Diagnostic Validity of Digital Imaging Fiber-Optic Transillumination (DIFOTI) and Near-Infrared Light Transillumination (NILT) for Caries in Dentine
Abstract
1. Introduction
2. Experimental Section
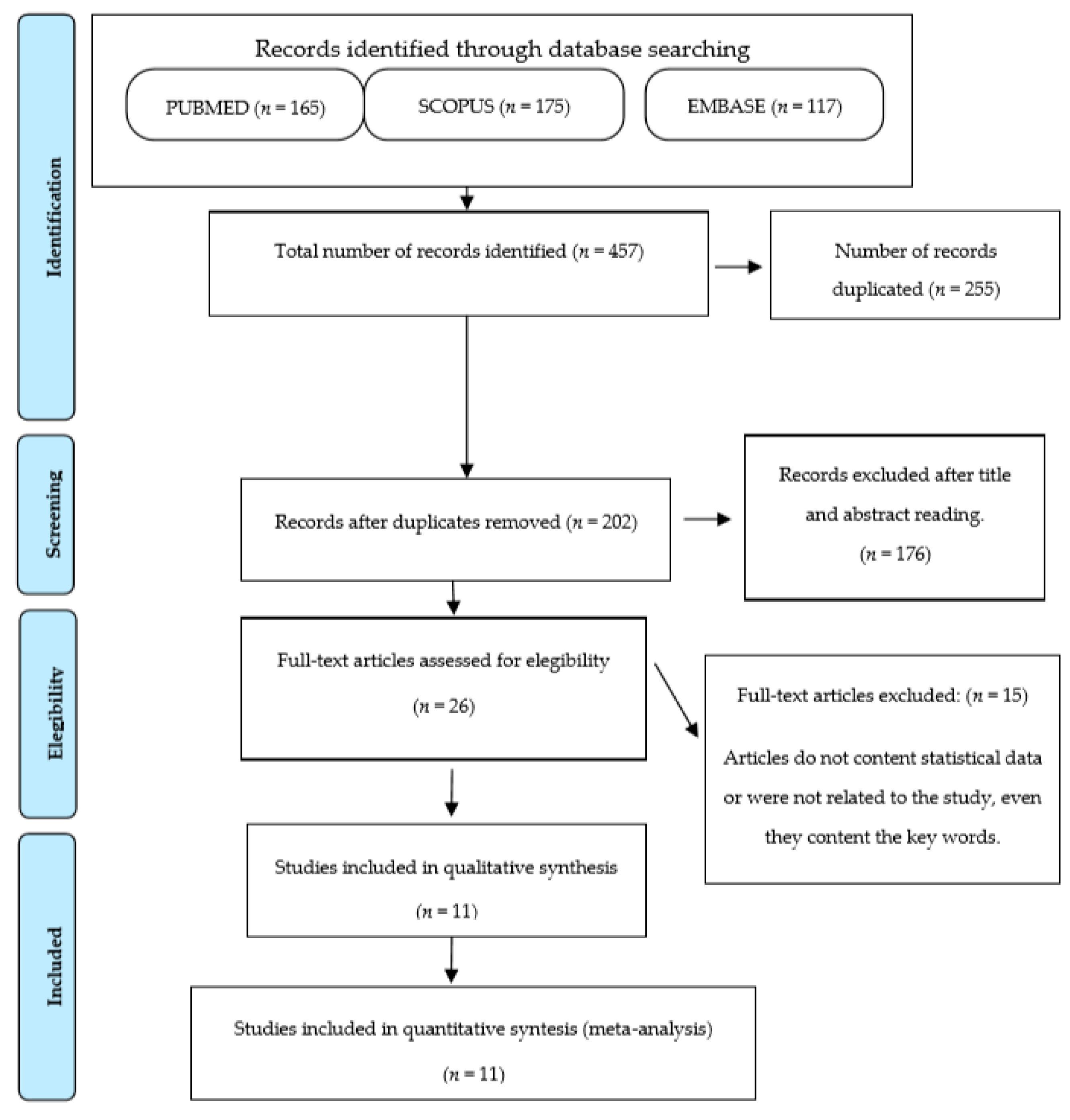
2.1. Article Search and Selection Strategies
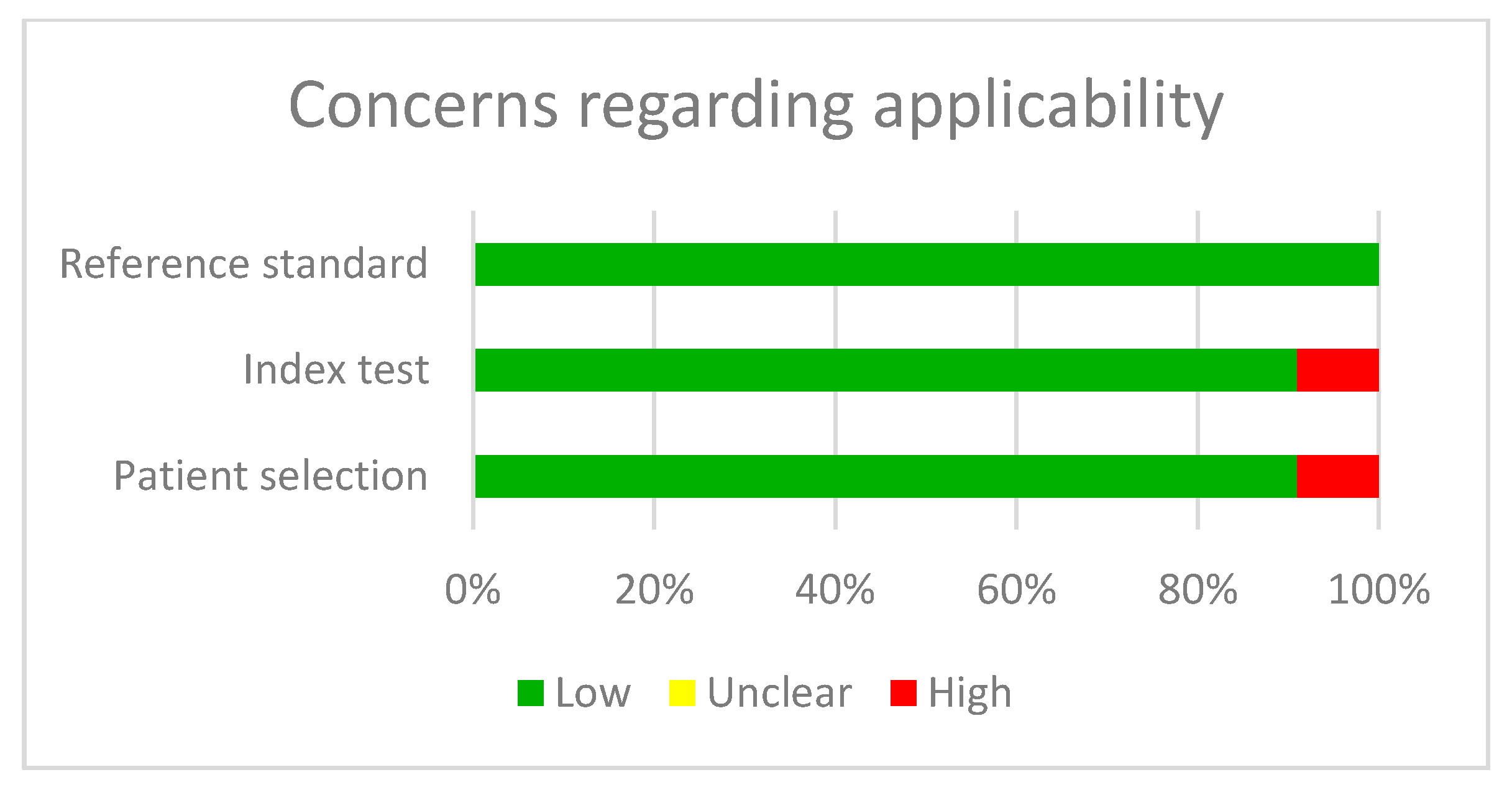
2.2. Article Quality Analysis
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Bibliographic Search
3.2. Qualitative Analysis
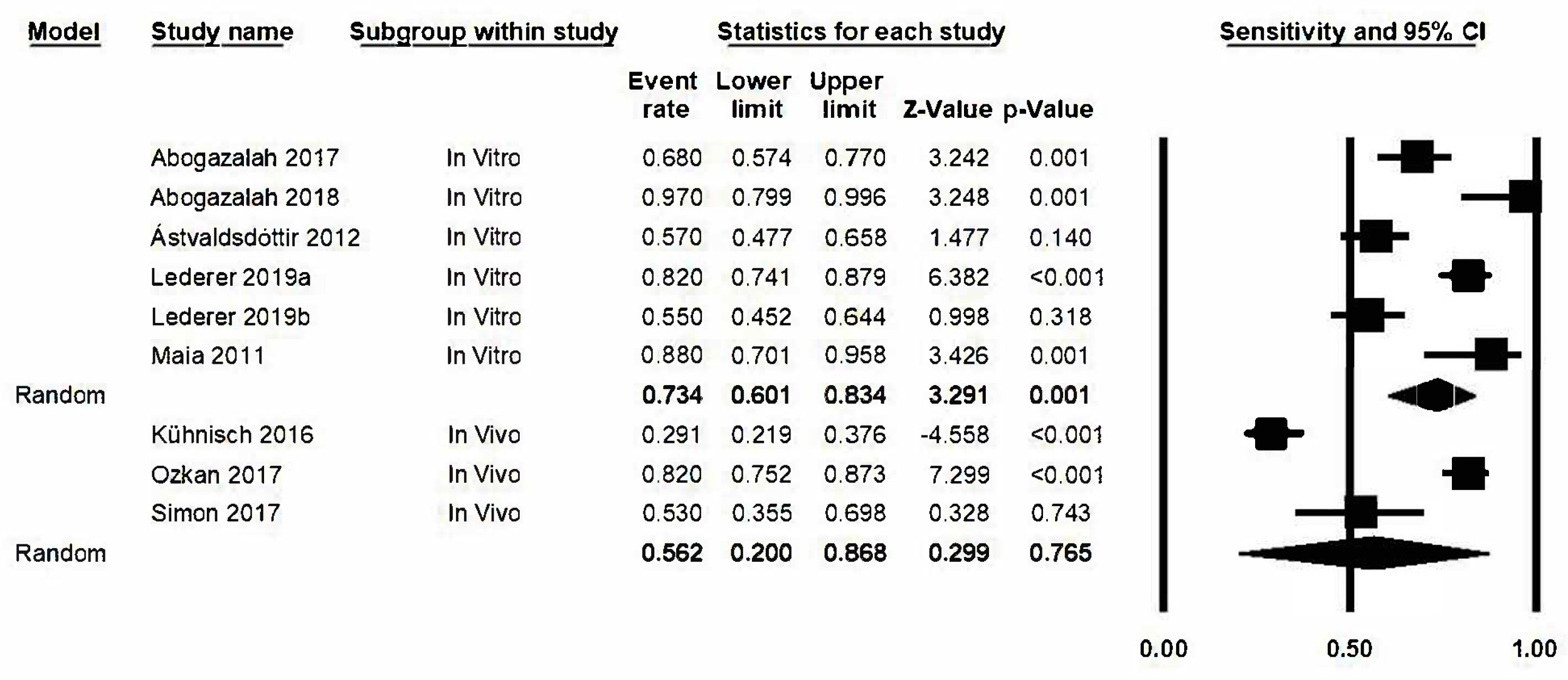
3.3. Quantitative Analysis
3.4. Sensitivity
3.5. Analysis Based on Subgroups and Metaregression
3.6. “One Study Removed” Analysis
3.7. Specificity
3.8. Analysis Based on Subgroups and Metaregression
3.9. “One Study Removed” Analysis
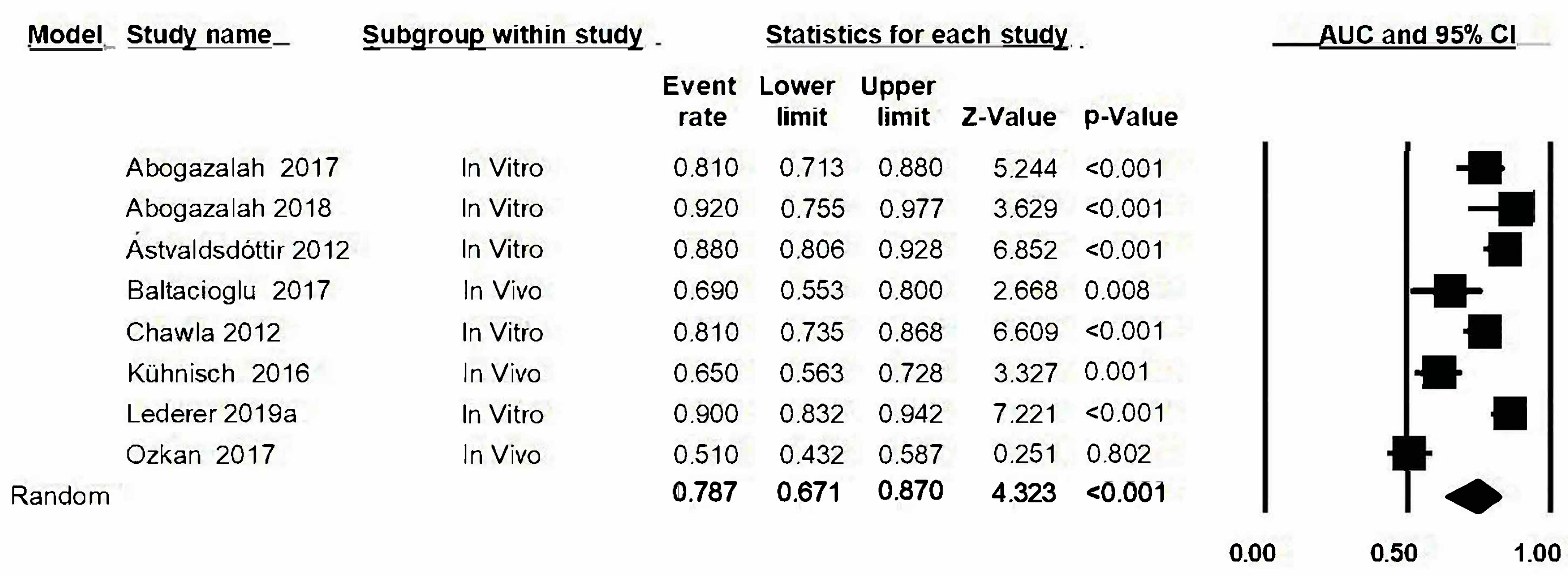
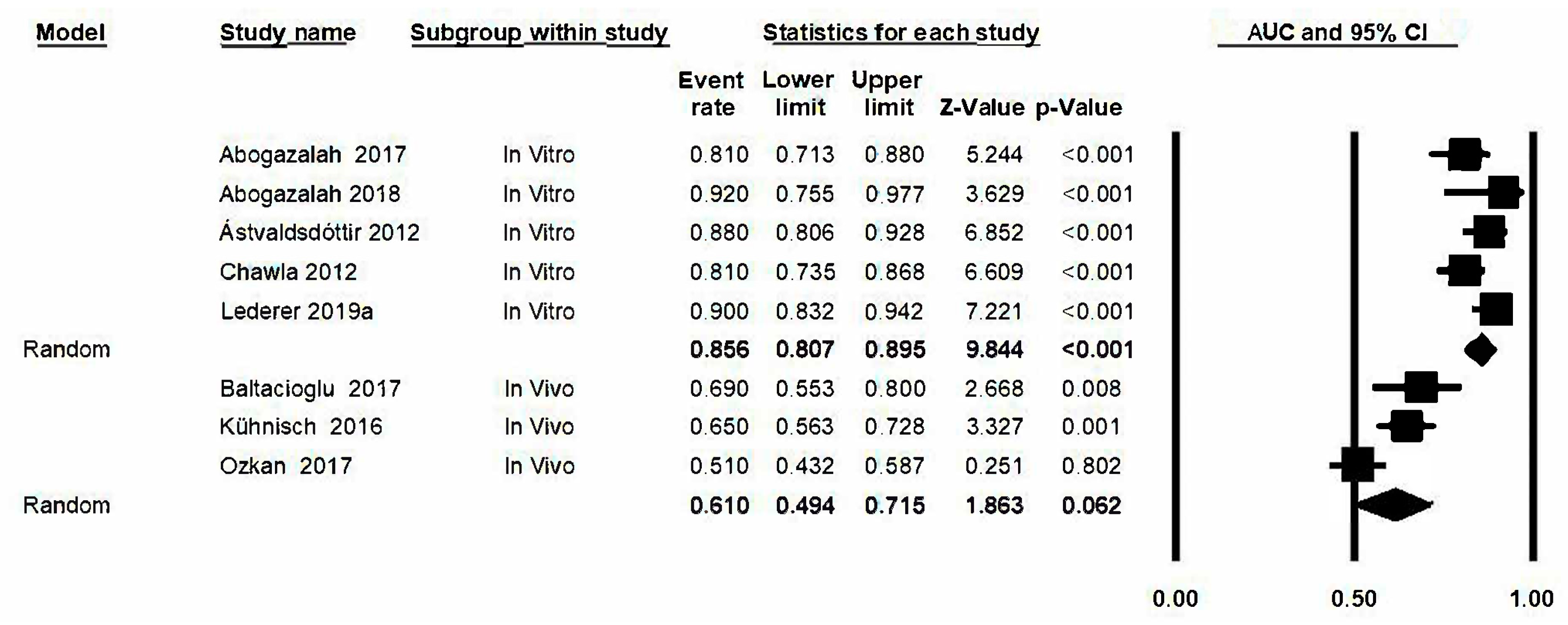
3.10. Area under the Curve (AUC)
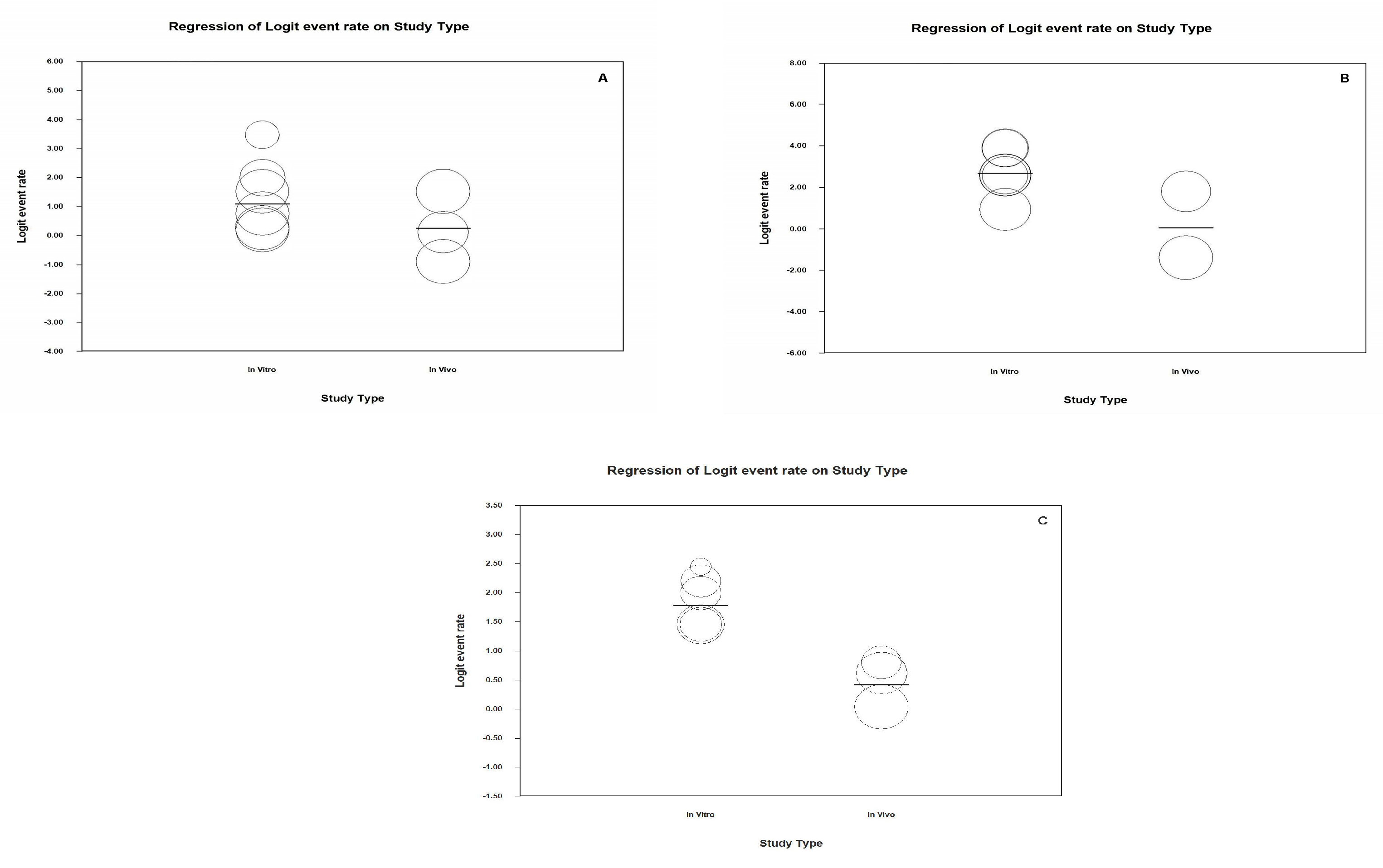
3.11. Analysis Based on Subgroups and Metaregression
3.12. Analysis “One Study Removed”
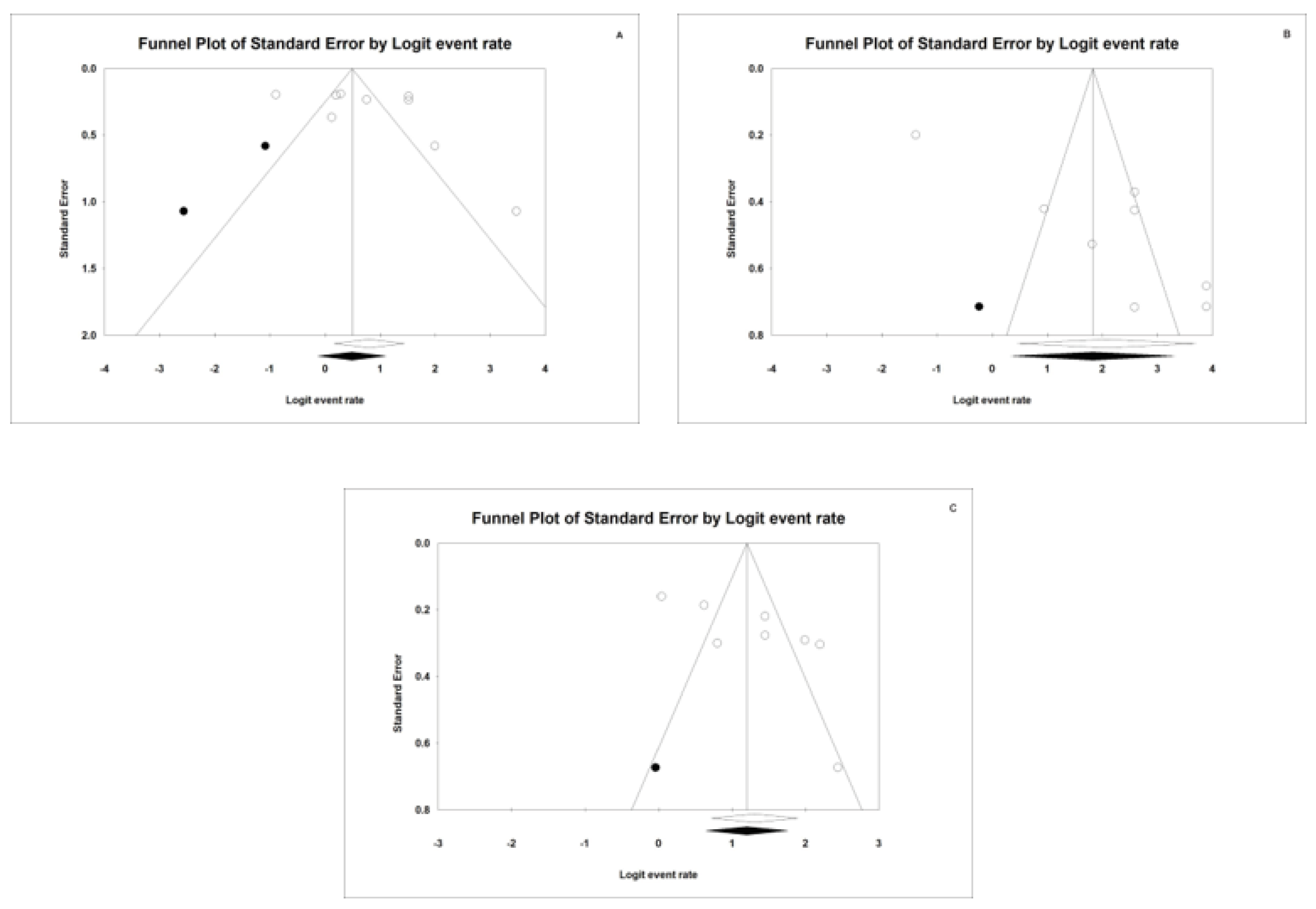
4. Publication Bias
4.1. Sensitivity
4.2. Specificity
4.3. AUC
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Featherstone, J.D. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Jablonski-Momeni, A.; Heinzel-Gutenbrunner, M.; Klein, S.M. In vivo performance of the VistaProof fluorescence-based camera for detection of occlusal lesions. Clin. Oral Investig. 2014, 18, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Lizmar, D.; Veitía, E.; Acevedo, A.M.; Rojas, F. Métodos convencionales y no convencionales para la detección de lesión inicial de caries. Revisión bibliográfica. Acta Odontológica Venezolana 2010, 49, 43–44. [Google Scholar]
- Cueto Rostom, V. Diagnóstico y tratamiento de lesiones cariosas incipientes en caras oclusales. Odontoestomatología 2009, 11, 4–15. [Google Scholar]
- Mohanraj, M.; Prabhu, V.R.; Senthil, R. Diagnostic methods for early detection of dental caries - A review. Int. J. Pedod. Rehabil. 2016, 1, 29–36. [Google Scholar]
- Baltacioglu, I.H.; Orhan, K. Comparison of diagnostic methods for early interproximal caries detection with near-infrared light transillumination: An in vivo study. BMC Oral Health 2017, 17, 130. [Google Scholar] [CrossRef]
- Ozkan, G.; Guzel, K. Clinical evaluation of near-infrared light transillumination in approximal dentin caries detection. Lasers Med Sci 2017, 32, 1417–1422. [Google Scholar] [CrossRef]
- Bussaneli, D.; Restrepo, M.; Boldieri, T.; Pretel, H.; Mancini, M.; Santos-Pinto, L.; Cordeiro, R. Assessment of a new infrared laser transillumination technology (808 nm) for the detection of occlusal caries—An in vitro study. Lasers Med Sci 2015, 30, 1873–1879. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; Altman, D.; et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Abogazalah, N.; Eckert, G.J.; Ando, M. In vitro performance of near infrared light transillumination at 780-nm and digital radiography for detection of non-cavitated approximal caries. J. Dent. 2017, 63, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Astvaldsdottir, A.; Ahlund, K.; Holbrook, W.P.; de Verdier, B.; Tranaeus, S. Approximal Caries Detection by DIFOTI: In Vitro Comparison of Diagnostic Accuracy/Efficacy with Film and Digital Radiography. Int. J. Dent. 2012, 2012, 326401. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.; Messer, L.B.; Adams, G.G.; Manton, D.J. An in vitro Comparison of Detection Methods for Approximal Carious Lesions in Primary Molars. Caries Res. 2012, 46, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.M.A.; Karlsson, L.; Margulis, W.; Gomes, A.S.L. Evaluation of two imaging techniques: Near-infrared transillumination and dental radiographs for the detection of early approximal enamel caries. Dentomaxillofac. Radiol. 2011, 40, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Abogazalah, N.; Eckert, G.J.; Ando, M. In vitro visual and visible light transillumination methods for detection of natural non-cavitated approximal caries. Clin. Oral Investig. 2018, 23, 1287–1294. [Google Scholar] [CrossRef]
- Lederer, A.; Kunzelmann, K.H.; Heck, K.; Hickel, R.; Litzenburger, F. In vitro validation of near-infrared transillumination at 780 nm for the detection of caries on proximal surfaces. Clin. Oral Investig. 2019. [Google Scholar] [CrossRef]
- Lederer, A.; Kunzelmann, K.H.; Heck, K.; Hickel, R.; Litzenburger, F. In-vitro validation of near-infrared reflection for proximal caries detection. Eur. J. Oral Sci. 2019, 127, 515–522. [Google Scholar] [CrossRef]
- Kühnisch, J.; Söchtig, F.; Pitchika, V.; Laubender, R.; Neuhaus, K.; Lussi, A.; Hickel, R. In vivo validation of near-infrared light transillumination for interproximal dentin caries detection. Clin. Oral Invest. 2016, 20, 821–829. [Google Scholar] [CrossRef]
- Simon, J.C.; Lucas, S.A.; Staninec, M.; Tom, H.; Chan, K.H.; Darling, C.L.; Cozin, M.J.; Lee, R.C.; Fried, D. Near-IR transillumination and reflectance imaging at 1300 nm and 1500–1700 nm for in vivo caries detection. Lasers Surg. Med. 2016, 48, 828–836. [Google Scholar] [CrossRef]
- Bin-Shuwaish, M.; Yaman, P.; Dennison, J.; Neiva, G. The Correlation of DIFOTI to Clinical and Radiographic Images in Class II Carious Lesions. J. Am. Dent. Assoc. 2008, 139, 1374–1381. [Google Scholar] [CrossRef]
- Young, D.A.; Featherstone, J.D.B. Digital imaging fiber-optic trans-illumination, F-speed radiographic film and depth of approximal lesions. J. Am. Dent. Assoc. 2005, 136, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Staninec, M.; Lee, C.; Darling, C.L.; Fried, D. In vivo near-IR imaging of approximal dental decay at 1,310 nm. Lasers Surg. Med. 2010, 42, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, A.; Elbaum, M.; Shultz, T.; Keem, S.; Greenebaum, M.; Driller, J. Assessment of dental caries with Digital Imaging Fiber-Optic TransIllumination (DIFOTI): In vitro study. Caries Res. 1997, 31, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Peers, A.; Hill, F.J.; Mitropoulos, C.M.; Holloway, P.J. Validity and Reproducibility of Clinical Examination, Fibre-Optic Transillumination, and Bite-Wing Radiology for the Diagnosis of Small Approximal Carious Lesions: An in vitro Study. Caries Res. 1993, 27, 307–311. [Google Scholar] [CrossRef]
- Jones, R.; Huynh, G.; Jones, G.; Fried, D. Near-infrared transillumination at 1310-nm for the imaging of early dental decay. Opt. Express 2003, 11, 2259. [Google Scholar] [CrossRef]
- Bühler, C.; Ngaotheppitak, P.; Fried, D. Imaging of occlusal dental caries (decay) with near-IR light at 1310-nm. Opt. Express 2005, 13, 573. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]




| AUTHOR/Year | n | Sn | Sp | AUC | GS | VV/VT |
|---|---|---|---|---|---|---|
| Abogazalah N. 2017 [11] | 85 | TRIL: 0.68 Rxd: 0.50 | TRIL: 0.93 Rxd: 0.64 | TRIL: 0.81 Rxd: 0.61 | T | VT |
| Abogazalah N. 2018 [15] | 30 | 0.97 | 0.93 | 0.92 | T | VT |
| Ástvaldsdóttir Á. 2012 [12] | 112 | TRIL D1: 0.7/D3: 0.57 Rxd D1: 0.37/D3: 0.47 | TRIL D1: 0.87/D3: 0.93 Rxd D1: 0.91/D3: 0.97 | TRIL D1: 0.82/D3: 0.88 Rxd D1: 0.64/D3: 0.84 | H | VT |
| Baltacioglu IH. 2017 [6] | 52 | TRIL: 0.81 Rxd: 0.69 | C | VV | ||
| Chawla N. 2012 [13] | 135 | TRIL D1: 0.66/D3: 0.81 Rxd D1:0.68/D3: 0.86 | H | VT | ||
| Kühnisch J. 2016 [18] | 127 | TRIL: 0.291 Rxd: 0.961 | TRIL: 0.65 Rxd: 0.984 | C | VV | |
| Lederer A. 2019a [16] | 120 | NILT D3: 0.82 | NILT D3: 0.98 | NILT D3: 0.90 | T | VT |
| Lederer A. 2019b [17] | 100 | NILT D3: 0.55 | NILT D3: 0.98 | T | VT | |
| Maia AMA. 2011 [14] | 28 | TRIL: 0.88 Rxd: 0.44 | TRIL: 0.72 Rxd: 0.61 | H | VT | |
| Ozkan G. 2017 [7] | 157 | TRIL: 0.82 Rxd:0.83 | TRIL:0.20 Rxd:0.60 | TRIL:0.51 Rxd: 0.71 | C | VV |
| Simon JC. 2017 [19] | 30 | TRIL: 0.53 Rxd: 0.23 | TRIL: 0.86 Rxd: 0.96 | H | VV |
| Study | Risk of Bias | Concerns Regarding Applicability | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Abogazalah N. 2017 [11] | ? | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ |
| Abogazalah N. 2018 [15] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ |
| Ástvaldsdóttir Á. 2012 [12] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | ☺ |
| Baltacioglu IH. 2017 [6] | ☹ | ? | ☺ | ☺ | ☺ | ☺ | ☺ |
| Chawla N. 2012 [13] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ |
| Kühnisch J. 2016 [18] | ☹ | ☺ | ? | ☺ | ☺ | ☺ | ☺ |
| Lederer A. 2019a [16] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ |
| Lederer A. 2019b [17] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ |
| Maia AMA. 2011 [14] | ? | ☺ | ☺ | ? | ☺ | ☺ | ☺ |
| Ozkan G. 2017 [7] | ☹ | ☺ | ? | ☺ | ☹ | ☺ | ☺ |
| Simon JC. 2017 [19] | ? | ☺ | ☺ | ☺ | ☺ | ☹ | ☺ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marmaneu-Menero, A.; Iranzo-Cortés, J.E.; Almerich-Torres, T.; Ortolá-Síscar, J.C.; Montiel-Company, J.M.; Almerich-Silla, J.M. Diagnostic Validity of Digital Imaging Fiber-Optic Transillumination (DIFOTI) and Near-Infrared Light Transillumination (NILT) for Caries in Dentine. J. Clin. Med. 2020, 9, 420. https://doi.org/10.3390/jcm9020420
Marmaneu-Menero A, Iranzo-Cortés JE, Almerich-Torres T, Ortolá-Síscar JC, Montiel-Company JM, Almerich-Silla JM. Diagnostic Validity of Digital Imaging Fiber-Optic Transillumination (DIFOTI) and Near-Infrared Light Transillumination (NILT) for Caries in Dentine. Journal of Clinical Medicine. 2020; 9(2):420. https://doi.org/10.3390/jcm9020420
Chicago/Turabian StyleMarmaneu-Menero, Ana, José Enrique Iranzo-Cortés, Teresa Almerich-Torres, José Carmelo Ortolá-Síscar, José María Montiel-Company, and José Manuel Almerich-Silla. 2020. "Diagnostic Validity of Digital Imaging Fiber-Optic Transillumination (DIFOTI) and Near-Infrared Light Transillumination (NILT) for Caries in Dentine" Journal of Clinical Medicine 9, no. 2: 420. https://doi.org/10.3390/jcm9020420
APA StyleMarmaneu-Menero, A., Iranzo-Cortés, J. E., Almerich-Torres, T., Ortolá-Síscar, J. C., Montiel-Company, J. M., & Almerich-Silla, J. M. (2020). Diagnostic Validity of Digital Imaging Fiber-Optic Transillumination (DIFOTI) and Near-Infrared Light Transillumination (NILT) for Caries in Dentine. Journal of Clinical Medicine, 9(2), 420. https://doi.org/10.3390/jcm9020420






