Plasma Lysyl-tRNA Synthetase 1 (KARS1) as a Novel Diagnostic and Monitoring Biomarker for Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Human Samples
2.2. Materials
2.3. Inflammatory-Induced Colorectal Colitis Cancer Model
2.4. Harvesting Mouse Plasma Samples
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Statistical Analysis
3. Results
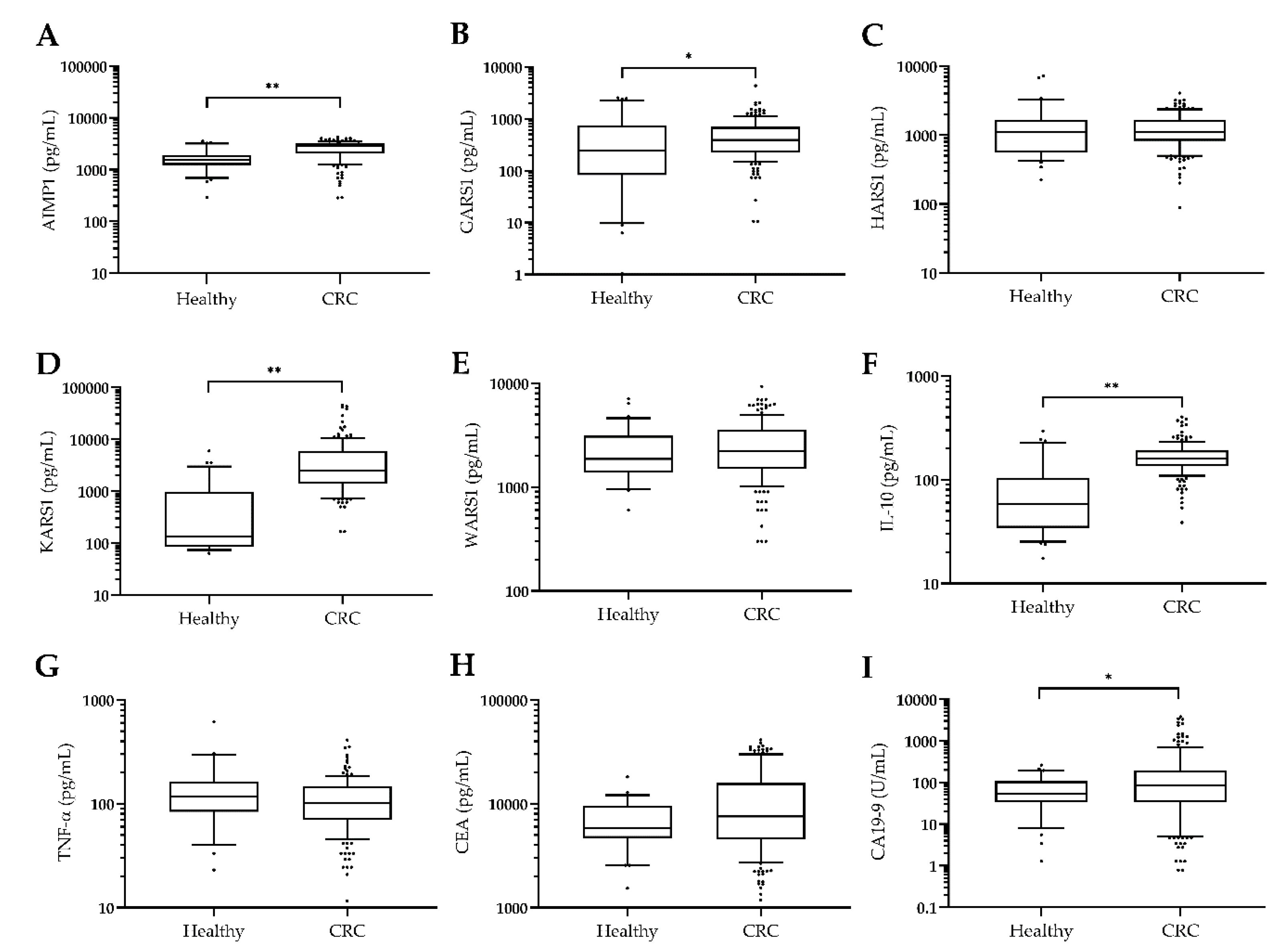
3.1. Plasma KARS1 Levels Were Increased in CRC Patients
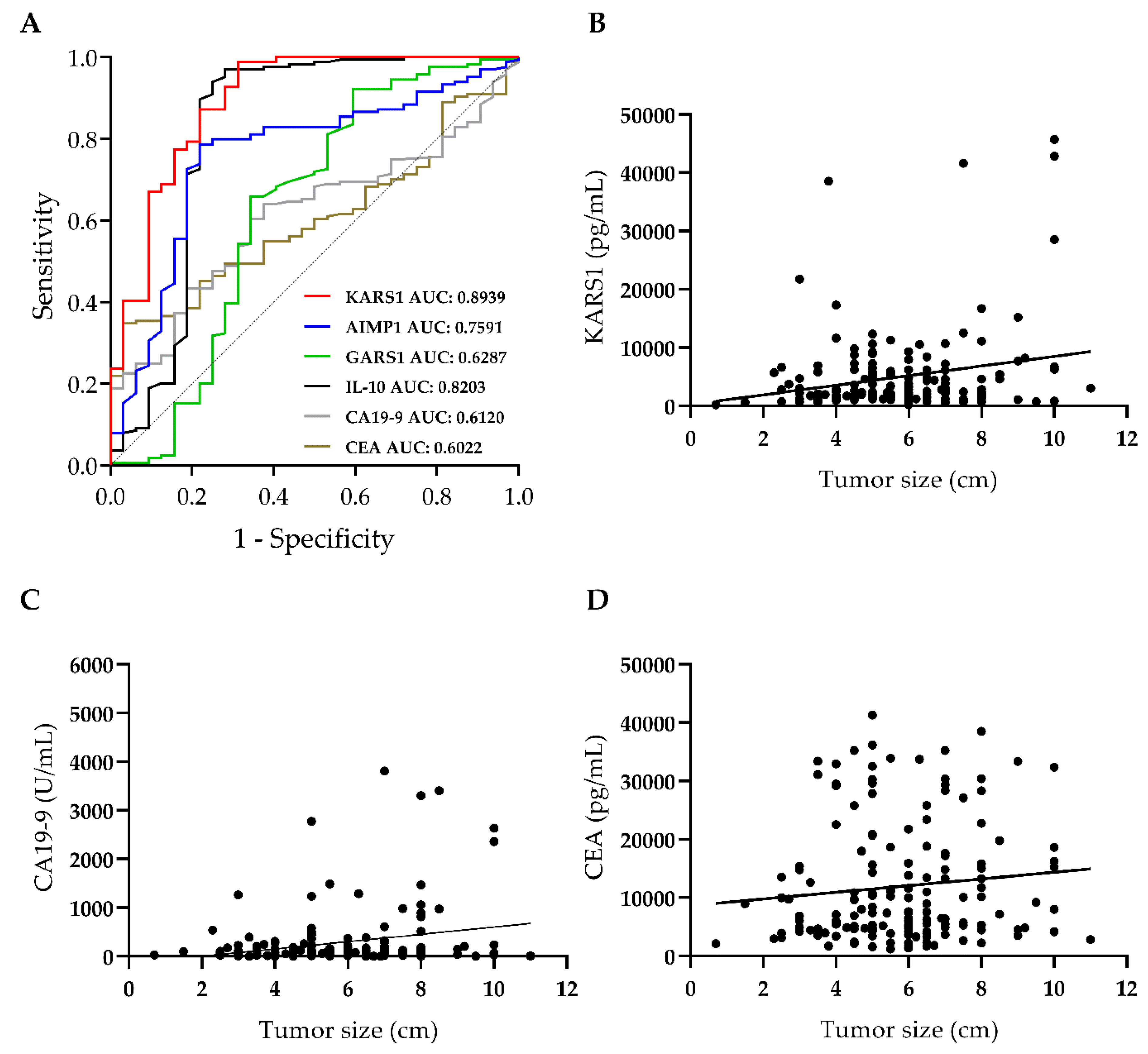
3.2. Plasma KARS1 Level Showed Diagnostic Potential in CRC
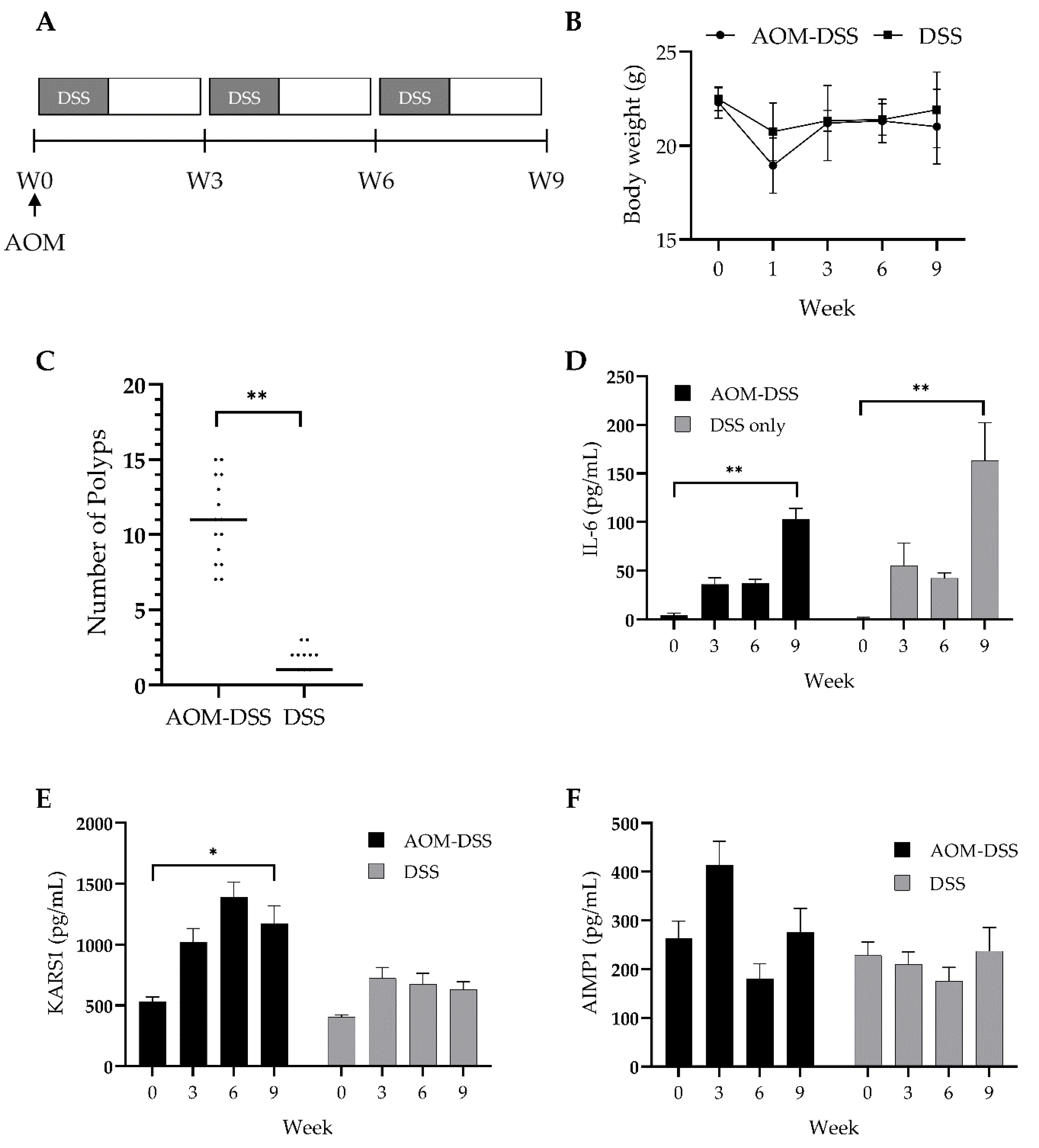
3.3. Validation of Diagnostic Potential of Plasma KARS1 Using Colitis-Induced CRC Mouse Model
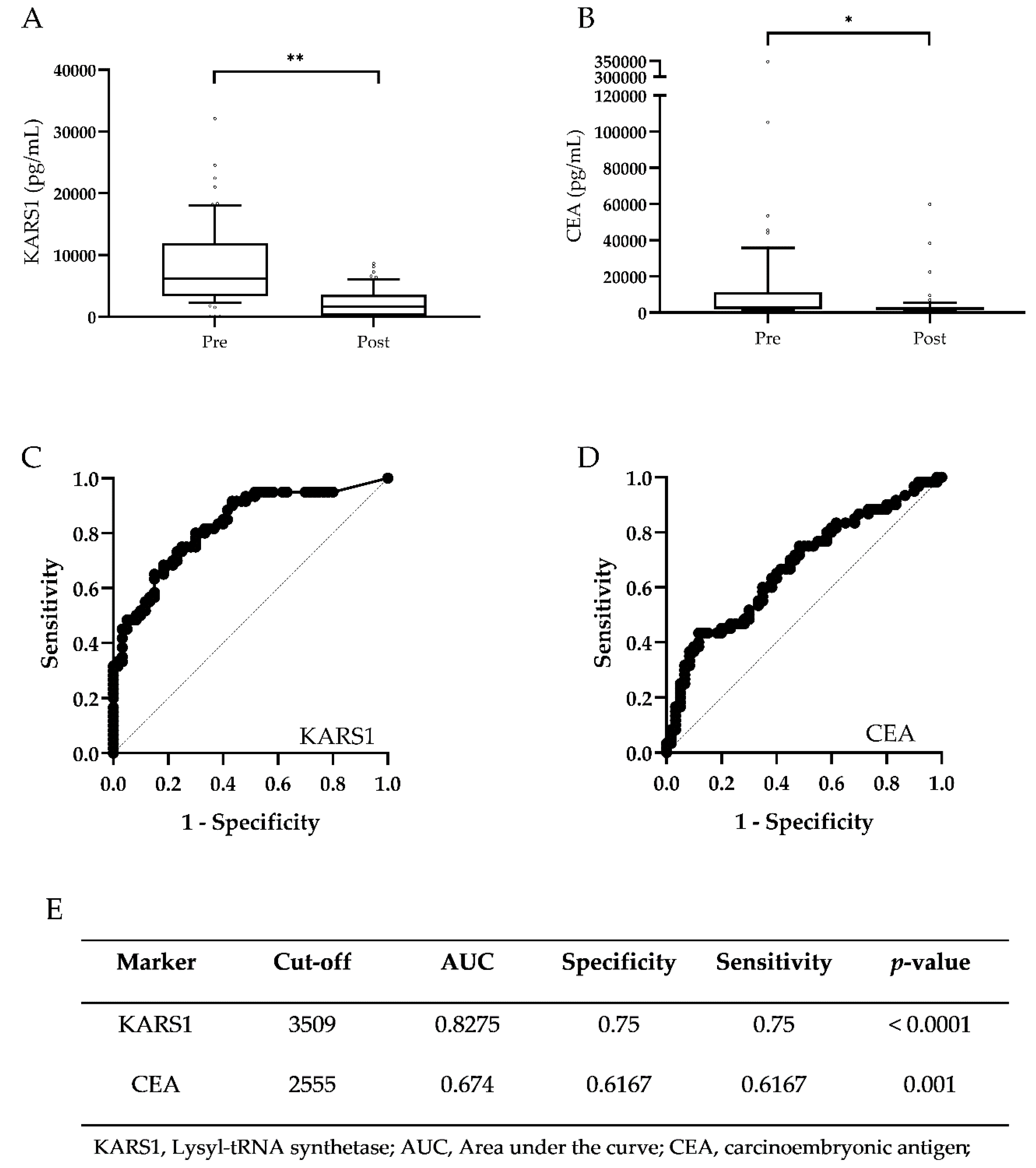
3.4. Validation of Monitoring Potential of Plasma KARS1 in CRC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal Cancer Statistics, 2017. Ca. Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global Patterns and Trends in Colorectal Cancer Incidence in Young Adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Hiom, S.C. Diagnosing Cancer Earlier: Reviewing the Evidence for Improving Cancer Survival. Br. J. Cancer 2015, 112, 1–5. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Colorectal Cancer Mortality Rates in Adults Aged 20 to 54 Years in the United States, 1970-2014. JAMA 2017, 318, 572–574. [Google Scholar] [CrossRef]
- Swiderska, M.; Choromańska, B.; Dąbrowska, E.; Konarzewska-Duchnowska, E.; Choromańska, K.; Szczurko, G.; Myśliwiec, P.; Dadan, J.; Ladny, J.R.; Zwierz, K. The Diagnostics of Colorectal Cancer. Contemp. Oncol. 2014, 18, 1–6. [Google Scholar]
- Robertson, J.D.; Lee, J.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Lieberman, D.; Levin, T.R.; Rex, D.K. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 152, 1217–1237. [Google Scholar]
- Zafar, H.M.; Yang, J.; Armstrong, K.; Groeneveld, P. Cost Differences after Initial Ct Colonography Versus Optical Colonoscopy in the Elderly. Acad. Radiol. 2015, 22, 807–813. [Google Scholar] [CrossRef][Green Version]
- Hamzehzadeh, L.; Yousefi, M.; Ghaffari, S.H. Colorectal Cancer Screening: A Comprehensive Review to Recent Non-Invasive Methods. Int. J. Hematol. Oncol. Stem. Cell Res. 2017, 11, 250–261. [Google Scholar]
- Vacante, M.; Borzì, A.M.; Basile, F.; Biondi, A. Biomarkers in Colorectal Cancer: Current Clinical Utility and Future Perspectives. World J. Clin. Cases 2018, 6, 869–881. [Google Scholar] [CrossRef]
- Chan, C.C.; Fan, C.W.; Kuo, Y.B.; Chen, Y.H.; Chang, P.Y.; Chen, K.T.; Hung, R.P.; Chan, E.C. Multiple Serological Biomarkers for Colorectal Cancer Detection. Int. J. Cancer 2010, 126, 1683–1690. [Google Scholar] [CrossRef]
- Duffy, M.J. Carcinoembryonic Antigen as a Marker for Colorectal Cancer: Is It Clinically Useful? Clin. Chem. 2001, 47, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Tabor, B.; Buckley, M.J.; Priebe, I.K.; Purins, L.; Pompeia, C.; Brierley, G.V.; Lockett, T.; Gibbs, P.; Tie, J.; et al. Blood- Based Protein Biomarker Panel for the Detection of Colorectal Cancer. PLoS ONE 2015, 10, e0120425. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bilbao, A.; Armananzas, R.; Ispizua, Z.; Calvo, B.; Alonso-Varona, A.; Inza, I.; Larranaga, P.; Lopez-Vivanco, G.; Suarez-Merino, B.; Betanzos, M. Identification of a Biomarker Panel for Colorectal Cancer Diagnosis. BMC Cancer 2012, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Robin, X.; Turck, N.; Hainard, A.; Lisacek, F.; Sanchez, J.C.; Muller, M. Bioinformatics for Protein Biomarker Panel Classification: What Is Needed to Bring Biomarker Panels into in Vitro Diagnostics? Expert Rev. Proteom. 2009, 6, 675–689. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Law, W.L.; Ng, E.K.O.; Chan, C.S.Y.; Lau, K.S.; Cheng, Y.Y.; Shin, V.Y.; Kwong, A.; Leung, W.K. Methylated Septin 9 and Carcinoembryonic Antigen for Serological Diagnosis and Monitoring of Patients with Colorectal Cancer after Surgery. Sci. Rep. 2019, 9, 10326. [Google Scholar] [CrossRef]
- Warren, J.D.; Xiong, W.; Bunker, A.M.; Vaughn, C.P.; Furtado, L.V.; Roberts, W.L.; Fang, J.C.; Samowitz, W.S.; Heichman, K.A. Septin 9 Methylated DNA Is a Sensitive and Specific Blood Test for Colorectal Cancer. BMC Med. 2011, 9, 133. [Google Scholar] [CrossRef]
- Sun, J.; Fei, F.; Zhang, M.; Li, Y.; Zhang, X.; Zhu, S.; Zhang, S. The Role of (M)Sept9 in Screening, Diagnosis, and Recurrence Monitoring of Colorectal Cancer. BMC Cancer 2019, 19, 450. [Google Scholar] [CrossRef]
- Kim, S.; You, S.; Hwang, D. Aminoacyl-Trna Synthetases and Tumorigenesis: More Than Housekeeping. Nat. Rev. Cancer 2011, 11, 708–718. [Google Scholar] [CrossRef]
- Park, S.G.; Schimmel, P.; Kim, S. Aminoacyl Trna Synthetases and Their Connections to Disease. Proc. Natl. Acad. Sci. USA 2008, 105, 11043–11049. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Park, S.; Choi, J.J.; Park, B.K.; Rhee, K.H.; Kang, E.; Ahn, S.; Lee, C.H.; Lee, J.S.; Inn, K.S.; et al. Secreted Tryptophanyl-Trna Synthetase as a Primary Defence System against Infection. Nat. Microbiol. 2016, 2, 16191. [Google Scholar] [CrossRef]
- Kim, D.G.; Choi, J.W.; Lee, J.Y.; Kim, H.; Oh, Y.S.; Lee, J.W.; Tak, Y.K.; Song, J.M.; Razin, E.; Yun, S.H.; et al. Interaction of Two Translational Components, Lysyl-Trna Synthetase and P40/37lrp, in Plasma Membrane Promotes Laminin-Dependent Cell Migration. Faseb. J. 2012, 26, 4142–4159. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Kim, H.J.; Min, Y.H.; Choi, E.C.; Shin, Y.K.; Park, B.J.; Lee, S.W.; Kim, S. Human Lysyl-Trna Synthetase Is Secreted to Trigger Proinflammatory Response. Proc. Natl. Acad. Sci. USA 2005, 102, 6356–6361. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Kim, D.; Lee, D.; Lee, H.M.; Song, D.G.; Jung, J.W.; Kim, J.E.; Kim, H.J.; Kwon, N.H.; Jo, E.K.; et al. Lysyl-Trna Synthetase-Expressing Colon Spheroids Induce M2 Macrophage Polarization to Promote Metastasis. J. Clin. Invest. 2018, 128, 5034–5055. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Kim, H.R.; Park, M.C.; Cho, S.; Goughnour, P.C.; Han, D.; Yoon, I.; Kim, Y.; Kang, T.; Song, E.; et al. Caspase-8 Controls the Secretion of Inflammatory Lysyl-Trna Synthetase in Exosomes from Cancer Cells. J. Cell Biol. 2017, 216, 2201–2216. [Google Scholar] [CrossRef] [PubMed]
- Bullwinkle, T.J.; Dewan, V.; Florentz, C.; Forsyth, K.-M.; Fox, P.L.; Gruic-Sovulj, I.; Guo, M.; Han, J.M.; Ibba, M.; Kim, D.; et al. Aminoacyl-tRNA synthetases in biology and medicine; Kim, S., Ed.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Zhou, J.J.; Wang, F.; Xu, Z.; Lo, W.S.; Lau, C.F.; Chiang, K.P.; Nangle, L.A.; Ashlock, M.A.; Mendlein, J.D.; Yang, X.L.; et al. Secreted Histidyl-Trna Synthetase Splice Variants Elaborate Major Epitopes for Autoantibodies in Inflammatory Myositis. J. Biol. Chem. 2014, 289, 19269–19275. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and Cancer: How Hot Is the Link? Biochem. Pharm. 2006, 72, 1605–1621. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N.; Richmond, A. The Role of Chemokines in Intestinal Inflammation and Cancer. Curr. Opin. Pharm. 2009, 9, 688–696. [Google Scholar] [CrossRef]
- Kundu, J.K.; Surh, Y.J. Emerging Avenues Linking Inflammation and Cancer. Free Radic. Biol. Med. 2012, 52, 2013–2037. [Google Scholar] [CrossRef]
- Neufert, C.; Becker, C.; Neurath, M.F. An Inducible Mouse Model of Colon Carcinogenesis for the Analysis of Sporadic and Inflammation-Driven Tumor Progression. Nat. Protoc. 2007, 2, 1998–2004. [Google Scholar] [CrossRef]
- Kinoshita, T.; Ito, H.; Miki, C. Serum Interleukin-6 Level Reflects the Tumor Proliferative Activity in Patients with Colorectal Carcinoma. Cancer 1999, 85, 2526–2531. [Google Scholar] [CrossRef]
- Galizia, G.; Orditura, M.; Romano, C.; Lieto, E.; Castellano, P.; Pelosio, L.; Imperatore, V.; Catalano, G.; Pignatelli, C.; de Vita, F. Prognostic Significance of Circulating Il-10 and Il-6 Serum Levels in Colon Cancer Patients Undergoing Surgery. Clin. Immunol. 2002, 102, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Abulafi, A.M.; Williams, N.S. Local Recurrence of Colorectal Cancer: The Problem, Mechanisms, Management and Adjuvant Therapy. Br. J. Surg. 1994, 81, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Buie, W.D.; Attard, J.A. Follow-up Recommendations for Colon Cancer. Clin. Colon. Rectal. Surg. 2005, 18, 232–243. [Google Scholar] [CrossRef][Green Version]
- Gold, P.; Freedman, S.O. Demonstration of Tumor-Specific Antigens in Human Colonic Carcinomata by Immunological Tolerance and Absorption Techniques. J. Exp. Med. 1965, 121, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Magnani, J.L.; Steplewski, Z.; Koprowski, H.; Ginsburg, V. Identification of the Gastrointestinal and Pancreatic Cancer-Associated Antigen Detected by Monoclonal Antibody 19–9 in the Sera of Patients as a Mucin. Cancer Res. 1983, 43, 5489–5492. [Google Scholar] [PubMed]
- Passerini, R.; Cassatella, M.C.; Boveri, S.; Salvatici, M.; Radice, D.; Zorzino, L.; Galli, C.; Sandri, M.T. The Pitfalls of Ca19-9: Routine Testing and Comparison of Two Automated Immunoassays in a Reference Oncology Center. Am. J. Clin. Pathol. 2012, 138, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Litvak, A.; Cercek, A.; Segal, N.; Reidy-Lagunes, D.; Stadler, Z.K.; Yaeger, R.D.; Kemeny, N.E.; Weiser, M.R.; Pessin, M.S.; Saltz, L. False-Positive Elevations of Carcinoembryonic Antigen in Patients with a History of Resected Colorectal Cancer. J. Natl. Compr. Canc. Netw. 2014, 12, 907–913. [Google Scholar] [CrossRef]
- Ardekani, A.E.; Amini, H.; Paymani, Z.; Fard-Esfahani, A. False-Positive Elevated Cea During Colon Cancer Surveillance: A Cholecystitis Case Report Diagnosed by Pet-Ct Scan. J. Surg. Case. Rep. 2019, 6, 138. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohn's Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Forbes, G.M. Colorectal Cancer Screening Tests: Pros and Cons, and for Whom? Expert Rev. Gastroenterol. Hepatol. 2008, 2, 197–205. [Google Scholar] [CrossRef]
- Jones, R.M.; Devers, K.J.; Kuzel, A.J.; Woolf, S.H. Patient-Reported Barriers to Colorectal Cancer Screening: A Mixed-Methods Analysis. Am. J. Prev. Med. 2010, 38, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, R.; Wang, H.; Li, W.; Pan, M.; Yao, X.; Zhan, W.; Yang, S.; Xu, L.; Ding, Y.; et al. Gut Microbiota-Stimulated Cathepsin K Secretion Mediates Tlr4-Dependent M2 Macrophage Polarization and Promotes Tumor Metastasis in Colorectal Cancer. Cell Death Differ. 2019, 26, 2447–2463. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Sun, L.; Xu, F.; Liu, L.; Hu, F.; Song, D.; Hou, Z.; Wu, W.; Luo, X.; Wang, J.; et al. M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer Res. 2019, 79, 146–158. [Google Scholar] [CrossRef] [PubMed]
| Healthy | CRC | |||
|---|---|---|---|---|
| No. | No. | |||
| Total(n) | 32 | 164 | ||
| Sex | Male(n) | 20 | 93 | |
| Female(n) | 12 | 71 | ||
| Age (years) | 44.3 ± 9.27 | 60.2 ± 12.3 | ||
| Tumor size (cm) | - | 5.75 ± 1.89 | ||
| Cancer Grade | I | - | 14 | |
| II | - | 50 | ||
| III | - | 50 | ||
| IV | - | 50 |
| CRC | ||
|---|---|---|
| No. | ||
| Total(n) | 60 | |
| Sex | Male(n) | 44 |
| Female(n) | 16 | |
| Age (years) | 58.45 ± 10.63 | |
| Tumor size (cm) | 1.88 ± 1.30 | |
| Cancer Grade | I | - |
| II | 3 | |
| III | 47 | |
| IV | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, J.H.; Park, M.C.; Goughnour, P.C.; Min, B.S.; Kim, S.B.; Lee, W.Y.; Cho, Y.B.; Cheon, J.H.; Lee, K.Y.; Nam, D.-H.; et al. Plasma Lysyl-tRNA Synthetase 1 (KARS1) as a Novel Diagnostic and Monitoring Biomarker for Colorectal Cancer. J. Clin. Med. 2020, 9, 533. https://doi.org/10.3390/jcm9020533
Suh JH, Park MC, Goughnour PC, Min BS, Kim SB, Lee WY, Cho YB, Cheon JH, Lee KY, Nam D-H, et al. Plasma Lysyl-tRNA Synthetase 1 (KARS1) as a Novel Diagnostic and Monitoring Biomarker for Colorectal Cancer. Journal of Clinical Medicine. 2020; 9(2):533. https://doi.org/10.3390/jcm9020533
Chicago/Turabian StyleSuh, Ji Hun, Min Chul Park, Peter C. Goughnour, Byung Soh Min, Sang Bum Kim, Woo Yong Lee, Yong Beom Cho, Jae Hee Cheon, Kang Young Lee, Do-Hyun Nam, and et al. 2020. "Plasma Lysyl-tRNA Synthetase 1 (KARS1) as a Novel Diagnostic and Monitoring Biomarker for Colorectal Cancer" Journal of Clinical Medicine 9, no. 2: 533. https://doi.org/10.3390/jcm9020533
APA StyleSuh, J. H., Park, M. C., Goughnour, P. C., Min, B. S., Kim, S. B., Lee, W. Y., Cho, Y. B., Cheon, J. H., Lee, K. Y., Nam, D.-H., & Kim, S. (2020). Plasma Lysyl-tRNA Synthetase 1 (KARS1) as a Novel Diagnostic and Monitoring Biomarker for Colorectal Cancer. Journal of Clinical Medicine, 9(2), 533. https://doi.org/10.3390/jcm9020533





