Head-to-Head Comparison of Consensus-Recommended Platelet Function Tests to Assess P2Y12 Inhibition—Insights for Multi-Center Trials
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Blood Collection
2.3. Platelet Function Testing
2.3.1. Analysis of VASP Phosphorylation by ELISA
2.3.2. Analysis of VASP Phosphorylation by Flow Cytometry
2.3.3. Multiplate® Aggregometry
2.3.4. Statistical Analysis
3. Results
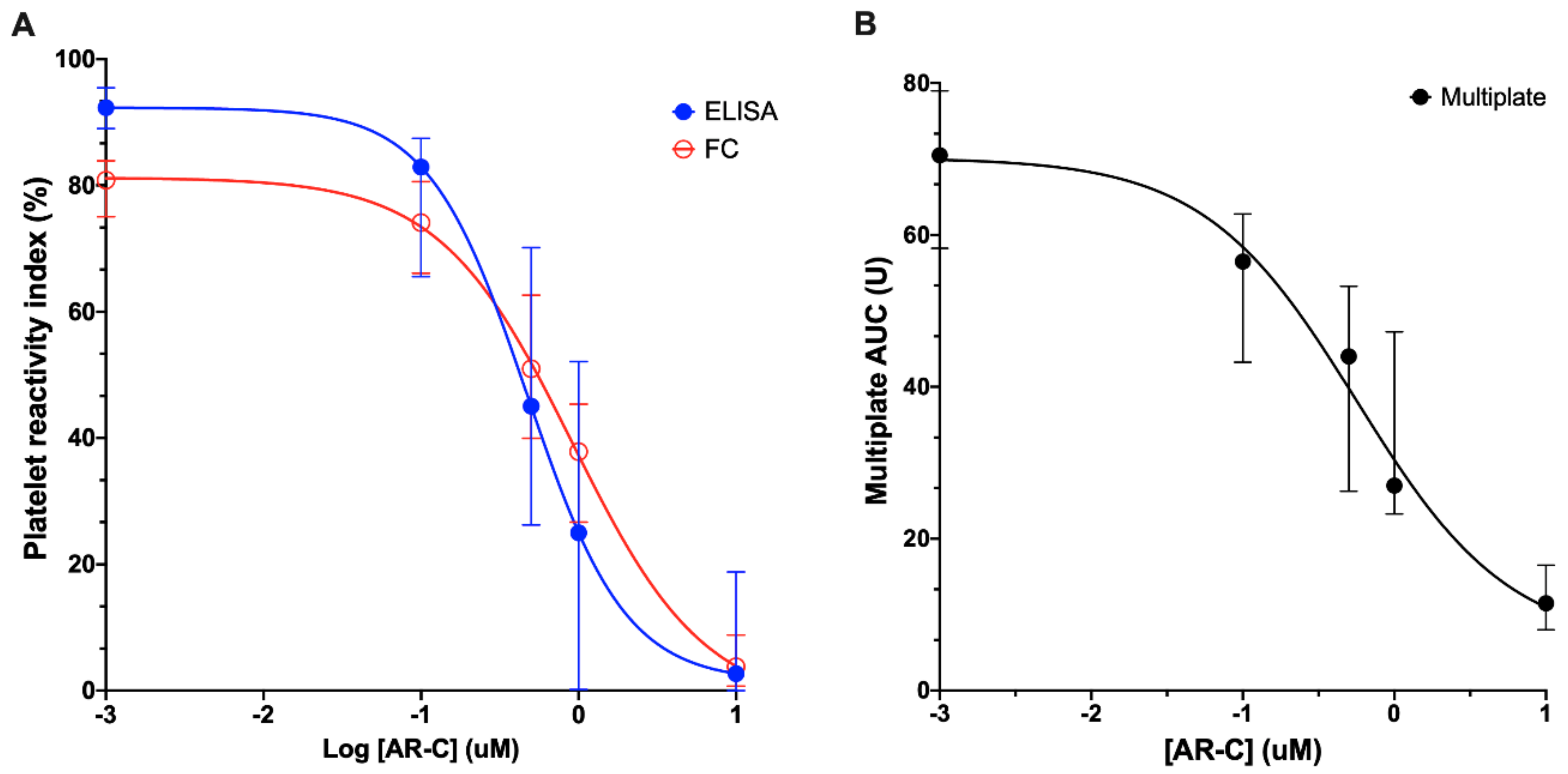
3.1. Inhibition of Platelet P2Y12 ADP Receptor Reactivity
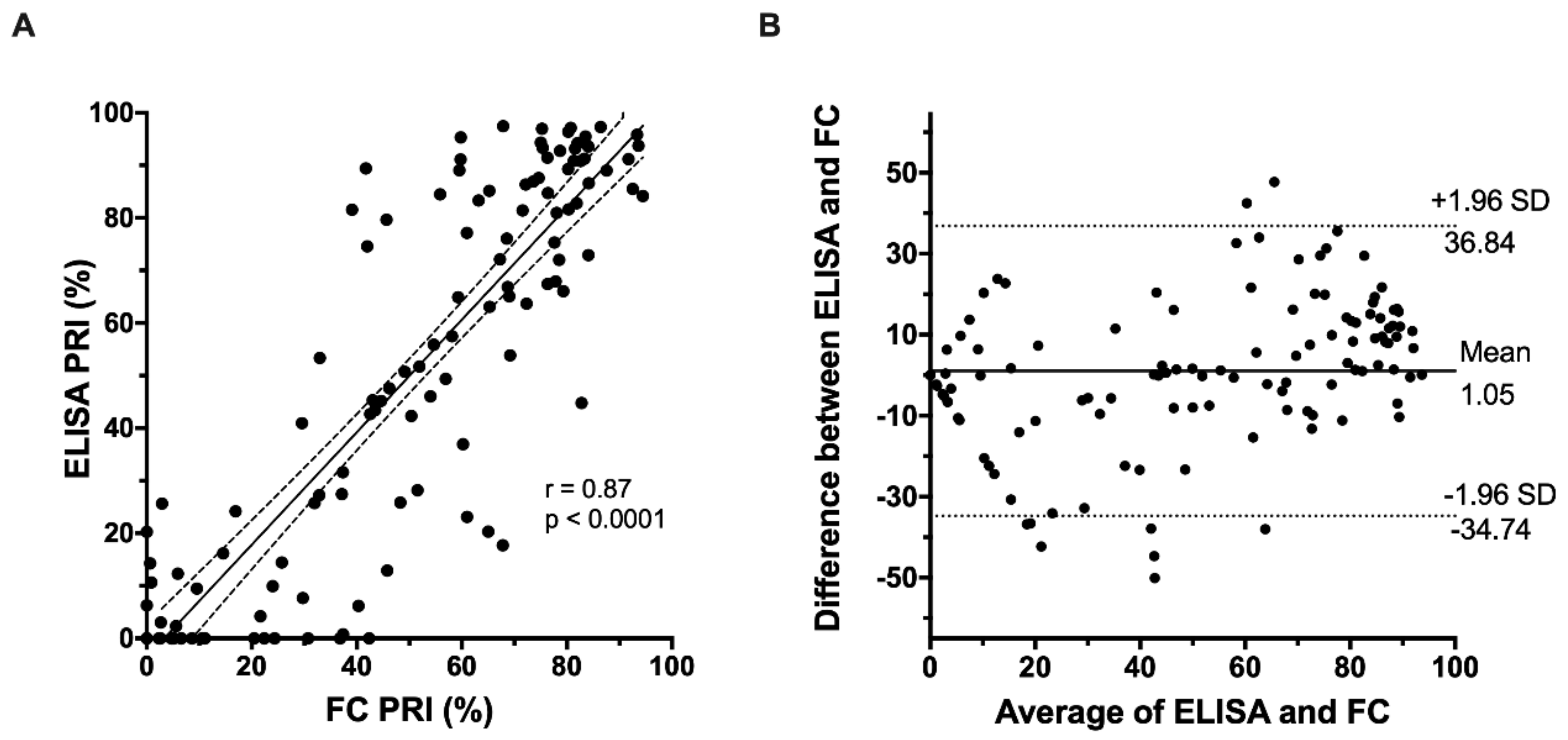
3.2. Correlation of FC-VASP with ELISA-VASP
3.3. Correlation of VASP Phosphorylation Assays with the Multiplate® Assay
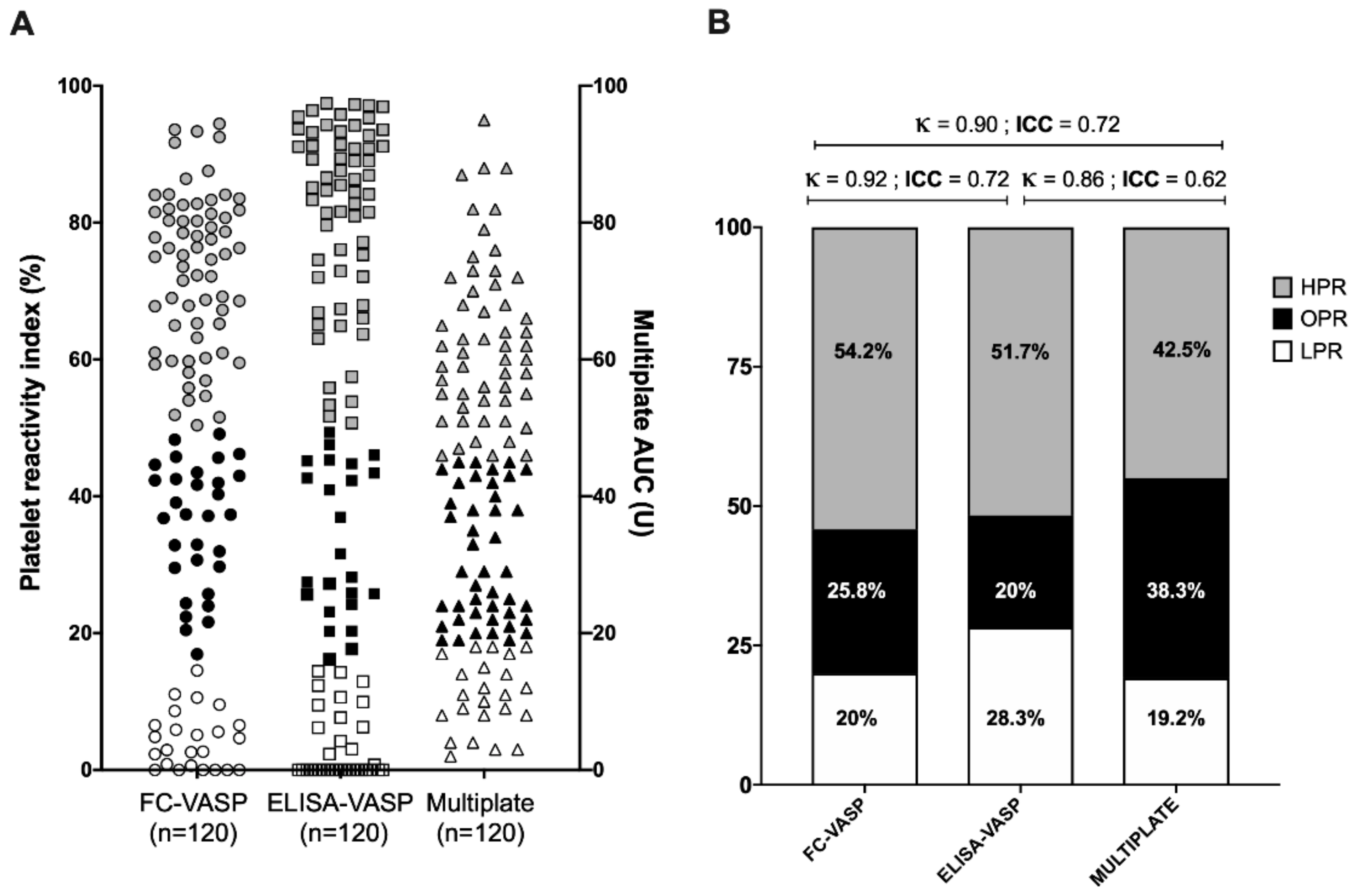
3.4. Classification into High, Optimal, and Low Platelet Reactivity Categories
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bonello, L.; Tantry, U.S.; Marcucci, R.; Blindt, R.; Angiolillo, D.J.; Becker, R.; Bhatt, D.L.; Cattaneo, M.; Collet, J.P.; Cuisset, T.; et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 2010, 56, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Aradi, D.; Storey, R.F.; Komocsi, A.; Trenk, D.; Gulba, D.; Kiss, R.G.; Husted, S.; Bonello, L.; Sibbing, D.; Collet, J.P.; et al. Expert position paper on the role of platelet function testing in patients undergoing percutaneous coronary intervention. Eur. Heart J. 2014, 35, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Aradi, D.; Alexopoulos, D.; Ten Berg, J.; Bhatt, D.L.; Bonello, L.; Collet, J.P.; Cuisset, T.; Franchi, F.; Gross, L.; et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Aradi, D.; Kirtane, A.; Bonello, L.; Gurbel, P.A.; Tantry, U.S.; Huber, K.; Freynhofer, M.K.; Ten Berg, J.; Janssen, P.; Angiolillo, D.J.; et al. Bleeding and stent thrombosis on P2Y12-inhibitors: Collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur. Heart J. 2015, 36, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Bouman, H.J.; Parlak, E.; van Werkum, J.W.; Breet, N.J.; ten Cate, H.; Hackeng, C.M.; ten Berg, J.M.; Taubert, D. Which platelet function test is suitable to monitor clopidogrel responsiveness? A pharmacokinetic analysis on the active metabolite of clopidogrel. J. Thromb. Haemost. 2010, 8, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Landry, S.; Tanguay, J.F.; Lordkipanidze, M. Personalizing antiplatelet therapies: What have we learned from recent trials? Platelets 2018, 29, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Lordkipanidzé, M. Platelet Function Tests. Semin. Thromb. Hemost. 2016, 42, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory, Approved Guideline, 3rd ed.; CLSI Document: Wayne, PA, USA, 2008; pp. 1–37. [Google Scholar]
- Cattaneo, M.; Cerletti, C.; Harrison, P.; Hayward, C.P.; Kenny, D.; Nugent, D.; Nurden, P.; Rao, A.K.; Schmaier, A.H.; Watson, S.P.; et al. Recommendations for the Standardization of Light Transmission Aggregometry: A Consensus of the Working Party from the Platelet Physiology Subcommittee of SSC/ISTH. J. Thromb. Haemost. 2013, 11, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, J.A.; Bourguet, N.; Boulay-Moine, D.; Sugidachi, A.; Yamaguchi, S.; Barragan, P.; Zhou, C.; Moulard, M. Comparison of a new ELISA assay with the flow cytometric assay for platelet vasodilator-associated stimulated phosphoprotein (VASP) phosphorylation in whole blood to assess P2Y(12) inhibition. Thromb. Haemost. 2012, 107, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Hobl, E.L.; Jilma, B.; Derhaschnig, U.; Schoergenhofer, C.; Schwameis, M.; Jilma-Stohlawetz, P. Comparison of a new ELISA-based with the flow cytometric assay for vasodilator-associated stimulated phosphoprotein phosphorylation to assess P2Y12 -inhibition after ticagrelor intake. Cytom. B Clin. Cytom. 2015, 88, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Wei, Y.; Chen, N.; Liu, H. Validation of a New ELISA-Based Vasodilator-Associated Stimulated Phosphoprotein Phosphorylation Assay to Assess Platelet Reactivity Index in a Chinese Population. Clin. Appl. Thromb. Hemost. 2018, 24, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Mingant, F.; Didier, R.; Gilard, M.; Martin, F.; Nicol, P.P.; Ugo, V.; Lippert, E.; Galinat, H. Comparison of four methods to assess high-on platelet reactivity under P2Y12 receptor inhibitor. Platelets 2018, 29, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, J.R.; Price, M.J.; Parise, H.; Gurbel, P.A. Evaluating the clinical usefulness of platelet function testing: Considerations for the proper application and interpretation of performance measures. Thromb. Haemost. 2013, 109, 808–816. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bélanger, J.-C.; Bandeira Ferreira, F.L.; Welman, M.; Boulahya, R.; Tanguay, J.-F.; Y.F. So, D.; Lordkipanidzé, M. Head-to-Head Comparison of Consensus-Recommended Platelet Function Tests to Assess P2Y12 Inhibition—Insights for Multi-Center Trials. J. Clin. Med. 2020, 9, 332. https://doi.org/10.3390/jcm9020332
Bélanger J-C, Bandeira Ferreira FL, Welman M, Boulahya R, Tanguay J-F, Y.F. So D, Lordkipanidzé M. Head-to-Head Comparison of Consensus-Recommended Platelet Function Tests to Assess P2Y12 Inhibition—Insights for Multi-Center Trials. Journal of Clinical Medicine. 2020; 9(2):332. https://doi.org/10.3390/jcm9020332
Chicago/Turabian StyleBélanger, Jean-Christophe, Fabio Luiz Bandeira Ferreira, Mélanie Welman, Rahma Boulahya, Jean-François Tanguay, Derek Y.F. So, and Marie Lordkipanidzé. 2020. "Head-to-Head Comparison of Consensus-Recommended Platelet Function Tests to Assess P2Y12 Inhibition—Insights for Multi-Center Trials" Journal of Clinical Medicine 9, no. 2: 332. https://doi.org/10.3390/jcm9020332
APA StyleBélanger, J.-C., Bandeira Ferreira, F. L., Welman, M., Boulahya, R., Tanguay, J.-F., Y.F. So, D., & Lordkipanidzé, M. (2020). Head-to-Head Comparison of Consensus-Recommended Platelet Function Tests to Assess P2Y12 Inhibition—Insights for Multi-Center Trials. Journal of Clinical Medicine, 9(2), 332. https://doi.org/10.3390/jcm9020332




