Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations
Abstract
1. Introduction
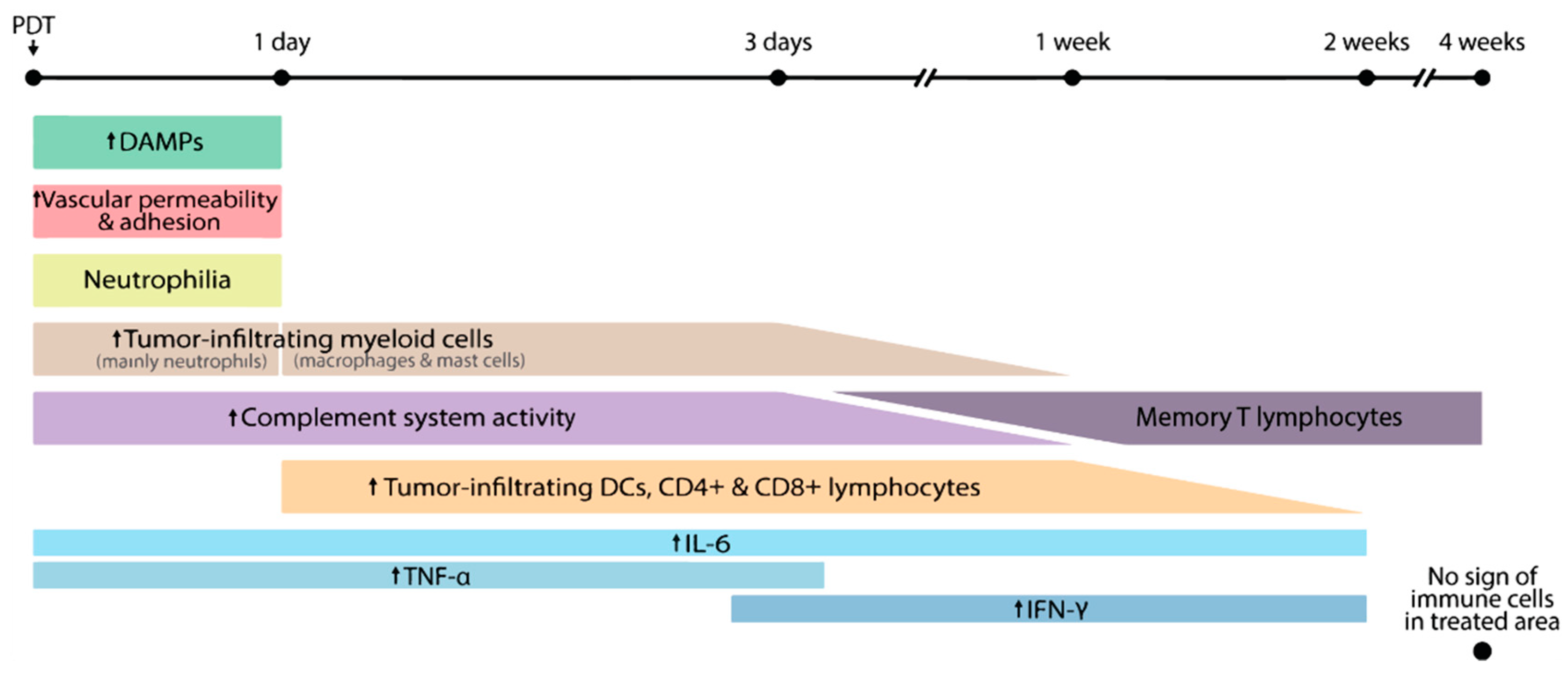
2. How PDT Induces Immune Responses against the Treated Tumor
3. Preclinical Evidence of Antitumor Immunity Induced by PDT
3.1. PDT and the Innate Immune System
3.1.1. Neutrophils
3.1.2. Complement
3.1.3. Immunogenic Cell Death
3.1.4. Dendritic Cells
3.2. PDT and the Adaptive Immune System
| Time Phase (after PDT) | Location | Major Immune Events | Tumor Cell Line/Model | Strain/Species | PS/Dose/Route | Illumination Protocol | Ref |
|---|---|---|---|---|---|---|---|
| Within 24 h | Tumor | -Localized neutrophil function increases at 1 h, and then decreases at 4 h -Increased influx of neutrophils at 24 h | AB12 mesothelioma | Balb/c mice | Photofrin 5 mg/kg i.v. | 135 J/cm2 75 mW/cm2 | [23] |
| Tumor | -Increase of neutrophils within 5 min -Increase of mast cells and other myeloid cells during 0–2 h -Increased cytotoxicity of tumor-associated macrophages at 2 h | SCCVII squamous cell carcinoma | C3H/HeN mice | Photofrin 25 mg/kg i.v. | 60 J/cm2 45 mW/cm2 | [22] | |
| Tumor | -Increase of neutrophils within 24 h -Increased release of myeloperoxidase at 24 h, which lasts at least 4 days | Rhabdomyosarcoma | WAG/Rij rats | ALA 200 mg/kg i.v. | 100 J/cm2 100 mW/cm2 | [28] | |
| Tumor | -Increased expression of TNF- α at 24 h | UVB induced squamous cell carcinoma | SKH-1 mice | Topical ALA, 8% cream | Multiple 30 J/cm2 20 mW/cm2 | [52] | |
| Peripheral blood; Peritoneal cells | -Increase of neutrophils within 24 h which is partly mediated by complement C3a -Increase of monocytes and B cells at 8 h -Increase of mast cells and macrophage in peritoneal at 8 h | FsaR fibrosarcoma | C3H/HeN mice | Photofrin, 10 mg/kg, i.v. | 150 J/cm2 100 mW/cm2 | [24] | |
| Peripheral blood | -Increase of neutrophils within 24 h | Rhabdomyosarcoma | WAG/Rij rats | ALA 200 mg/kg i.v. | 100 J/cm2 100 mW/cm2 | [28] | |
| Tumor draining lymph node | -Increase of IL-12 expressing APC at 24 h -Increased cross-activation of T cells by APC at 24 h | EMT6, mammary sarcoma; CT26, colon carcinoma | BALB/C | Photofrin 5 mg/kg i.v. | 135 J/cm2 75 mW/cm2 | [51] | |
| 1 week | Tumor tissue | -Increase of DC, CD4+, and CD8+ T cells at 7 days | UVB induced squamous cell carcinoma | SKH-1 mice | Topical ALA, 8% cream | Multiple 30 J/cm2 20 mW/cm2 | [52] |
| Tumor tissue | -Infiltration CD4+/CD8+ T cells at 7 days | PECA squamous cell carcinoma | SKH-1 mice | ALA 0.5 mM (in vitro killing for the production of cancer vaccine) | 0.5 J/cm2 10 mW/cm2 | [61] | |
| >1 week | Lymph nodes; Spleen | -Increased production of IFN- by CD4+ and CD8+ T cells in lymph nodes at 2 weeks -Increased production of IFN− by CD8+ T cells in spleen at 2 weeks -Upregulated CD107 (marker for cytolytic activity) in splenocytes at 2 weeks | EMT6 mammary sarcoma | BALB/C | Photofrin, 10 mg/kg, i.p. | 65 J/cm2, 47 mW/cm2 | [59] |
| No specified time point available | Tumor tissue; Spleen; Lymph node; Serum | -Inhibited tumor metastases -Reduced growth of tumor re-challenge -CD4+CD8+ T cells accumulate in tumor, being mostly central memory T cells (CD44hiCD62Lhi) -No significant change of CD3+ T cells in spleen -Elevated serum levels of TNF-α and IFN-ƴ -Restore immune balance to healthy state and prolong relapse-free survival | B16 metastatic melanoma | C57BL6j mice | Topical ALA 20% w/w, loaded in CDG2/HA-contructed nanoparticles | 25 mW/cm2, 5 min | [60] |
4. Clinical Evidence of Antitumor Immunity Induced by PDT
4.1. Acute Immune Response
4.2. Adaptive Immune Response
| Disease (Stage/Subtypes) | PS/Dose | Illumination Protocol | No. of Patients | * Prior Treatment | Immune Events and Time Points Post PDT | Samples | Ref |
|---|---|---|---|---|---|---|---|
| BCC (superficial and nodular BCC) | Topical ALA (10% emulsion) | 75 J/cm2 70–126 mW/cm2 | 10 | N/A | -Increase of Langerhans cells associated with lymphocytes in tumor at 1 week -No sign of immune cells in tumor at 4 weeks | Tumor | [65] |
| BCC (superficial BCC) | Topical ALA (20% emulsion) | 100 J/cm2 for 10 min | 15 | NA | -Neutrophils increase at 4 h, and declines to basal levels after 48 h -Mast cells tend to increase up to 72 h -Lymphocytes increase at 24 h -Macrophages continuously increase at 48 and 72 h | Tumor biopsy | [62] |
| BCC (ulcerating, superficial and nodular BCC) | Topical ALA (10% emulsion) | 100 J/cm2 150 mW/cm2 | 17 | Yes (only surgical excision) | -Increased neutrophil activity in blood at 4 h -Decreased expression of IL-1β by lymphocytes at 4 h -No significant changes in IL-6, IL-2 and TNF-α by lymphocytes -Decreased TGF-β1 levels in serum at 4 h | Peripheral blood; Serum | [63] |
| BCC (superficial and nodular BCC) | Topical ALA (20% emulsion) or Photofrin i.v. (1 mg/kg) | 100–260 J/cm2 150 mW/cm2 | 21 | Yes, 12 patients | -Increased tumor antigen-specific T cell response at 1 and 2 weeks -Anecdotal regression of lesions outside the treated area (ALA) | Peripheral blood | [70] |
| BCC (superficial and nodular BCC) | Topical MAL | 37 J/cm2 for 7 min 40 s (2 sections with 1-week interval) | 10 | No | 30 min to 2 h: -Increase of peritumoral inflammatory cells -Increased levels of IL-23, IFN-γ, IL-22 and IL-17 1–12 weeks: -Decrease of peritumoral inflammatory cells -Decreased levels of IL-23, IFN-γ, IL-22, and IL-17 | Tumor biopsy | [64] |
| BCC (superficial BCC) | Topical MAL (2 g) | 37 J/cm2, 70 mW/cm2 | 8 | N/A | -Increased infiltration of neutrophils at 1 and 24 h -Increased expression of E-selectin in blood vessels at 1 and 24 h -Decrease of Langerhans cell numbers at 1 and 24 h | Tumor biopsy | [69] |
| VIN (high-grade) | Topical ALA (20% emulsion) | 50–100 J/cm2 | 32 | Yes, 6 patients | -Loss of HLA class I in PDT nonresponders -Increase of CD8 T cell infiltration in PDT responders compared with nonresponders | Tumor biopsy | [71] |
| VIN (high-grade) | Topical MAL | 50 J/cm2 (2 sections with a month interval) | 11 | Yes | -No statistically significant differences in CD4, CD8, CD1a, and CD68 cells was detected at 26 w | Tumor biopsy | [66] |
| ESCC (early stages) | Photofrin (1 mg/kg) | 80 J/cm2 | 8 | N/A | -Increased of peripheral granulocyte at 1 and 2 weeks -increase of peripheral monocyte at 1 week -Increased levels of IL-6 in serum at 1 week -Increased number of peripheral Treg at 2 weeks -Inhibited immunosuppressive function of peripheral Treg at 2 w -No significant change of peripheral lymphocyte numbers or systemic CD4+ T cell numbers at 1 week -No significant changes of tumor-infiltrating Treg at 1 and 2 weeks | Peripheral blood; Serum; Tumor biopsy | [67] |
| HNSCC (Stage 1–4) | Temoporfin (Foscan) | N/A | 9 | Yes | 24 h, 1 week, and 4–6 weeks: -Increased frequency of NK cells at 4–6 weeks -Increased frequency of Treg up to 6 weeks -Increased concentration of HMGB1 (peak at 1 week), IL-6 (peak at 24 h), and IL-10 (peak at 24 h) -Decreased concentration of Perforin (lowest at 24 h and 1 week) | Peripheral blood; Serum | [68] |
4.3. Systemic Immune Response
5. Potentiating PDT with Immune Modulation
5.1. Immunostimulants
5.2. Blocking or Depleting Immunosuppressive (Cellular) Factors
5.3. Importance of Tumor Antigens
5.4. Immune Potentiating Therapeutic Vaccines
6. Future Directions for Clinical Research
6.1. Recommendations for Immune Monitoring
6.2. Recommendations for Optimizing PDT Outcome
7. Conclusions
Funding
Conflicts of Interest
References
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Velez-montoya, R.; Oliver, S.C.N.; Olson, J.L.; Fine, S.L.; Mandava, N.; Quiroz-mercado, H. Current knowledge and trends in age-related macular degeneration: Today’s and future treatments. Retina 2013, 33, 1487–1502. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, A.; Sandborn, W.; Melhem, H.; Bolotine, L.; Chamaillard, M.; Peyrin-Biroulet, L. Photodynamic therapy as a new treatment modality for inflammatory and infectious conditions. Expert Rev. Clin. Immunol. 2015, 11, 637–657. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Institutes of Health ClinicalTrials. Available online: https://clinicaltrials.gov/ (accessed on 1 September 2019).
- Chen, B.; Pogue, B.; Hoopes, P.; Hasan, T. Vascular and cellular targeting for photodynamic therapy. Crit. Rev. Eukaryot. Gene Expr. 2006, 16, 279–305. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.; Foster, T.; Girotti, A.; Gollnick, S.; Hahn, S.; Hamblin, M.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of cancer: An update. CA Cancer J Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part three-Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef]
- Garg, A.D.; Nowis, D.; Golab, J.; Agostinis, P. Photodynamic therapy: Illuminating the road from cell death towards anti-tumour immunity. Apoptosis 2010, 15, 1050–1071. [Google Scholar] [CrossRef]
- Garg, A.D.; Agostinis, P. ER stress, autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses. Photochem. Photobiol. Sci. 2014, 13, 474–487. [Google Scholar] [CrossRef]
- Jalili, A.; Makowski, M.; Switaj, T.; Nowis, D.; Wilczek, E.; Chora, M. Effective Photoimmunotherapy of Murine Colon Carcinoma Induced by the Combination of Photodynamic Therapy and Dendritic Cells. Clin. Cancer Res. 2004, 10, 4498–4508. [Google Scholar] [CrossRef]
- Korbelik, M.; Sun, J.; Cecic, I. Photodynamic Therapy—Induced Cell Surface Expression and Release of Heat Shock Proteins: Relevance for Tumor Response. Cancer Res. 2005, 65, 1018–1026. [Google Scholar]
- Ji, J.; Fan, Z.; Zhou, F.; Wang, X.; Shi, L.; Zhang, H.; Wang, P.; Yang, D.; Zhang, L.; Chen, W.R.; et al. Improvement of DC vaccine with ALA-PDT induced immunogenic apoptotic cells for skin squamous cell carcinoma. Oncotarget 2015, 6, 17135–17146. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M. Role of cell stress signaling networks in cancer cell death and antitumor immune response following proteotoxic injury inflicted by photodynamic therapy. Lasers Surg. Med. 2018, 50, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Villanueva, J.F.; Díaz-Molina, R.; García-González, V. Protein folding and mechanisms of proteostasis. Int. J. Mol. Sci. 2015, 16, 17193–17230. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- van Vliet, A.R.; Martin, S.; Garg, A.D.; Agostinis, P. The PERKs of damage-associated molecular patterns mediating cancer immunogenicity: From sensor to the plasma membrane and beyond. Semin. Cancer Biol. 2015, 33, 74–85. [Google Scholar] [CrossRef]
- Janssens, S.; Pulendran, B.; Lambrecht, B.N. Emerging functions of the unfolded protein response in immunity. Nat. Immunol. 2014, 15, 910–919. [Google Scholar] [CrossRef]
- Starck, S.R.; Tsai, J.C.; Chen, K.; Shodiya, M.; Wang, L.; Yahiro, K.; Martins-Green, M.; Shastri, N.; Walter, P. Translation from the 5’ untranslated region shapes the integrated stress response. Science 2016. [Google Scholar] [CrossRef]
- Starck, S.R.; Shastri, N. Nowhere to hide: Unconventional translation yields cryptic peptides for immune surveillance. Immunol. Rev. 2016, 272, 8–16. [Google Scholar] [CrossRef]
- Keisari, Y. Tumor abolition and antitumor immunostimulation by physico-chemical tumor ablation. Front. Biosci. Landmark 2017, 22, 310–347. [Google Scholar] [CrossRef]
- Seitz, C.; Rückert, M.; Deloch, L.; Weiss, E.-M.; Utz, S.; Izydor, M.; Ebel, N.; Schlücker, E.; Fietkau, R.; Gaipl, U.S.; et al. Tumor Cell-Based Vaccine Generated With High Hydrostatic Pressure Synergizes With Radiotherapy by Generating a Favorable Anti-tumor Immune Microenvironment. Front. Oncol. 2019, 9, 805. [Google Scholar] [CrossRef]
- Krosl, G.; Korbelik, M.; Dougherty, G.J. Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy. Br. J. Cancer 1995, 71, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.W.; Snyder, E.; Miller, J.; Carter, S.; Houser, C.; Klampatsa, A.; Albelda, S.M.; Cengel, K.A.; Busch, T.M. Luminol Chemiluminescence Reports Photodynamic Therapy-Generated Neutrophil Activity In Vivo and Serves as a Biomarker of Therapeutic Efficacy. Photochem. Photobiol. 2019, 95, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Korbelik, M. Role of Complement Anaphylatoxin C3a in Photodynamic Therapy-elicited Engagement of Host Neutrophils and Other Immune Cells. Photochem. Photobiol. 2006, 82, 558–562. [Google Scholar] [CrossRef]
- Cecic, I.; Parkins, C.S.; Korbelik, M.; Cecic, I.; Parkins, C.S.; Korbelik, M. Induction of Systemic Neutrophil Response in Mice by Photodynamic Therapy of Solid Tumors. Photochem. Photobiol. 2001, 74, 712–720. [Google Scholar] [CrossRef]
- Cecic, I.; Stott, B.; Korbelik, M. Acute phase response-associated systemic neutrophil mobilization in mice bearing tumors treated by photodynamic therapy. Int. Immunopharmacol. 2006, 6, 1259–1266. [Google Scholar] [CrossRef]
- De Vree, W.J.A.; Essers, M.C.; Koster, J.F.; Koster, F. Role of Interleukin 1 and Granulocyte Colony-Stimulating Factor in Photofrin-based Photodynamic Therapy of Rat Rhabdomyosarcoma Tumors. Cancer Res. 1997, 57, 2555–2558. [Google Scholar]
- de Bruijn, H.S.; Sluiter, W.; van der Ploeg-van den Heuvel, A.; Sterenborg, H.J.C.M.; Robinson, D.J. Evidence for a bystander role of neutrophils in the response to systemic 5-aminolevulinic acid-based photodynamic therapy. Photodermatol. Photoimmunol. Photomed. 2006, 22, 238–246. [Google Scholar] [CrossRef]
- Cecic, I.; Korbelik, M. Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer Lett. 2002, 183, 43–51. [Google Scholar] [CrossRef]
- Sun, J.; Cecic, I.; Parkins, C.S.; Korbelik, M. Neutrophils as inflammatory and immune effectors in photodynamic therapy-treated mouse SCCVII tumours. Photochem. Photobiol. Sci. 2002, 1, 690–695. [Google Scholar] [CrossRef]
- Korbelik, M.; Sun, J.; Posakony, J.J. Interaction between photodynamic therapy and BCG immunotherapy responsible for the reduced recurrence of treated mouse tumors. Photochem. Photobiol. 2001, 73, 403–409. [Google Scholar] [CrossRef]
- Gollnick, S.O.; Evans, S.S.; Baumann, H.; Owczarczak, B.; Maier, P.; Vaughan, L.; Wang, W.C.; Unger, E.; Henderson, B.W. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br. J. Cancer 2003, 88, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Schacht, V.; Szeimies, R.-M.; Abels, C. Photodynamic therapy with 5-aminolevulinic acid induces distinct microcirculatory effects following systemic or topical application. Photochem. Photobiol. Sci. 2006, 5, 452–458. [Google Scholar] [CrossRef] [PubMed]
- De Vree, W.J.A.; Essers, M.C.; De Bruijn, H.S.; Star, W.M.; Koster, J.F.; Sluiter, W. Evidence for an Important Role of Neutrophils in the Efficacy of Photodynamic Therapy in Vivo. Cancer Res. 1996, 56, 2908–2911. [Google Scholar] [PubMed]
- Mamidi, S.; Höne, S.; Kirschfink, M. The complement system in cancer: Ambivalence between tumour destruction and promotion. Immunobiology 2017, 222, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Cecic, I.; Serrano, K.; Gyongyossy-issa, M.; Korbelik, M. Characteristics of complement activation in mice bearing Lewis lung carcinomas treated by photodynamic therapy. Cancer Lett. 2005, 225, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Cecic, I. Complement activation cascade and its regulation: Relevance for the response of solid tumors to photodynamic therapy. J. Photochem. Photobiol. B Biol. 2008, 93, 53–59. [Google Scholar] [CrossRef]
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-Stubbs, S.; Obeid, M.; et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005, 202, 1691–1701. [Google Scholar] [CrossRef]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]
- Garg, A.D.; Vandenberk, L.; Fang, S.; Fasche, T.; Van Eygen, S.; Maes, J.; Van Woensel, M.; Koks, C.; Vanthillo, N.; Graf, N.; et al. Pathogen response-like recruitment and activation of neutrophils by sterile immunogenic dying cells drives neutrophil-mediated residual cell killing. Cell Death Differ. 2017, 24, 832–843. [Google Scholar] [CrossRef]
- Adkins, I.; Fucikova, J.; Garg, A.D.; Agostinis, P.; Špíšek, R. Physical modalities inducing immunogenic tumor cell death for cancer immunotherapy. Oncoimmunology 2014, 3. [Google Scholar] [CrossRef]
- Bezu, L.; Sauvat, A.; Humeau, J.; Leduc, M.; Kepp, O.; Kroemer, G. eIF2α phosphorylation: A hallmark of immunogenic cell death. Oncoimmunology 2018, 7, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Dudek, A.M.; Agostinis, P. Cancer immunogenicity, danger signals, and DAMPs: What, when, and how? Biofactors 2013, 39, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Zhang, W.; Merchant, S. Involvement of damage-associated molecular patterns in tumor response to photodynamic therapy: Surface expression of calreticulin and high-mobility group box-1 release. Cancer Immunol. Immunother. 2011, 60, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, J.; Zhang, H.; Fan, Z.; Zhang, L.; Shi, L.; Zhou, F.; Chen, W.R.; Wang, H.; Wang, X. Stimulation of dendritic cells by DAMPs in ALA-PDT treated SCC tumor cells. Oncotarget 2015, 6, 44688–44702. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013. [Google Scholar] [CrossRef]
- Humeau, J.; Lévesque, S.; Kroemer, G.; Pol, J.G. Gold standard assessment of immunogenic cell death in oncological mouse models. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Gollnick, S.O.; Vaughan, L.; Henderson, B.W. Generation of Effective Antitumor Vaccines Using Photodynamic Therapy. Cancer Res. 2002, 62, 1604–1608. [Google Scholar]
- Zhou, F.; Xing, D.; Chen, W.R. Regulation of HSP70 on activating macrophages using PDT- induced apoptotic cells. Int. J. Cancer 2009, 125, 1380–1389. [Google Scholar] [CrossRef]
- Korbelik, M. Cancer vaccines generated by photodynamic therapy. Photochem. Photobiol. Sci. 2011. [Google Scholar] [CrossRef]
- Gollnick, S.O.; Owczarczak, B.; Maier, P. Photodynamic therapy and anti-tumor immunity. Lasers Surg. Med. 2006, 38, 509–515. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Lv, T.; Tu, Q.; Huang, Z.; Wang, X. Therapeutic and immune effects of 5-aminolevulinic acid photodynamic therapy on UVB-induced squamous cell carcinomas in hairless mice. Exp. Dermatol. 2013, 22, 358–379. [Google Scholar] [CrossRef]
- Audiger, C.; Rahman, M.J.; Yun, T.J.; Tarbell, K.V.; Lesage, S. The Importance of Dendritic Cells in Maintaining Immune Tolerance. J. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Peters, C.; Lakbir, D.; Bünemann, E.; Börger, V.; Sabel, M.C.; Hänggi, D.; Steiger, H.J.; Stummer, W.; Sorg, R.V. Heat-shock protein 70-dependent dendritic cell activation by 5-aminolevulinic acid-mediated photodynamic treatment of human glioblastoma spheroids in vitro. Br. J. Cancer 2011, 105, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Dougherty, G.J. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999, 59, 1941–1946. [Google Scholar]
- Korbelik, M.; Krosl, G.; Krosl, J.; Dougherty, G.J. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 1996, 56, 5647–5652. [Google Scholar]
- Korbelik, M.; Cecic, I. Contribution of myeloid and lymphoid host cells to the curative outcome of mouse sarcoma treatment by photodynamic therapy. Cancer Lett. 1999, 137, 91–98. [Google Scholar] [CrossRef]
- Kabingu, E.; Vaughan, L.; Owczarczak, B.; Ramsey, K.; Gollnick, S. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br. J. Cancer 2007, 96, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Gabrysiak, M.; Muchowicz, A.; Bednarek, W.; Barankiewicz, J.; Rygiel, T.; Boon, L.; Mroz, P.; Hamblin, M.R.; Golab, J. 5-Aza-2′-deoxycytidine potentiates antitumour immune response induced by photodynamic therapy. Eur. J. Cancer 2014, 50, 1370–1381. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Deng, H.; Wu, J.; Huang, H.; Sun, R.; Zhang, H.; Xiong, X.; Feng, M. Robust Photodynamic Therapy Using 5-ALA-Incorporated Nanocomplexes Cures Metastatic Melanoma through Priming of CD4 + CD8 + Double Positive T Cells. Adv. Sci. 2019. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, P.; Wang, X.; Shi, L.; Fan, Z.; Zhang, G.; Yang, D.; Bahavar, C.F.; Zhou, F.; Chen, W.R.; et al. Antitumor effects of DC vaccine with ALA-PDT-induced immunogenic apoptotic cells for skin squamous cell carcinoma in mice. Technol. Cancer Res. Treat. 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Prignano, F.; Lotti, T.; Spallanzani, A.; Berti, S.; De Giorgi, V.; Moretti, S. Sequential effects of photodynamic treatment of basal cell carcinoma. J. Cutan. Pathol. 2009, 36, 409–416. [Google Scholar] [CrossRef]
- Adamek, M.; Kawczyk-Krupka, A.; Mostowy, A.; Czuba, Z.; Krol, W.; Kasperczyk, S.; Jakobisiak, M.; Golab, J.; Sieron, A. Topical ALA-PDT modifies neutrophils’ chemiluminescence, lymphocytes’ interleukin-1beta secretion and serum level of transforming growth factor beta1 in patients with nonmelanoma skin malignancies: A clinical study. Photodiagnosis Photodyn. Ther. 2005, 2, 65–72. [Google Scholar] [CrossRef]
- Pellegrini, C.; Orlandi, A.; Costanza, G.; Di Stefani, A.; Piccioni, A.; Di Cesare, A.; Chiricozzi, A.; Ferlosio, A.; Peris, K.; Fargnoli, M.C. Expression of IL-23/Th17-related cytokines in basal cell carcinoma and in the response to medical treatments. PLoS ONE 2017. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Casari, A.; Pepe, P.; Moscarella, E.; Zalaudek, I.; Argenziano, G.; Pellacani, G. Confocal microscopy insights into the treatment and cellular immune response of Basal cell carcinoma to photodynamic therapy. Dermatology 2012, 225, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Winters, U.; Daayana, S.; Lear, J.T.; Tomlinson, A.E.; Elkord, E.; Stern, P.L.; Kitchener, H.C. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clin. Cancer Res. 2008. [Google Scholar] [CrossRef] [PubMed]
- Reginato, E.; Lindenmann, J.; Langner, C.; Schweintzger, N.; Bambach, I.; Smolle-Jüttner, F.; Wolf, P. Photodynamic therapy downregulates the function of regulatory T cells in patients with esophageal squamous cell carcinoma. Photochem. Photobiol. Sci. 2014, 13, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.N.; Lorenz, K.; Lotfi, R.; Fürst, D.; Tsamadou, C.; Jaekle, S.; Mytilineos, J.; Brunner, C.; Theodorakis, J.; Hoffmann, T.K.; et al. Influence of photodynamic therapy on peripheral immune cell populations and cytokine concentrations in head and neck cancer. Photodiagnosis Photodyn. Ther. 2017. [Google Scholar] [CrossRef]
- Evangelou, G.; Farrar, M.D.; Cotterell, L.; Andrew, S.; Tosca, A.D.; Watson, R.E.B.; Rhodes, L.E. Topical photodynamic therapy significantly reduces epidermal Langerhans cells during clinical treatment of basal cell carcinoma. Br. J. Dermatol. 2012, 166, 1112–1115. [Google Scholar] [CrossRef]
- Kabingu, E.; Oseroff, A.; Wilding, G.; Gollnick, S. Enhanced systemic immune reactivity to a Basal cell carcinoma associated antigen following photodynamic therapy. Clin. cancer Res. 2009, 15, 4460–4466. [Google Scholar] [CrossRef]
- Abdel-Hady, E.S.; Martin-Hirsch, P.; Duggan-Keen, M.; Stern, P.L.; Moore, J.V.; Corbitt, G.; Kitchener, H.C.; Hampson, I.N. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001. [Google Scholar]
- Thong, P.S.P.; Ong, K.W.; Goh, N.S.G.; Kho, K.W.; Manivasager, V.; Bhuvaneswari, R.; Olivo, M.; Soo, K.C. Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol. 2007. [Google Scholar] [CrossRef]
- Anzengruber, F.; Avci, P.; De Freitas, L.F.; Hamblin, M.R. T-cell mediated anti-tumor immunity after photodynamic therapy: Why does it not always work and how can we improve it? Photochem. Photobiol. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013. [Google Scholar] [CrossRef] [PubMed]
- Kalathil, S.G.; Thanavala, Y. High immunosuppressive burden in cancer patients: A major hurdle for cancer immunotherapy. Cancer Immunol. Immunother. 2016. [Google Scholar] [CrossRef] [PubMed]
- Circelli, L.; Tornesello, M.; Buonaguro, F.M.; Buonaguro, L. Use of adjuvants for immunotherapy. Hum. Vaccines Immunother. 2017. [Google Scholar] [CrossRef]
- Bae, S.M.; Kim, Y.W.; Kwak, S.Y.; Kim, Y.W.; Ro, D.Y.; Shin, J.C.; Park, C.H.; Han, S.J.; Oh, C.H.; Kim, C.K.; et al. Photodynamic therapy-generated tumor cell lysates with CpG-oligodeoxynucleotide enhance immunotherapy efficacy in human papillomavirus 16 (E6/E7) immortalized tumor cells. Cancer Sci. 2007. [Google Scholar] [CrossRef]
- Xia, Y.; Gupta, G.K.; Castano, A.P.; Mroz, P.; Avci, P.; Hamblin, M.R. CpG oligodeoxynucleotide as immune adjuvant enhances photodynamic therapy response in murine metastatic breast cancer. J. Biophotonics 2014. [Google Scholar] [CrossRef]
- Bhatta, A.K.; Wang, P.; Keyal, U.; Zhao, Z.; Ji, J.; Zhu, L.; Wang, X.; Zhang, G. Therapeutic effect of Imiquimod enhanced ALA-PDT on cutaneous squamous cell carcinoma. Photodiagnosis Photodyn. Ther. 2018. [Google Scholar] [CrossRef]
- Korbelik, M.; Sun, J.; Cecic, I.; Serrano, K. Adjuvant treatment for complement activation increases the effectiveness of photodynamic therapy of solid tumors. Photochem. Photobiol. Sci. 2004, 3, 812–816. [Google Scholar] [CrossRef]
- Korbelik, M.; Cecic, I. Enhancement of tumour response to photodynamic therapy by adjuvant mycobacterium cell-wall treatment. J. Photochem. Photobiol. B Biol. 1998, 44, 151–158. [Google Scholar] [CrossRef]
- Korbelik, M.; Banáth, J.; Zhang, W.; Gallagher, P.; Hode, T.; Lam, S.S.K.; Chen, W.R. N-dihydrogalactochitosan as immune and direct antitumor agent amplifying the effects of photodynamic therapy and photodynamic therapy-generated vaccines. Int. Immunopharmacol. 2019. [Google Scholar] [CrossRef]
- Korbelik, M.; Banáth, J.; Saw, K.M.; Zhang, W.; Čiplys, E. Calreticulin as cancer treatment adjuvant: Combination with photodynamic therapy and photodynamic therapy-generated vaccines. Front. Oncol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Korbelik, M.; Bartels, K.E.; Liu, H.; Sun, J.; Nordquist, R.E. Enhancement of Laser Cancer Treatment by a Chitosan-derived Immunoadjuvant¶. Photochem. Photobiol. 2005. [Google Scholar] [CrossRef]
- Korbelik, M.; Naraparaju, V.R.; Yamamoto, N. Macrophage-directed immunotherapy as adjuvant to photodynamic therapy of cancer. Br. J. Cancer 1997. [Google Scholar] [CrossRef] [PubMed]
- Gołab, J.; Wilczyński, G.; Zagozdzon, R.; Stokłosa, T.; Dabrowska, A.; Rybczyńska, J.; Wasik, M.; Machaj, E.; Ołda, T.; Kozar, K.; et al. Potentiation of the anti-tumour effects of Photofrin-based photodynamic therapy by localized treatment with G-CSF. Br. J. Cancer 2000, 82, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Krosl, G.; Korbelik, M. Potentiation of photodynamic therapy by immunotherapy: The effect of schizophyllan (SPG). Cancer Lett. 1994, 84, 43–49. [Google Scholar] [CrossRef]
- Ji, J.; Wang, P.; Zhou, Q.; Zhu, L.; Zhang, H.; Zhang, Y.; Zheng, Z.; Bhatta, A.K.; Zhang, G.; Wang, X. CCL8 enhances sensitivity of cutaneous squamous cell carcinoma to photodynamic therapy by recruiting M1 macrophages. Photodiagnosis Photodyn. Ther. 2019. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J. Am. Chem. Soc. 2016. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, M.J.; Murray, K.S.; La Rosa, S.P.; Budhu, S.; Merghoub, T.; Somma, A.; Monette, S.; Kim, K.; Corradi, R.B.; Scherz, A.; et al. Systemic antitumor immunity by PD-1/PD-L1 inhibition is potentiated by vascular-targeted photodynamic therapy of primary tumors. Clin. Cancer Res. 2018. [Google Scholar] [CrossRef]
- Kleinovink, J.W.; Fransen, M.F.; Löwik, C.W.; Ossendorp, F. Photodynamic-immune checkpoint therapy eradicates local and distant tumors by CD8+ T cells. Cancer Immunol. Res. 2017. [Google Scholar] [CrossRef]
- Muchowicz, A.; Wachowska, M.; Stachura, J.; Tonecka, K.; Gabrysiak, M.; Wołosz, D.; Pilch, Z.; Kilarski, W.W.; Boon, L.; Klaus, T.J.; et al. Inhibition of lymphangiogenesis impairs antitumour effects of photodynamic therapy and checkpoint inhibitors in mice. Eur. J. Cancer 2017. [Google Scholar] [CrossRef]
- Bao, R.; Wang, Y.; Lai, J.; Zhu, H.; Zhao, Y.; Li, S.; Li, N.; Huang, J.; Yang, Z.; Wang, F.; et al. Enhancing Anti-PD-1/PD-L1 Immune Checkpoint Inhibitory Cancer Therapy by CD276-Targeted Photodynamic Ablation of Tumor Cells and Tumor Vasculature. Mol. Pharm. 2019, 16, 339–348. [Google Scholar] [CrossRef]
- Nagaya, T.; Friedman, J.; Maruoka, Y.; Ogata, F.; Okuyama, S.; Clavijo, P.E.; Choyke, P.L.; Allen, C.; Kobayashi, H. Host immunity following near-infrared photoimmunotherapy is enhanced with PD-1 checkpoint blockade to eradicate established antigenic tumors. Cancer Immunol. Res. 2019, 7, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Monu, N.R.; Frey, A.B. Myeloid-derived suppressor cells and anti-tumor T cells: A complex relationship. Immunol. Invest. 2012. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular mechanisms oftreg-mediatedt cell suppression. Front. Immunol. 2012. [Google Scholar] [CrossRef]
- Reginato, E.; Mroz, P.; Chung, H.; Kawakubo, M.; Wolf, P.; Hamblin, M.R. Photodynamic therapy plus regulatory T-cell depletion produces immunity against a mouse tumour that expresses a self-antigen. Br. J. Cancer 2013, 109, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Banáth, J.; Zhang, W. Mreg activity in tumor response to photodynamic therapy and photodynamic therapy-generated cancer vaccines. Cancers 2016, 8, 94. [Google Scholar] [CrossRef]
- Kumai, T.; Oikawa, K.; Aoki, N.; Kimura, S.; Harabuchi, Y.; Celis, E.; Kobayashi, H. Tumor-derived TGF-β and prostaglandin E2 attenuate anti-tumor immune responses in head and neck squamous cell carcinoma treated with EGFR inhibitor. J. Transl. Med. 2014. [Google Scholar] [CrossRef]
- Robbins, P.F.; El-Gamil, M.; Li, Y.F.; Kawakami, Y.; Loftus, D.; Appella, E.; Rosenberg, S.A. A mutated β-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J. Exp. Med. 1996. [Google Scholar] [CrossRef]
- Boon, T.; Old, L. Cancer Tumor antigens. Curr. Opin. Immunol. 1997. [Google Scholar] [CrossRef]
- Wang, X.G.; Revskaya, E.; Bryan, R.A.; Strickler, H.D.; Burk, R.D.; Casadevall, A.; Dadachova, E. Treating cancer as an infectious disease-viral antigens as novel targets for treatment and potential prevention of tumors of viral etiology. PLoS ONE 2007. [Google Scholar] [CrossRef]
- Melief, C.J.M.; Van Hall, T.; Arens, R.; Ossendorp, F.; Van Der Burg, S.H. Therapeutic cancer vaccines. J. Clin. Invest. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Tajiri, T.; Tomioka, Y.; Awazu, K. Photodynamic therapy induces interleukin secretion from dendritic cells. Int. J. Clin. Exp. Med. 2010, 3, 110–114. [Google Scholar] [PubMed]
- Zhang, F.; Zhu, Y.; Fan, G.; Hu, S. Photodynamic therapy reduces the inhibitory effect of osteosarcoma cells on dendritic cells by upregulating HSP70. Oncol. Lett. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhu, G.; Wang, S.; Yu, G.; Yang, Z.; Lin, L.; Zhou, Z.; Liu, Y.; Dai, Y.; Zhang, F.; et al. In Situ Dendritic Cell Vaccine for Effective Cancer Immunotherapy. ACS Nano 2019. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yin, G.; Le, V.; Zhang, A.; Chen, S.; Liang, X.; Liu, J. Photodynamic-therapy Activates Immune Response by disrupting Immunity Homeostasis of Tumor Cells, which Generates Vaccine for Cancer Therapy. Int. J. Biol. Sci. 2016, 12, 120–132. [Google Scholar] [CrossRef]
- Jung, N.C.; Kim, H.J.; Kang, M.S.; Lee, J.H.; Song, J.Y.; Seo, H.G.; Bae, Y.S.; Lim, D.S. Photodynamic therapy-mediated DC immunotherapy is highly effective for the inhibition of established solid tumors. Cancer Lett. 2012. [Google Scholar] [CrossRef]
- Zhang, N.; Bai, S.; Cai, X.; Li, L. Inhibitory and immunological effects induced by the combination of photodynamic therapy and dendritic cells on mouse transplanted hepatoma. Photodiagnosis Photodyn. Ther. 2015, 8–11. [Google Scholar] [CrossRef]
- Kleinovink, J.W.; van Driel, P.B.; Snoeks, T.J.; Prokopi, N.; Fransen, M.F.; Cruz, L.J.; Mezzanotte, L.; Chan, a.; Lowik, C.W.; Ossendorp, F. Combination of Photodynamic Therapy and Specific Immunotherapy Efficiently Eradicates Established Tumors. Clin. Cancer Res. 2015, 1–11. [Google Scholar] [CrossRef]
- Zom, G.G.; Willems, M.M.J.H.P.; Khan, S.; Van Der Sluis, T.C.; Kleinovink, J.W.; Camps, M.G.M.; Van Der Marel, G.A.; Filippov, D.V.; Melief, C.J.M.; Ossendorp, F. Novel TLR2-binding adjuvant induces enhanced T cell responses and tumor eradication. J. Immunother. Cancer 2018. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Xu, J.; Zhang, R.; Song, G.; Chao, Y.; Feng, L.; Han, F.; Dong, Z.; Li, B.; et al. Smart Nanoreactors for pH-Responsive Tumor Homing, Mitochondria-Targeting, and Enhanced Photodynamic-Immunotherapy of Cancer. Nano Lett. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, R.; Buchner, A.; Palluch, P.; Pongratz, T.; Oboukhovskij, K.; Beyer, W.; Johansson, A.; Stepp, H.; Baumgartner, R.; Zimmermann, W. Induction of immune mediators in glioma and prostate cancer cells by non-lethal photodynamic therapy. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Brackett, C.M.; Owczarczak, B.; Ramsey, K.; Maier, P.G.; Gollnick, S.O. IL-6 potentiates tumor resistance to photodynamic therapy (PDT). Lasers Surg. Med. 2011, 43, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Skivka, L.M.; Gorobets, O.B.; Kutsenok, V.V.; Lozinsky, M.O.; Borisevich, A.N.; Fedorchuk, A.G.; Kholin, V.V.; Gamaleya, N.F. 5-aminolevulinic acid mediated photodynamic therapy of Lewis lung carcinoma: A role of tumor infiltration with different cells of immune system. Exp. Oncol. 2004, 26, 312–315. [Google Scholar]
- Shams, M.; Owczarczak, B.; Manderscheid-Kern, P.; Bellnier, D.A.; Gollnick, S.O. Development of photodynamic therapy regimens that control primary tumor growth and inhibit secondary disease. Cancer Immunol. Immunother. 2015, 64, 287–297. [Google Scholar] [CrossRef]
- Sitnik, T.M.; Hampton, J.A.; Henderson, B.W. Reduction of tumour oxygenation during and after photodynamic therapy in vivo: Effects of fluence rate. Br. J. Cancer 1998, 77, 1386–1394. [Google Scholar] [CrossRef]
- Henderson, B.W.; Busch, T.M.; Vaughan, L.A.; Frawley, N.P.; Babich, D.; Sosa, T.A.; Zollo, J.D.; Dee, A.S.; Cooper, M.T.; Bellnier, D.A.; et al. Photofrin photodynamic therapy can significantly deplete or preserve oxygenation in human basal cell carcinomas during treatment, depending on fluence rate. Cancer Res. 2000, 60, 525–529. [Google Scholar]
- Henderson, B.W.; Gollnick, S.O.; Snyder, J.W.; Busch, T.M.; Kousis, P.C.; Cheney, R.T.; Morgan, J. Choice of Oxygen-Conserving Treatment Regimen Determines the Inflammatory Response and Outcome of Photodynamic Therapy of Tumors. Cancer Res. 2004, 64, 2120–2126. [Google Scholar] [CrossRef]
- De Vijlder, H.C.; Sterenborg, H.J.C.M.; Martino Neumann, H.A.; Robinson, D.J.; De Haas, E.R.M. Light fractionation significantly improves the response of superficial basal cell carcinoma to aminolaevulinic acid photodynamic therapy: Five-year follow-up of a randomized, prospective trial. Acta Derm. Venereol. 2012, 92, 641–647. [Google Scholar] [CrossRef]

| Photosensitizer (PS) | Porfimer Sodium | ALA |
|---|---|---|
| Trade name | Photofrin | Levulan |
| Composition | Mixture of hematoporphyrin derivatives | Heme precursor (prodrug) converted to Protoporphyrin IX |
| Maximum absorption | 630 nm | 630–635 nm |
| Adsorption at maximum wavelength | 3000 M−1cm−1 (weak) | 5000 M−1cm−1 (weak) |
| Administration | Systemic (i.v.) | Systemic (i.v.), oral, topical |
| Time of illumination after PS administration | 40–50 h | Within 24 h |
| Clinical dose | 1 mg/kg | 10–20% ALA emulsion (topical) |
| Illumination conditions | 80–260 J/cm2 150 mW/cm2 | 75–260 J/cm2 150 mW/cm2 |
| Singlet oxygen quantum yield | 0.89 (high) | 0.56 (moderate) |
| PS localization | Mitochondria | Cell membrane, mitochondria, lysosome |
| Induced cell death | Apoptosis (mainly) * | Apoptosis (mainly) * |
| Disadvantages | Limited tissue penetration Skin photosensitization (6 weeks) | Limited tissue penetration Moderate pain if skin treatment |
| Approved indications | Bladder, esophageal, skin, and non-small cell lung and cancer | Actinic keratosis and other non-oncologic indications |
| Ongoing clinical trials | Brain, cervical, breast and head and neck cancer, among others | Basal cell carcinoma, cervical neoplasia, and head and neck cancer, among others |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán Hernández, I.; Yu, Y.; Ossendorp, F.; Korbelik, M.; Oliveira, S. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations. J. Clin. Med. 2020, 9, 333. https://doi.org/10.3390/jcm9020333
Beltrán Hernández I, Yu Y, Ossendorp F, Korbelik M, Oliveira S. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations. Journal of Clinical Medicine. 2020; 9(2):333. https://doi.org/10.3390/jcm9020333
Chicago/Turabian StyleBeltrán Hernández, Irati, Yingxin Yu, Ferry Ossendorp, Mladen Korbelik, and Sabrina Oliveira. 2020. "Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations" Journal of Clinical Medicine 9, no. 2: 333. https://doi.org/10.3390/jcm9020333
APA StyleBeltrán Hernández, I., Yu, Y., Ossendorp, F., Korbelik, M., & Oliveira, S. (2020). Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations. Journal of Clinical Medicine, 9(2), 333. https://doi.org/10.3390/jcm9020333





