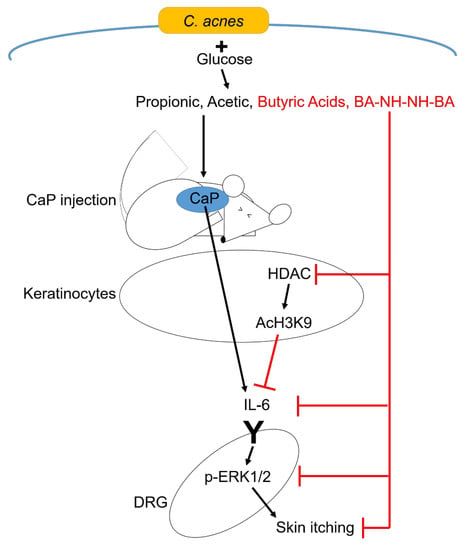
Skin Cutibacterium acnes Mediates Fermentation to Suppress the Calcium Phosphate-Induced Itching: A Butyric Acid Derivative with Potential for Uremic Pruritus
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Bacterial Culture and Fermentation
2.3. GC-MS Analysis
2.4. CaP Degradation
2.5. RT-PCR
2.6. Administration of CaP into Cells and Mice
2.7. Flow Cytometry
2.8. NGS Analysis
2.9. Statistical Analysis
3. Results
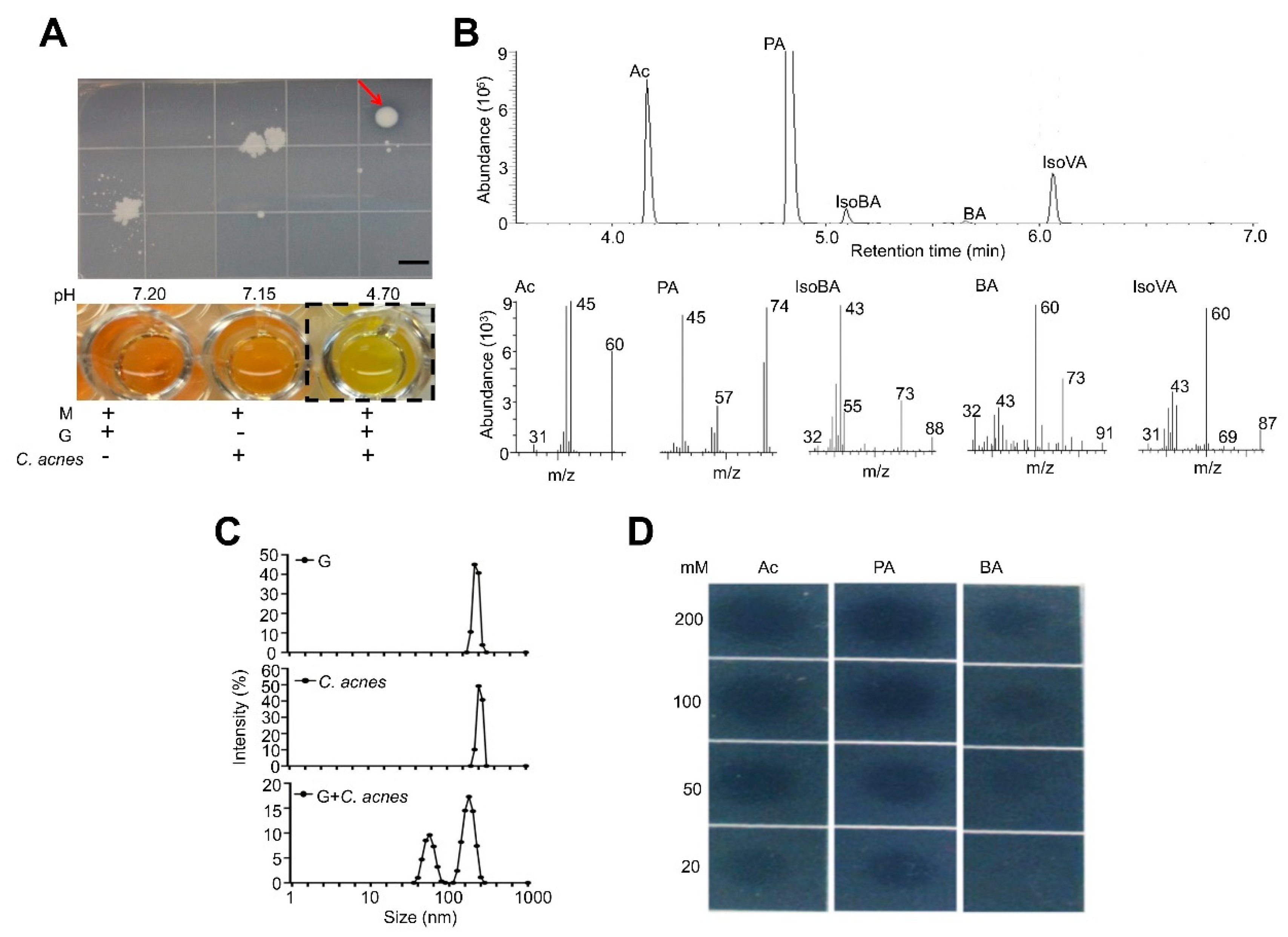
3.1. C. acnes Fermentation and CaP Solubilization by Fermentation Metabolites
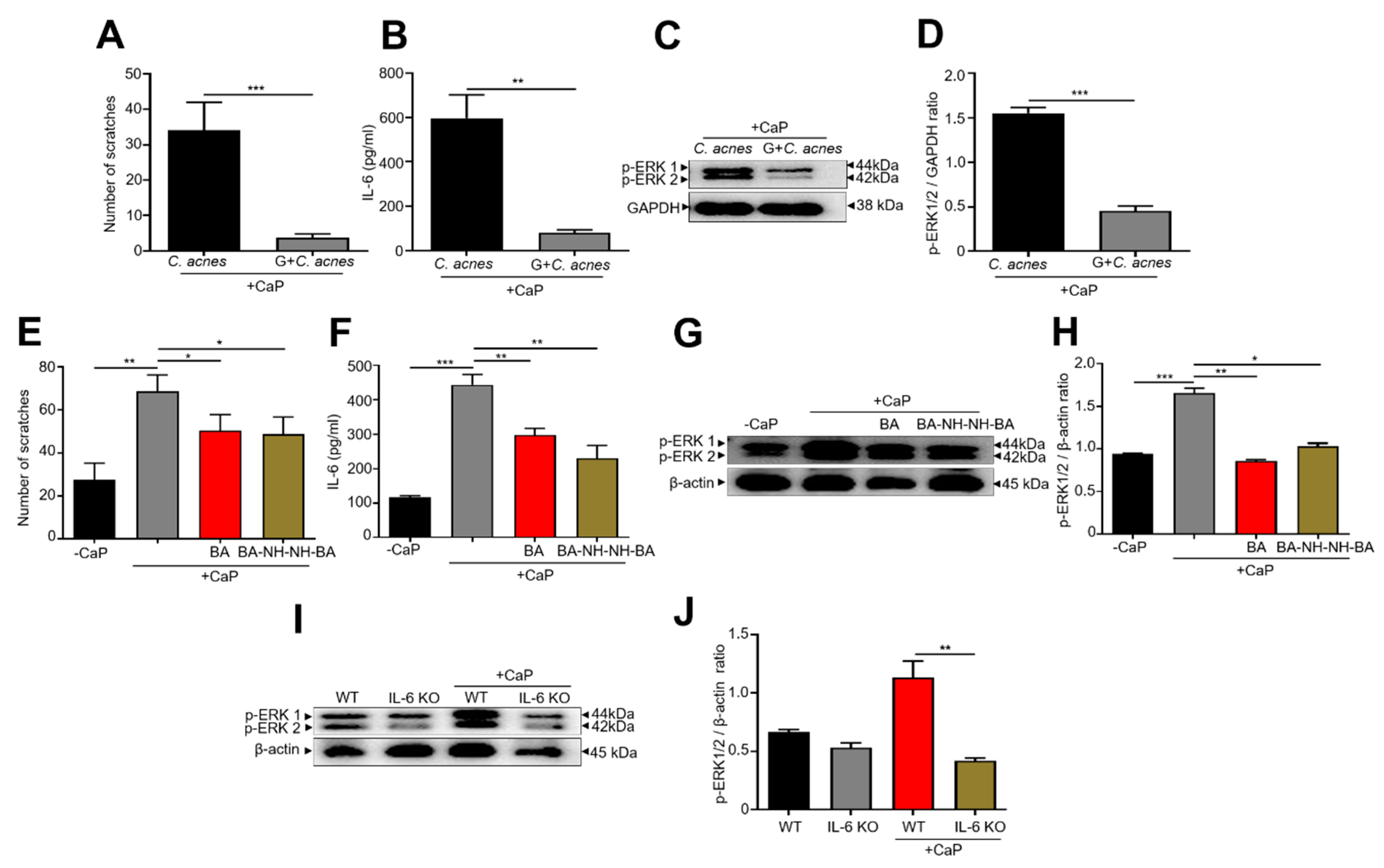
3.2. Regulation of CaP-Induced IL-6 and Histone H3 Lysine 9 Acetylation (AcH3K9) by Butyric Acid and Butyric Acid N-[2-(2-Butyrylamino-ethoxy)-ethyl]-butyramide, BA-NH-NH-BA
3.3. Inhibition of CaP-Induced Itching, IL-6, and p-ERK 1/2 by BA-NH-NH-BA in Mice
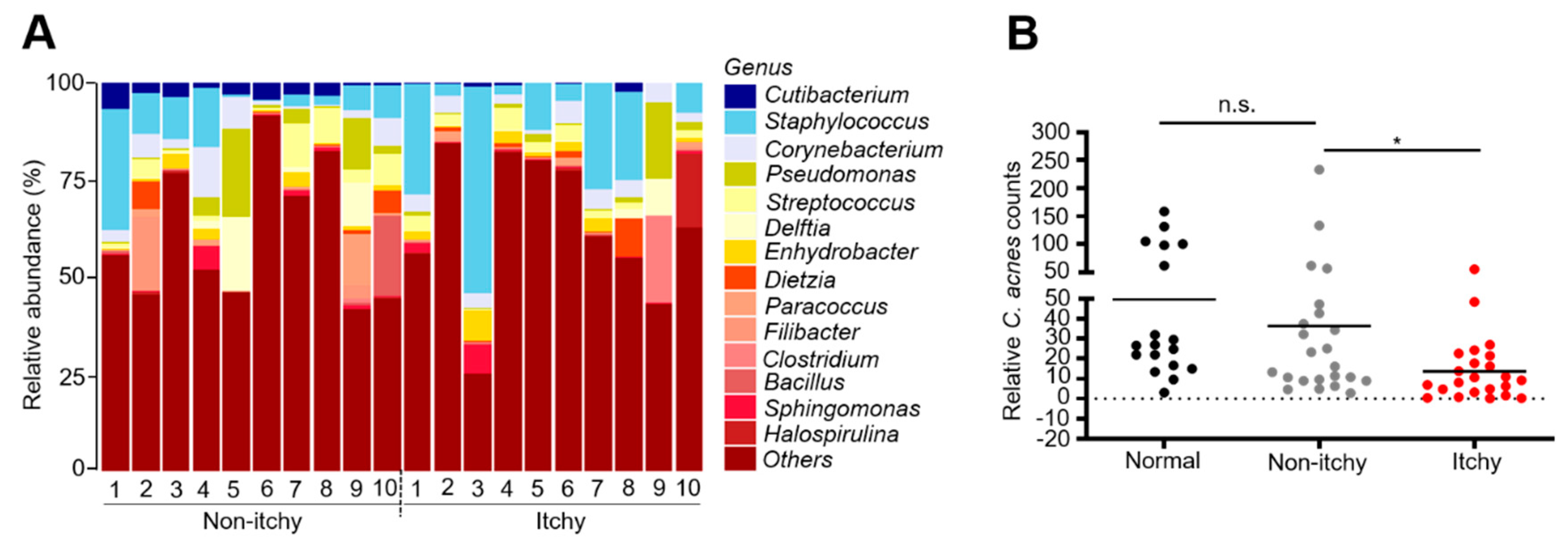
3.4. Reduction of the Abundance of Genus Cutibacterium in Itchy Skin of Patients with CKD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AcH3K9 | histone H3 lysine 9 acetylation |
| BA-NH-NH-BA | butyric acid N-[2-(2-Butyrylamino-ethoxy)-ethyl]-butyramide |
| B. shackletonii | Bacillus shackletonii |
| CaP | calcium phosphate |
| C. acnes | Cutibacterium acnes |
| CKD | chronic kidney disease |
| DOPPS | Dialysis Outcomes and Practice Patterns Study |
| DRG | dorsal root ganglion |
| ERK | extracellular signal-regulated kinases |
| GPCR | G protein-coupled receptor |
| HDAC | histone deacetylase |
| K10 | keratin 10 |
| LN | lupus nephritis |
| NGS | next-generation sequencing |
| OD | optical density |
| OTUs | operational taxonomic units |
| RR | relative risk |
| S. aureus | Staphylococcus aureus |
| SCFAs | short-chain fatty acids |
| S. epidermidis | Staphylococcus epidermidis |
| SE | standard error |
References
- Lavery, M.J.; Stull, C.; Kinney, M.O.; Yosipovitch, G. Nocturnal Pruritus: The Battle for a Peaceful Night’s Sleep. Int. J. Mol. Sci. 2016, 17, 425. [Google Scholar] [CrossRef]
- Berger, T.G.; Steinhoff, M. Pruritus and renal failure. Semin. Cutan. Med. Surg. 2011, 30, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Shirazian, S.; Aina, O.; Park, Y.; Chowdhury, N.; Leger, K.; Hou, L.; Miyawaki, N.; Mathur, V.S. Chronic kidney disease-associated pruritus: Impact on quality of life and current management challenges. Int. J. Nephrol. Renov. Dis. 2017, 10, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Heisig, M.; Reich, A.; Szepietowski, J.C. Is uremic pruritus still an important clinical problem in maintenance hemodialysis patients? J. Eur. Acad. Dermatol. Venereol. 2016, 30, e198–e199. [Google Scholar] [CrossRef] [PubMed]
- Solak, B.; Acikgoz, S.B.; Sipahi, S.; Erdem, T. Epidemiology and determinants of pruritus in pre-dialysis chronic kidney disease patients. Int. Urol. Nephrol. 2016, 48, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Susel, J.; Batycka-Baran, A.; Reich, A.; Szepietowski, J.C. Uraemic pruritus markedly affects the quality of life and depressive symptoms in haemodialysis patients with end-stage renal disease. Acta Derm. Venereol. 2014, 94, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Mettang, T.; Tschulena, U.; Passlick-Deetjen, J.; Weisshaar, E. Prevalence of chronic itch and associated factors in haemodialysis patients: A representative cross-sectional study. Acta Derm. Venereol. 2015, 95, 816–821. [Google Scholar] [CrossRef]
- Pisoni, R.L.; Wikstrom, B.; Elder, S.J.; Akizawa, T.; Asano, Y.; Keen, M.L.; Saran, R.; Mendelssohn, D.C.; Young, E.W.; Port, F.K. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transpl. 2006, 21, 3495–3505. [Google Scholar] [CrossRef]
- Wong, L.Y.; Liew, A.S.T.; Weng, W.T.; Lim, C.K.; Vathsala, A.; Toh, M. Projecting the Burden of Chronic Kidney Disease in a Developed Country and Its Implications on Public Health. Int. J. Nephrol. 2018, 2018, 5196285. [Google Scholar] [CrossRef]
- Collins, A.J.; Foley, R.N.; Gilbertson, D.T.; Chen, S.C. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int. Suppl. (2011) 2015, 5, 2–7. [Google Scholar] [CrossRef]
- Saliba, W.; El-Haddad, B. Secondary Hyperparathyroidism: Pathophysiology and Treatment. J. Am. Board Fam. Med. JABFM 2009, 22, 574–581. [Google Scholar] [CrossRef]
- Tentori, F.; Blayney, M.J.; Albert, J.M.; Gillespie, B.W.; Kerr, P.G.; Bommer, J.; Young, E.W.; Akizawa, T.; Akiba, T.; Pisoni, R.L.; et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2008, 52, 519–530. [Google Scholar] [CrossRef]
- Tajbakhsh, R.; Joshaghani, H.; Bayzayi, F.; Haddad, M.; Qorbani, M. Association between pruritus and serum concentrations of parathormone, calcium and phosphorus in hemodialysis patients. Saudi J. Kidney Dis. Transpl. 2013, 24, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Kurban, M.S.; Boueiz, A.; Kibbi, A.G. Cutaneous manifestations of chronic kidney disease. Clin Derm. 2008, 26, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Le, C.; Bedocs, P.M. Calcinosis Cutis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Blachley, J.D.; Blankenship, D.M.; Menter, A.; Parker, T.F., III; Knochel, J.P. Uremic pruritus: Skin divalent ion content and response to ultraviolet phototherapy. Am. J. Kidney Dis. 1985, 5, 237–241. [Google Scholar] [CrossRef]
- Momose, A.; Kudo, S.; Sato, M.; Saito, H.; Nagai, K.; Katabira, Y.; Funyu, T. Calcium ions are abnormally distributed in the skin of haemodialysis patients with uraemic pruritus. Nephrol. Dial. Transpl. 2004, 19, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Wilson, S.R.; Hoon, M.A. Why we scratch an itch: The molecules, cells and circuits of itch. Nat. Neurosci. 2014, 17, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ji, R.R. New insights into the mechanisms of itch: Are pain and itch controlled by distinct mechanisms? Pflug. Arch. 2013, 465, 1671–1685. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, G.Y.; Song, N.J.; Huang, Y.; Chen, J.Y.; Wang, Q.X.; Ding, Y.Q. Extracellular signal-regulated kinase (ERK) activation is required for itch sensation in the spinal cord. Mol. Brain 2014, 7, 25. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, S.; Liu, Y.; Xiong, J.; Liang, J.; Ji, W. Role of ERK1/2 activation on itch sensation induced by bradykinin B1 activation in inflamed skin. Exp. Med. 2016, 12, 627–632. [Google Scholar] [CrossRef][Green Version]
- Amdur, R.L.; Mukherjee, M.; Go, A.; Barrows, I.R.; Ramezani, A.; Shoji, J.; Reilly, M.P.; Gnanaraj, J.; Deo, R.; Roas, S.; et al. Interleukin-6 Is a Risk Factor for Atrial Fibrillation in Chronic Kidney Disease: Findings from the CRIC Study. PLoS ONE 2016, 11, e0148189. [Google Scholar] [CrossRef] [PubMed]
- Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Temmar, M.; Lemke, H.D.; Tribouilloy, C.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010, 77, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Yamamura, K.; Kido-Nakahara, M.; Nakahara, T.; Fukui, Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy 2018, 73, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.D.; Oussedik, E.; D’Amber, V.; Feldman, S.R. Interleukin-31 pathway and its role in atopic dermatitis: A systematic review. J. Dermatol. Treat. 2017, 28, 591–599. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Patsinakidis, N.; Raap, U. Role of the Pruritic Cytokine IL-31 in Autoimmune Skin Diseases. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef]
- Yang, X.O.; Panopoulos, A.D.; Nurieva, R.; Chang, S.H.; Wang, D.; Watowich, S.S.; Dong, C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007, 282, 9358–9363. [Google Scholar] [CrossRef]
- Regna, N.L.; Chafin, C.B.; Hammond, S.E.; Puthiyaveetil, A.G.; Caudell, D.L.; Reilly, C.M. Class I and II histone deacetylase inhibition by ITF2357 reduces SLE pathogenesis in vivo. Clin. Immunol. 2014, 151, 29–42. [Google Scholar] [CrossRef]
- Bek-Thomsen, M.; Lomholt, H.B.; Kilian, M. Acne is not associated with yet-uncultured bacteria. J. Clin. Microbiol. 2008, 46, 3355–3360. [Google Scholar] [CrossRef]
- Keshari, S.; Kumar, M.; Balasubramaniam, A.; Chang, T.W.; Tong, Y.; Huang, C.-M. Prospects of acne vaccines targeting secreted virulence factors of Cutibacterium acnes. Expert Rev. Vaccines 2019, 18, 433–437. [Google Scholar] [CrossRef]
- Shi, B.; Bangayan, N.J.; Curd, E.; Taylor, P.A.; Gallo, R.L.; Leung, D.Y.M.; Li, H. The skin microbiome is different in pediatric versus adult atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Tseng, C.H.; Strober, B.E.; Pei, Z.; Blaser, M.J. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS ONE 2008, 3, e2719. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Program, N.C.S.; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef]
- Chang, H.W.; Yan, D.; Singh, R.; Liu, J.; Lu, X.; Ucmak, D.; Lee, K.; Afifi, L.; Fadrosh, D.; Leech, J.; et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018, 6, 154. [Google Scholar] [CrossRef]
- Fyhrquist, N.; Muirhead, G.; Prast-Nielsen, S.; Jeanmougin, M.; Olah, P.; Skoog, T.; Jules-Clement, G.; Feld, M.; Barrientos-Somarribas, M.; Sinkko, H.; et al. Microbe-host interplay in atopic dermatitis and psoriasis. Nat. Commun. 2019, 10, 4703. [Google Scholar] [CrossRef]
- Francuzik, W.; Franke, K.; Schumann, R.R.; Heine, G.; Worm, M. Propionibacterium acnes Abundance Correlates Inversely with Staphylococcus aureus: Data from Atopic Dermatitis Skin Microbiome. Acta Derm. Venereol. 2018, 98, 490–495. [Google Scholar] [CrossRef]
- Shu, M.; Wang, Y.; Yu, J.; Kuo, S.; Coda, A.; Jiang, Y.; Gallo, R.L.; Huang, C.M. Fermentation of Propionibacterium acnes, a Commensal Bacterium in the Human Skin Microbiome, as Skin Probiotics against Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2013, 8, e55380. [Google Scholar] [CrossRef]
- Kao, M.S.; Huang, S.; Chang, W.L.; Hsieh, M.F.; Huang, C.J.; Gallo, R.L.; Huang, C.M. Microbiome precision editing: Using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Elmariah, S.B.; Lerner, E.A. Topical therapies for pruritus. Semin. Cutan. Med. Surg. 2011, 30, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Traisaeng, S.; Herr, D.R.; Kao, H.J.; Chuang, T.H.; Huang, C.M. A Derivative of Butyric Acid, the Fermentation Metabolite of Staphylococcus epidermidis, Inhibits the Growth of a Staphylococcus aureus Strain Isolated from Atopic Dermatitis Patients. Toxins 2019, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Keshari, S.; Sipayung, A.D.; Hsieh, C.C.; Su, L.J.; Chiang, Y.R.; Chang, H.C.; Yang, W.C.; Chuang, T.H.; Chen, C.L.; Huang, C.M. The IL-6/p-BTK/p-ERK signaling mediates the calcium phosphate-induced pruritus. FASEB J. 2019, 33, 12036–12046. [Google Scholar] [CrossRef]
- Gröne, A. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002, 88, 1–12. [Google Scholar] [CrossRef]
- Olaru, F.; Jensen, L.E. Chemokine expression by human keratinocyte cell lines after activation of Toll-like receptors. Exp. Derm. 2010, 19, e314–e316. [Google Scholar] [CrossRef]
- Wagner Mackenzie, B.; Waite, D.W.; Taylor, M.W. Evaluating variation in human gut microbiota profiles due to DNA extraction method and inter-subject differences. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4516–4522. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Turan, M.; Ataoğlu, N.; Şahιn, F. Evaluation of the Capacity of Phosphate Solubilizing Bacteria and Fungi on Different Forms of Phosphorus in Liquid Culture. J. Sustain. Agric. 2006, 28, 99–108. [Google Scholar] [CrossRef]
- Yadav, B.K.; Tarafdar, J.C. Efficiency of Bacillus coagulans as P biofertilizer to mobilize native soil organic and poorly soluble phosphates and increase crop yield. Arch. Agron. Soil Sci. 2012, 58, 1099–1115. [Google Scholar] [CrossRef]
- Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus Solubilization by Bacillus Species. Molecules 2018, 23, 2897. [Google Scholar] [CrossRef]
- Jikare, A.; Chavan, M. Siderophore produced by Bacillus shackletonii. Gn-09 and showed its plant growth promoting activity. Int. J. Pharm. Biol. Sci. 2013, 3, 198–202. [Google Scholar]
- Rathore, D.P. Chelation effect on phosphate solubilizing activity by citrobacter freundii mtcc 6738. Int. J. Appl. Nat. Sci. 2014, 3, 103–108. [Google Scholar]
- Sharon, J.; Hathwaik, L.; Glenn, G.M.; Imam, S.; Lee, C.C. Isolation of efficient phosphate solubilizing bacteria capable of enhancing tomato plant growth. J. Soil Sci. Plant Nutr. 2016, 16. [Google Scholar] [CrossRef]
- Chen, Y.; Rekha, P.; Arun, A.; Shen, F.; Lai, W.A.; Young, C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Eddington, H.; Heaf, J.G. Clinical management of disturbances of calcium and phosphate metabolism in dialysis patients. NDT Plus 2009, 2, 267–272. [Google Scholar] [CrossRef][Green Version]
- Kimmel, M.; Alscher, D.M.; Dunst, R.; Braun, N.; Machleidt, C.; Kiefer, T.; Stulten, C.; van der Kuip, H.; Pauli-Magnus, C.; Raub, U.; et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol. Dial. Transpl. 2006, 21, 749–755. [Google Scholar] [CrossRef]
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 715–721. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Ivanek, R.; Grohn, Y.T.; Geornaras, I.; Sofos, J.N.; Wiedmann, M. Quantitative risk assessment for Listeria monocytogenes in selected categories of deli meats: Impact of lactate and diacetate on listeriosis cases and deaths. J. Food Prot. 2009, 72, 978–989. [Google Scholar] [CrossRef]
- Collins, E.B. Preservatives in dairy foods. J. Dairy Sci. 1971, 54, 148–152. [Google Scholar] [CrossRef]
- Banasiewicz, T.; Krokowicz, L.; Stojcev, Z.; Kaczmarek, B.F.; Kaczmarek, E.; Maik, J.; Marciniak, R.; Krokowicz, P.; Walkowiak, J.; Drews, M. Microencapsulated sodium butyrate reduces the frequency of abdominal pain in patients with irritable bowel syndrome. Colorectal. Dis. 2013, 15, 204–209. [Google Scholar] [CrossRef]
- Pituch, A.; Walkowiak, J.; Banaszkiewicz, A. Butyric acid in functional constipation. Gastroenterol. Rev. Przegląd Gastroenterol. 2013, 8, 295–298. [Google Scholar] [CrossRef]
- Christensen, G.J.; Scholz, C.F.; Enghild, J.; Rohde, H.; Kilian, M.; Thurmer, A.; Brzuszkiewicz, E.; Lomholt, H.B.; Bruggemann, H. Antagonism between Staphylococcus epidermidis and Propionibacterium acnes and its genomic basis. BMC Genom. 2016, 17, 152. [Google Scholar] [CrossRef]
- Collier, C.N.; Harper, J.C.; Cafardi, J.A.; Cantrell, W.C.; Wang, W.; Foster, K.W.; Elewski, B.E. The prevalence of acne in adults 20 years and older. J. Am. Acad. Derm. 2008, 58, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.C.; Maglione, J.; Hillebrand, G.G.; Miyamoto, K.; Kimball, A.B. Acne vulgaris in women: Prevalence across the life span. J. Womens Health 2012, 21, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Chiu, Y.L.; Hsu, S.P.; Pai, M.F.; Yang, J.Y.; Peng, Y.S. Relationship between Fetuin A, Vascular Calcification and Fracture Risk in Dialysis Patients. PLoS ONE 2016, 11, e0158789. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Hung, B.; Kempf, M.; Liu, P.Y.; Dalley, A.; Saunders, N.; Kimble, R. Fetuin-A promotes primary keratinocyte migration: Independent of epidermal growth factor receptor signalling. Exp. Dermatol. 2009, 19, e289–e292. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, G.A.; Cummins, K.M.; Wassel, C.L.; Daniels, L.B.; Ix, J.H. The association of fetuin-A with cardiovascular disease mortality in older community-dwelling adults: The Rancho Bernardo study. J. Am. Coll. Cardiol. 2012, 59, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Maria De, A.; Gabriella, G.; Fabio, M.; Leonilde, B.; Piero, P.; Marco, G. The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019, 26, 3567–3583. [Google Scholar] [CrossRef]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef]
- Scheppach, W.; Bartram, H.P.; Richter, F. Role of short-chain fatty acids in the prevention of colorectal cancer. Eur. J. Cancer 1995, 31A, 1077–1080. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Morera, R.; Ciccocioppo, R.; Cazzola, P.; Gotti, S.; Tinozzi, F.P.; Tinozzi, S.; Corazza, G.R. Oral butyrate for mildly to moderately active Crohn’s disease. Aliment. Pharmacol. 2005, 22, 789–794. [Google Scholar] [CrossRef]
- Lisiero, D.N.; Soto, H.; Everson, R.G.; Liau, L.M.; Prins, R.M. The histone deacetylase inhibitor, LBH589, promotes the systemic cytokine and effector responses of adoptively transferred CD8+ T cells. J. Immunother. Cancer 2014, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhuang, S. Treatment of chronic kidney diseases with histone deacetylase inhibitors. Front. Physiol. 2015, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Chriett, S.; Dąbek, A.; Wojtala, M.; Vidal, H.; Balcerczyk, A.; Pirola, L. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci. Rep. 2019, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Finnin, M.S.; Donigian, J.R.; Cohen, A.; Richon, V.M.; Rifkind, R.A.; Marks, P.A.; Breslow, R.; Pavletich, N.P. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 1999, 401, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J.; et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investig. Derm. 2013, 133, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Saggini, A.; Chimenti, S.; Chiricozzi, A. IL-6 as a Druggable Target in Psoriasis: Focus on Pustular Variants. J. Immunol. Res. 2014, 2014, 10. [Google Scholar] [CrossRef]
- Grossman, R.M.; Krueger, J.; Yourish, D.; Granelli-Piperno, A.; Murphy, D.P.; May, L.T.; Kupper, T.S.; Sehgal, P.B.; Gottlieb, A.B. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc. Natl. Acad. Sci. USA 1989, 86, 6367–6371. [Google Scholar] [CrossRef]
- Toshitani, A.; Ansel, J.C.; Chan, S.C.; Li, S.H.; Hanifin, J.M. Increased Interleukin 6 Production by T Cells Derived from Patients with Atopic Dermatitis. J. Investig. Dermatol. 1993, 100, 299–304. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Song, X.; Jiang, W.; Tang, S.; Shen, F.; Xie, S. Association between IL-4, IL-6, IL-18 polymorphisms and atopic dermatitis risk: A meta-analysis. Int. J. Clin. Exp. Med. 2017, 10, 7375–7386. [Google Scholar]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Kuvandik, G.; Cetin, M.; Genctoy, G.; Horoz, M.; Duru, M.; Akcali, C.; Satar, S.; Kiykim, A.A.; Kaya, H. The prevalance, epidemiology and risk factors for onychomycosis in hemodialysis patients. BMC Infect. Dis. 2007, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Noverr, M.C.; Huffnagle, G.B. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect. Immun. 2004, 72, 6206–6210. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Song, J.H.; Kim, I.J.; Joo, S.Y.; Eom, G.H.; Kim, I.; Cha, H.; Cho, J.M.; Ma, S.K.; Kim, S.W.; et al. Histone deacetylase inhibitor, CG200745 attenuates renal fibrosis in obstructive kidney disease. Sci. Rep. 2018, 8, 11546. [Google Scholar] [CrossRef]
- Duvic, M.; Guitart, J.; Huen, A.; Porcu, P.; LeBoeuf, N.R.; Skolnik, J.; Owen, K.; Ohd, J.; Kim, Y.H. 607 Anti-pruritic properties of remetinostat (SHAPE), a topical histone deacetylase inhibitor (HDACi); data from a randomized phase 2 study in patients with stage IA-IIA mycosis fungoides. J. Investig. Dermatol. 2018, 138, S103. [Google Scholar] [CrossRef]
- Vandecasteele, S.J.; Boelaert, J.R.; De Vriese, A.S. Staphylococcus aureus Infections in Hemodialysis: What a Nephrologist Should Know. Clin. J. Am. Soc. Nephrol. 2009, 4, 1388. [Google Scholar] [CrossRef]
- Piraino, B. Staphylococcus aureus infections in dialysis patients: Focus on prevention. ASAIO J. 2000, 46, S13–S17. [Google Scholar] [CrossRef]
- McLaughlin, J.; Watterson, S.; Layton, A.M.; Bjourson, A.J.; Barnard, E.; McDowell, A. Propionibacterium acnes and Acne Vulgaris: New Insights from the Integration of Population Genetic, Multi-Omic, Biochemical and Host-Microbe Studies. Microorganisms 2019, 7, 128. [Google Scholar] [CrossRef]
- Barnard, E.; Shi, B.; Kang, D.; Craft, N.; Li, H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci. Rep. 2016, 6, 39491. [Google Scholar] [CrossRef]
- Galperin, T.A.; Cronin, A.J.; Leslie, K.S. Cutaneous manifestations of ESRD. Clin. J. Am. Soc. Nephrol. 2014, 9, 201–218. [Google Scholar] [CrossRef]
- Kuypers, D.R. Skin problems in chronic kidney disease. Nat. Rev. Nephrol. 2009, 5, 157. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Kalantar-Zadeh, K.; Vaziri, N.D. The Gut as a Source of Inflammation in Chronic Kidney Disease. Nephron 2015, 130, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Gannesen, A.V.; Borrel, V.; Lefeuvre, L.; Netrusov, A.I.; Plakunov, V.K.; Feuilloley, M.G.J. Effect of two cosmetic compounds on the growth, biofilm formation activity, and surface properties of acneic strains of Cutibacterium acnes and Staphylococcus aureus. Microbiologyopen 2019, 8, e00659. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Serizawa, S.; Ito, M.; Sato, Y. Effect of aging on sebaceous gland activity and on the fatty acid composition of wax esters. J. Investig. Derm. 1987, 89. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keshari, S.; Wang, Y.; Herr, D.R.; Wang, S.-M.; Yang, W.-C.; Chuang, T.-H.; Chen, C.-L.; Huang, C.-M. Skin Cutibacterium acnes Mediates Fermentation to Suppress the Calcium Phosphate-Induced Itching: A Butyric Acid Derivative with Potential for Uremic Pruritus. J. Clin. Med. 2020, 9, 312. https://doi.org/10.3390/jcm9020312
Keshari S, Wang Y, Herr DR, Wang S-M, Yang W-C, Chuang T-H, Chen C-L, Huang C-M. Skin Cutibacterium acnes Mediates Fermentation to Suppress the Calcium Phosphate-Induced Itching: A Butyric Acid Derivative with Potential for Uremic Pruritus. Journal of Clinical Medicine. 2020; 9(2):312. https://doi.org/10.3390/jcm9020312
Chicago/Turabian StyleKeshari, Sunita, Yanhan Wang, Deron Raymond Herr, Sung-Min Wang, Wu-Chang Yang, Tsung-Hsien Chuang, Chien-Lung Chen, and Chun-Ming Huang. 2020. "Skin Cutibacterium acnes Mediates Fermentation to Suppress the Calcium Phosphate-Induced Itching: A Butyric Acid Derivative with Potential for Uremic Pruritus" Journal of Clinical Medicine 9, no. 2: 312. https://doi.org/10.3390/jcm9020312
APA StyleKeshari, S., Wang, Y., Herr, D. R., Wang, S.-M., Yang, W.-C., Chuang, T.-H., Chen, C.-L., & Huang, C.-M. (2020). Skin Cutibacterium acnes Mediates Fermentation to Suppress the Calcium Phosphate-Induced Itching: A Butyric Acid Derivative with Potential for Uremic Pruritus. Journal of Clinical Medicine, 9(2), 312. https://doi.org/10.3390/jcm9020312