, D.W.C. Cancer Biomarker Assays: Performance Standards. In Biomarkers in Cancer Screening and Early Detection, 1st ed.; Srivastava, S., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 267–276. [Google Scholar]
- Tan, W.S.; Tan, W.P.; Tan, M.Y.; Khetrapal, P.; Dong, L.; de Winter, P.; Feber, A.; Kelly, J.D. Novel urinary biomarkers for the detection of bladder cancer: A systematic review. Cancer Treat. Rev. 2018, 69, 39–52. [Google Scholar] [CrossRef]
- Chihara, Y.; Kanai, Y.; Fujimoto, H.; Sugano, K.; Kawashima, K.; Liang, G.; Jones, P.A.; Fujimoto, K.; Kuniyasu, H.; Hirao, Y. Diagnostic markers of urothelial cancer based on DNA methylation analysis. BMC Cancer 2013, 13, 275. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Ye, R.; Zhang, D.; Li, Q.; An, D.; Fang, L.; Lin, Y.; Hou, Y.; Xu, A.; et al. An epigenetic biomarker combination of PCDH17 and POU4F2 detects bladder cancer accurately by methylation analyses of urine sediment DNA in Han Chinese. Oncotarget 2016, 7, 2754–2764. [Google Scholar] [CrossRef]
- Yegin, Z.; Gunes, S.; Buyukalpelli, R. Hypermethylation of TWIST1 and NID2 in tumor tissues and voided urine in urinary bladder cancer patients. DNA Cell Biol. 2013, 32, 386–392. [Google Scholar] [CrossRef]
- Renard, I.; Joniau, S.; van Cleynenbreugel, B.; Collette, C.; Naome, C.; Vlassenbroeck, I.; Nicolas, H.; de Leval, J.; Straub, J.; Van Criekinge, W.; et al. Identification and validation of the methylated TWIST1 and NID2 genes through real-time methylation-specific polymerase chain reaction assays for the noninvasive detection of primary bladder cancer in urine samples. Eur. Urol. 2010, 58, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, T.; Wang, Z.; Zhang, H.; Qian, Z.; Xu, H.; Gao, B.; Wang, W.; Gu, L.; Meng, J.; et al. A novel set of DNA methylation markers in urine sediments for sensitive/specific detection of bladder cancer. Clin. Cancer Res. 2007, 13, 7296–7304. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, Z.; Zhu, T.; Yu, J.; Ma, K.; Zhang, H.; He, Y.; Luo, X.; Zhu, J. Hypermethylated SFRP1, but none of other nine genes “informative” for western countries, is valuable for bladder cancer detection in Mainland China. J. Cancer Res. Clin. Oncol. 2009, 135, 1717–1727. [Google Scholar] [CrossRef]
- Chan, M.W.; Chan, L.W.; Tang, N.L.; Tong, J.H.; Lo, K.W.; Lee, T.L.; Cheung, H.Y.; Wong, W.S.; Chan, P.S.; Lai, F.M.; et al. Hypermethylation of multiple genes in tumor tissues and voided urine in urinary bladder cancer patients. Clin. Cancer Res. 2002, 8, 464–470. [Google Scholar] [PubMed]
- Roperch, J.P.; Grandchamp, B.; Desgrandchamps, F.; Mongiat-Artus, P.; Ravery, V.; Ouzaid, I.; Roupret, M.; Phe, V.; Ciofu, C.; Tubach, F.; et al. Promoter hypermethylation of HS3ST2, SEPTIN9 and SLIT2 combined with FGFR3 mutations as a sensitive/specific urinary assay for diagnosis and surveillance in patients with low or high-risk non-muscle-invasive bladder cancer. BMC Cancer 2016, 16, 704. [Google Scholar] [CrossRef]
- Dahmcke, C.M.; Steven, K.E.; Larsen, L.K.; Poulsen, A.L.; Abdul-Al, A.; Dahl, C.; Guldberg, P. A Prospective Blinded Evaluation of Urine-DNA Testing for Detection of Urothelial Bladder Carcinoma in Patients with Gross Hematuria. Eur. Urol. 2016, 70, 916–919. [Google Scholar] [CrossRef]
- Brimo, F.; Vollmer, R.T.; Case, B.; Aprikian, A.; Kassouf, W.; Auger, M. Accuracy of urine cytology and the significance of an atypical category. Am. J. Clin. Pathol. 2009, 132, 785–793. [Google Scholar] [CrossRef]
- Lavery, H.J.; Zaharieva, B.; McFaddin, A.; Heerema, N.; Pohar, K.S. A prospective comparison of UroVysion FISH and urine cytology in bladder cancer detection. BMC Cancer 2017, 17, 247. [Google Scholar] [CrossRef]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 2011, 68, 3033. [Google Scholar] [CrossRef]
- Singh, S.; Sadacharan, S.; Su, S.; Belldegrun, A.; Persad, S.; Singh, G. Overexpression of vimentin: Role in the invasive phenotype in an androgen-independent model of prostate cancer. Cancer Res. 2003, 63, 2306–2311. [Google Scholar]
- Kokkinos, M.I.; Wafai, R.; Wong, M.K.; Newgreen, D.F.; Thompson, E.W.; Waltham, M. Vimentin and Epithelial-Mesenchymal Transition in Human Breast Cancer—Observations in vitro and in vivo. Cells Tissues Organs 2007, 185, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Al-Saad, S.; Al-Shibli, K.; Donnem, T.; Persson, M.; Bremnes, R.M.; Busund, L.T. The prognostic impact of NF-kappaB p105, vimentin, E-cadherin and Par6 expression in epithelial and stromal compartment in non-small-cell lung cancer. Br. J. Cancer 2008, 99, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, C.; Henrique, R. Epigenetic biomarkers in urological tumors: A systematic review. Cancer Lett. 2014, 342, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Zhang, P.; Dong, W.; He, L. MicroRNA-663 suppresses cell invasion and migration by targeting transforming growth factor beta 1 in papillary thyroid carcinoma. Tumour Biol. 2015, 37, 7633–7644. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, C.; Yu, S.; Liu, Q.; Rao, J.; Zhang, H.R.; Xiao, H.L.; Fu, T.W.; Long, H.; He, Z.; et al. MiR-663 suppresses oncogenic function of CXCR4 in glioblastoma. Clin. Cancer Res. 2015, 21, 4004–4013. [Google Scholar] [CrossRef]
- Jiao, L.; Deng, Z.; Xu, C.; Yu, Y.; Li, Y.; Yang, C.; Chen, J.; Liu, Z.; Huang, G.; Li, L.C.; et al. MiR-663 induces castration-resistant prostate cancer transformation and predicts clinical recurrence. J. Cell Physiol. 2014, 229, 834–844. [Google Scholar] [CrossRef]
- Huang, C.; Sun, Y.; Ma, S.; Vadamootoo, A.S.; Wang, L.; Jin, C. Identification of circulating miR-663a as a potential biomarker for diagnosing osteosarcoma. Pathol. Res. Pract. 2019, 215, 152411. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Zhang, M.; Wang, X.; Bai, X.; Li, H.; Kan, L.; Zhou, Y.; Niu, H.; He, P. MicroRNA-663a is downregulated in non-small cell lung cancer and inhibits proliferation and invasion by targeting JunD. BMC Cancer 2016, 16, 315. [Google Scholar] [CrossRef]
- Huang, W.; Li, J.; Guo, X.; Zhao, Y.; Yuan, X. MiR-663a inhibits hepatocellular carcinoma cell proliferation and invasion by targeting HMGA2. Biomed. Pharmacother. 2016, 81, 431–438. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, B.; Jiao, A.; Li, F.; Sun, N.; Zhang, G.; Zhang, J. MiR-663a inhibits tumor growth and invasion by regulating TGF-β1 in hepatocellular carcinoma. BMC Cancer 2018, 18, 1179. [Google Scholar] [CrossRef]

| Tissues | Urines | ||||||
|---|---|---|---|---|---|---|---|
| Testing Set | Validation Sets | ||||||
| Clinicopaphological Features | Bladder UC | Normal Bladder Mucosae | Bladder UC | Healthy Donors | Bladder UC | Healthy Donors (#1) | Inflammatory Controls (#2) |
| Patients, n | 94 | 19 | 27 | 24 | 100 | 57 | 174 |
| Gender, n | |||||||
| Males | 78 | 19 | 20 | 13 | 79 | 16 | 132 |
| Females | 16 | 0 | 7 | 12 | 21 | 41 | 42 |
| Median age, yrs (range) | 69 (45–91) | 63 (48–75) | 69 (47–88) | 45 (39–61) | 68 (38–91) | 49 (41–64) | 64 (18–92) |
| Grade, n | |||||||
| Papillary, low-grade | 34 | n.a. | 13 | n.a. | 51 | n.a. | n.a. |
| Papillary, high-grade | 33 | n.a. | 8 | n.a. | 26 | n.a. | n.a. |
| Invasive, high-grade | 27 | n.a. | 6 | n.a. | 23 | n.a. | n.a. |
| Invasion of Muscular Layer, n | |||||||
| NMIBC | 67 | n.a. | 19 | n.a. | 77 | n.a. | n.a. |
| MIBC | 27 | n.a. | 8 | n.a. | 23 | n.a. | n.a. |
| Samples | Biomarker Performance | miR663ame-VIMme (%) |
|---|---|---|
| Validation #1 | Sensitivity | 87.0 |
| Specificity | 86.0 | |
| PPV | 91.6 | |
| NPV | 79.0 | |
| Accuracy | 86.6 | |
| Validation #2 | Sensitivity | 80.0 |
| Specificity | 75.3 | |
| PPV | 65.0 | |
| NPV | 86.8 | |
| Accuracy | 77.0 |
| Disease-specific Survival | Variables | Hazard Ratio (HR) | 95% CI for OR | p |
|---|---|---|---|---|
| Univariate | Invasion of muscular layer | 6.15 | 2.76–13.72 | 0.0001 |
| Grade | ||||
| PLG vs. PHG | 15.59 | 2.03–119.94 | 0.008 | |
| PLG vs. IHG | 32.83 | 4.31–250.06 | 0.001 | |
| Age | 2.34 | 0.98–5.59 | 0.060 | |
| Gender | 1.02 | 0.39–2.70 | 0.970 | |
| miR663a methylation ≤ median | 1.61 | 0.75–3.48 | 0.225 | |
| VIM methylation ≤ median | 1.07 | 0.50–2.28 | 0.861 | |
| Multivariate | Invasion of muscular layer | 3.54 | 1.12–11.19 | 0.031 |
| Grade | ||||
| PLG vs. PHG | 8.03 | 0.97–66.32 | 0.053 | |
| PLG vs. IHG | 11.89 | 1.18–119.37 | 0.035 | |
| miR663a methylation ≤ median | 2.67 | 1.05–6.81 | 0.040 | |
| VIM methylation ≤ median | 1.12 | 0.51–2.42 | 0.783 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro-Reis, S.; Blanca, A.; Tedim-Moreira, J.; Carneiro, I.; Montezuma, D.; Monteiro, P.; Oliveira, J.; Antunes, L.; Henrique, R.; Lopez-Beltran, A.; et al. A Multiplex Test Assessing MiR663ame and VIMme in Urine Accurately Discriminates Bladder Cancer from Inflammatory Conditions. J. Clin. Med. 2020, 9, 605. https://doi.org/10.3390/jcm9020605
Monteiro-Reis S, Blanca A, Tedim-Moreira J, Carneiro I, Montezuma D, Monteiro P, Oliveira J, Antunes L, Henrique R, Lopez-Beltran A, et al. A Multiplex Test Assessing MiR663ame and VIMme in Urine Accurately Discriminates Bladder Cancer from Inflammatory Conditions. Journal of Clinical Medicine. 2020; 9(2):605. https://doi.org/10.3390/jcm9020605
Chicago/Turabian StyleMonteiro-Reis, Sara, Ana Blanca, Joana Tedim-Moreira, Isa Carneiro, Diana Montezuma, Paula Monteiro, Jorge Oliveira, Luís Antunes, Rui Henrique, António Lopez-Beltran, and et al. 2020. "A Multiplex Test Assessing MiR663ame and VIMme in Urine Accurately Discriminates Bladder Cancer from Inflammatory Conditions" Journal of Clinical Medicine 9, no. 2: 605. https://doi.org/10.3390/jcm9020605
APA StyleMonteiro-Reis, S., Blanca, A., Tedim-Moreira, J., Carneiro, I., Montezuma, D., Monteiro, P., Oliveira, J., Antunes, L., Henrique, R., Lopez-Beltran, A., & Jerónimo, C. (2020). A Multiplex Test Assessing MiR663ame and VIMme in Urine Accurately Discriminates Bladder Cancer from Inflammatory Conditions. Journal of Clinical Medicine, 9(2), 605. https://doi.org/10.3390/jcm9020605







