Increased Diagnostic Accuracy of Adnexal Tumors with A Combination of Established Algorithms and Biomarkers
Abstract
1. Introduction
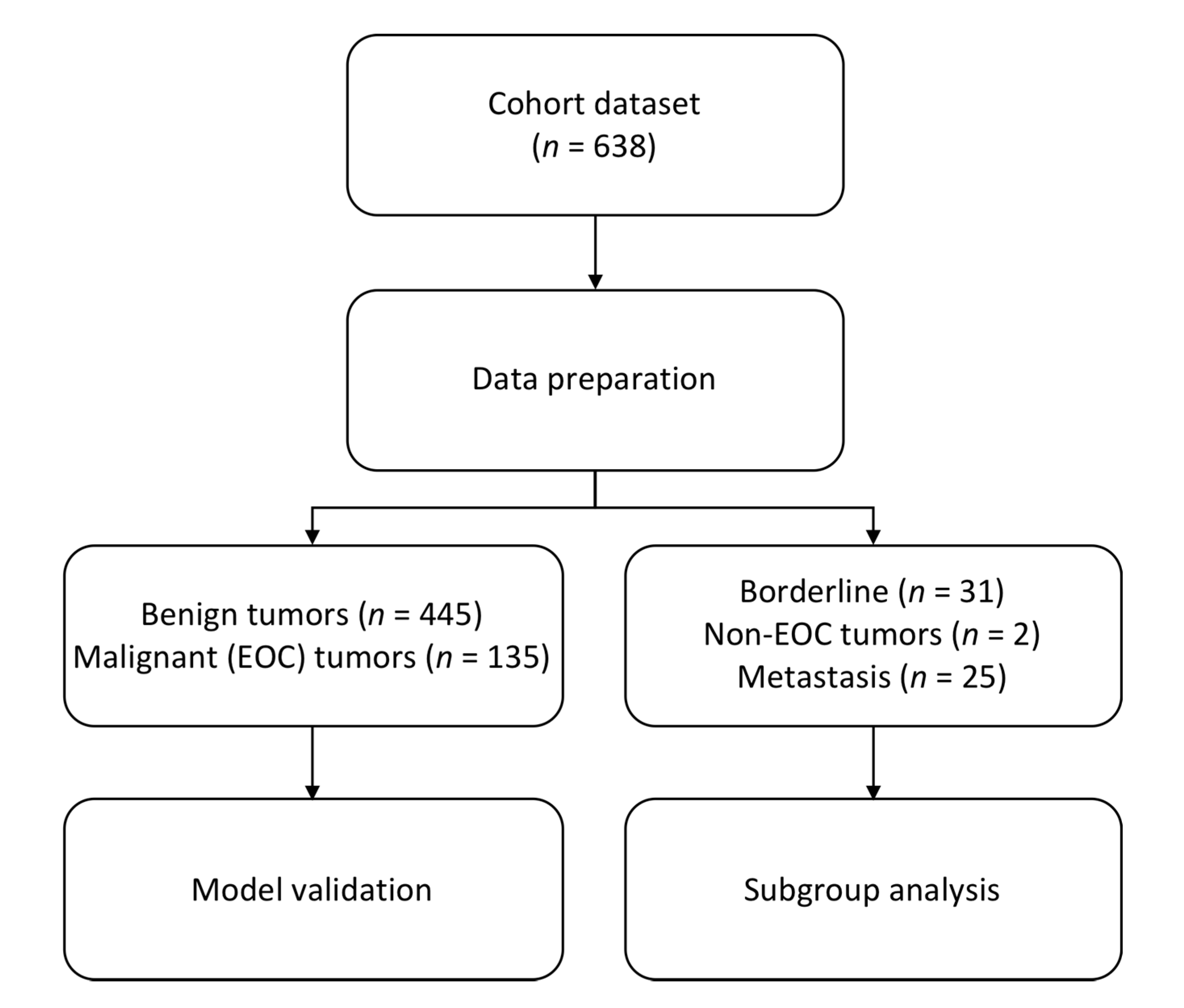
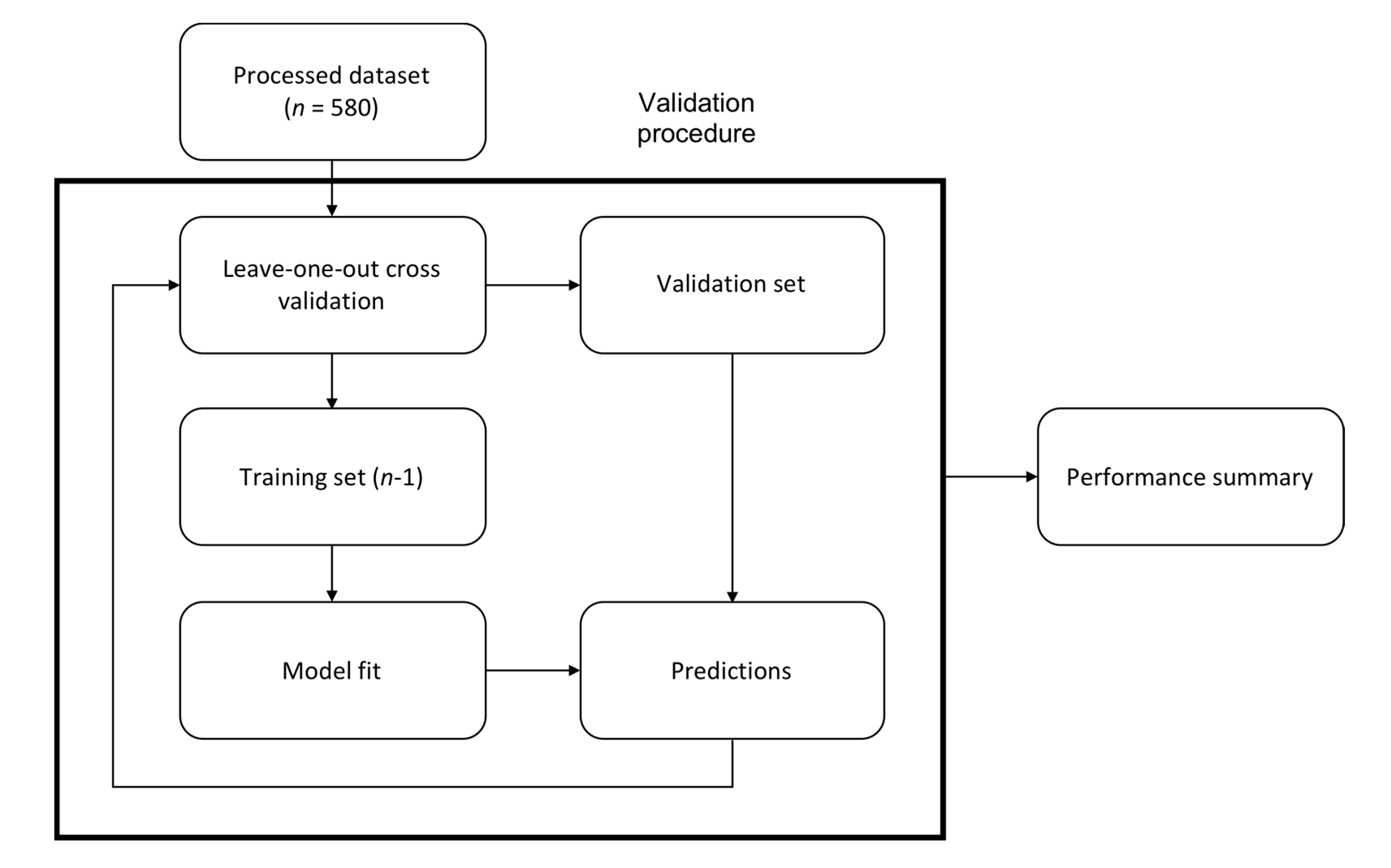
2. Materials and Methods
3. Results
3.1. Model Performance—Gothenburg Index (GOT)
3.1.1. Risk of Malignancy Index with the Addition of HE4—GOT-1
3.1.2. CA125 with the Addition of HE4—GOT-2
3.1.3. Risk of Ovarian Malignancy Algorithm with Addition of Transvaginal Ultrasound—GOT-3
3.2. Subgroup Analyses for GOT-1 and GOT-2 Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lavoue, V.; Huchon, C.; Akladios, C.; Alfonsi, P.; Bakrin, N.; Ballester, M.; Bendifallah, S.; Bolze, P.; Bonnet, F.; Bourgin, C.; et al. Management of epithelial cancer of the ovary, fallopian tube, and primary peritoneum. Long text of the Joint French Clinical Practice Guidelines issued by FRANCOGYN, CNGOF, SFOG, and GINECO-ARCAGY, and endorsed by INCa. Part 1: Diagnostic exploration and staging, surgery, perioperative care, and pathology. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 369–378. [Google Scholar] [PubMed]
- Lycke, M.; Kristjansdottir, B.; Sundfeldt, K. A multicenter clinical trial validating the performance of HE4, CA125, risk of ovarian malignancy algorithm and risk of malignancy index. Gynecol. Oncol. 2018, 151, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Partheen, K.; Kristjansdottir, B.; Sundfeldt, K. Evaluation of ovarian cancer biomarkers HE4 and CA-125 in women presenting with a suspicious cystic ovarian mass. J. Gynecol. Oncol. 2011, 22, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.W.; Pavlik, E.J.; Long, A.; Miller, R.W.; DeSimone, C.P.; Hoff, J.T.; Ueland, W.R.; Kryscio, R.J.; van Nagell, J.R.; Ueland, F.R. Serial ultrasonographic evaluation of ovarian abnormalities with a morphology index. Gynecol. Oncol. 2014, 135, 8–12. [Google Scholar] [CrossRef]
- du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the arbeitsgemeinschaft gynaekologische onkologie studiengruppe ovarialkarzinom (ago-ovar) and the groupe d’investigateurs nationaux pour les etudes des cancers de l’ovaire (gineco). Cancer 2009, 115, 1234–1244. [Google Scholar]
- Dahm-Kähler, P.; Palmqvist, C.; Staf, C.; Holmberg, E.; Johannesson, L. Centralized primary care of advanced ovarian cancer improves complete cytoreduction and survival—A population-based cohort study. Gynecol. Oncol. 2016, 142, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Aletti, G.D.; Cliby, W.A. Time for centralizing patients with ovarian cancer: What are we waiting for? Gynecol. Oncol. 2016, 142, 209–210. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Jacobs, I.; Oram, D.; Fairbanks, J.; Turner, J.; Frost, C.; Grudzinskas, J.G. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. BJOG Int. J. Obstet. Gynaecol. 1990, 97, 922–929. [Google Scholar] [CrossRef]
- Akker, P.A.V.D.; Aalders, A.L.; Snijders, M.P.; Kluivers, K.B.; Samlal, R.A.; Vollebergh, J.H.; Massuger, L.F. Evaluation of the risk of malignancy index in daily clinical management of adnexal masses. Gynecol. Oncol. 2010, 116, 384–388. [Google Scholar] [CrossRef]
- Hellström, I.; Raycraft, J.; Hayden-Ledbetter, M.; Ledbetter, J.A.; Schummer, M.; McIntosh, M.; Drescher, C.; Urban, N.; Hellström, K.E. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003, 63, 3695–3700. [Google Scholar]
- Drapkin, R.; Pathak, A.P.; Artemov, D.; Ward, B.D.; Jackson, D.G.; Neeman, M.; Bhujwalla, Z.M. Human Epididymis Protein 4 (HE4) Is a Secreted Glycoprotein that Is Overexpressed by Serous and Endometrioid Ovarian Carcinomas. Cancer Res. 2005, 65, 2162–2169. [Google Scholar] [CrossRef]
- Galgano, M.T.; Hampton, G.M.; Frierson, H.F.; Jr, H.F.F. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod. Pathol. 2006, 19, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, J.; Yao, H.; Wu, Z.; Wang, M.; Qi, J. Diagnostic accuracy of serum HE4, CA125 and ROMA in patients with ovarian cancer: A meta-analysis. Tumor Biol. 2014, 35, 6127–6138. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, S.; Schiumarini, D.; Panteghini, M. Human epididymis protein 4: Factors of variation. Clin. Chim. Acta 2015, 438, 171–177. [Google Scholar] [CrossRef]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C., Jr.; Skates, S.J. A novel multiple marker bioassay utilizing he4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009, 112, 40–46. [Google Scholar] [CrossRef]
- Menon, U.; Griffin, M.; Gentry-Maharaj, A. Ovarian cancer screening-current status, future directions. Gynecol. Oncol. 2014, 132, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Buamah, P. Benign conditions associated with raised serum CA-125 concentration. J. Surg. Oncol. 2000, 75, 264–265. [Google Scholar] [CrossRef]
- Tingulstad, S.; Hagen, B.; Skjeldestad, F.E.; Halvorsen, T.; Nustad, K.; Onsrud, M. The risk-of-malignancy index to evaluate potential ovarian cancers in local hospitals. Obstet. Gynecol. 1999, 93, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; van Calster, B.; Testa, A.; Savelli, L.; Fischerova, D.; Froyman, W.; Wynants, L.; van Holsbeke, C.; Epstein, E.; Franchi, D.; et al. Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am. J. Obstet. Gynecol. 2016, 214, 424–437. [Google Scholar] [CrossRef]
- Wynants, L.; Timmerman, D.; Verbakel, J.Y.; Testa, A.; Savelli, L.; Fischerova, D.; Franchi, D.; van Holsbeke, C.; Epstein, E.; Froyman, W.; et al. Clinical Utility of Risk Models to Refer Patients with Adnexal Masses to Specialized Oncology Care: Multicenter External Validation Using Decision Curve Analysis. Clin. Cancer Res. 2017, 23, 5082–5090. [Google Scholar] [CrossRef] [PubMed]
- Manegold-Brauer, G.; Buechel, J.; Knipprath-Mészaros, A.; Schoetzau, A.; Hacker, N.F.; Tercanli, S.; Lapaire, O.; Heinzelmann-Schwarz, V. Improved Detection Rate of Ovarian Cancer Using a 2-Step Triage Model of the Risk of Malignancy Index and Expert Sonography in an Outpatient Screening Setting. Int. J. Gynecol. Cancer 2016, 26, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Kristjansdottir, B.; Levan, K.; Partheen, K.; Sundfeldt, K. Diagnostic performance of the biomarkers HE4 and CA125 in type I and type II epithelial ovarian cancer. Gynecol. Oncol. 2013, 131, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Enroth, S.; Berggrund, M.; Lycke, M.; Lundberg, M.; Assarsson, E.; Olovsson, M.; Stålberg, K.; Sundfeldt, K.; Gyllensten, U. A two-step strategy for identification of plasma protein biomarkers for endometrial and ovarian cancer. Clin. Proteomics 2018, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Enroth, S.; Berggrund, M.; Lycke, M.; Broberg, J.; Lundberg, M.; Assarsson, E.; Olovsson, M.; Stålberg, K.; Sundfeldt, K.; Gyllensten, U. High throughput proteomics identifies a high-accuracy 11 plasma protein biomarker signature for ovarian cancer. Commun. Biol. 2019, 2, 221. [Google Scholar] [CrossRef] [PubMed]
- Chudecka-Głaz, A.; Cymbaluk-Płoska, A.; Jastrzębska, J.; Menkiszak, J. Can ROMA algorithm stratify ovarian tumor patients better when being based on specific age ranges instead of the premenopausal and postmenopausal status? Tumor Biol. 2016, 37, 8879–8887. [Google Scholar] [CrossRef]
- Coleman, R.L.; Herzog, T.J.; Chan, D.W.; Munroe, D.G.; Pappas, T.C.; Smith, A.; Zhang, Z.; Wolf, J. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am. J. Obstet. Gynecol. 2016, 215, 82.e1–82.e11. [Google Scholar] [CrossRef]
- Karlsen, M.A.; Høgdall, E.V.; Christensen, I.J.; Borgfeldt, C.; Kalapotharakos, G.; Zdrazilova-Dubska, L.; Chovanec, J.; Lok, C.A.; Stiekema, A.; Mutz-Dehbalaie, I.; et al. A novel diagnostic index combining HE4, CA125 and age may improve triage of women with suspected ovarian cancer—An international multicenter study in women with an ovarian mass. Gynecol. Oncol. 2015, 138, 640–646. [Google Scholar] [CrossRef]
- Kristjansdottir, B.; Levan, K.; Partheen, K.; Carlsohn, E.; Sundfeldt, K. Potential tumor biomarkers identified in ovarian cyst fluid by quantitative proteomic analysis, iTRAQ. Clin. Proteomics 2013, 10, 4. [Google Scholar] [CrossRef]
- Meys, E.M.J.; Kaijser, J.; Kruitwagen, R.F.P.M.; Slangen, B.F.M.; van Calster, B.; Aertgeerts, B.; Verbakel, J.Y.; Timmerman, D.; van Gorp, T. Subjective assessment versus ultrasound models to diagnose ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer 2016, 58, 17–29. [Google Scholar] [CrossRef]
- Bristow, R.E.; Smith, A.; Zhang, Z.; Chan, D.W.; Crutcher, G.; Fung, E.T.; Munroe, D.G. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol. Oncol. 2013, 128, 252–259. [Google Scholar] [CrossRef]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J. Ovarian Res. 2019, 12, 28. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.-M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Elecsys he4-Human Epididymal Protein 4. 2020. Available online: http://www.diagnostics.roche.com (accessed on 17 December 2019).
- Lachenbruch, P.A.; Mickey, M.R. Estimation of error rates in discriminant analysis. Technometrics 1968, 10, 1–11. [Google Scholar] [CrossRef]
- Moore, R.G.; Brown, A.K.; Miller, M.C.; Skates, S.; Allard, W.J.; Verch, T.; Steinhoff, M.; Messerlian, G.; DiSilvestro, P.; Granai, C.; et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol. Oncol. 2008, 108, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Blackman, A.; Miller, M.C.; Robison, K.; DiSilvestro, P.A.; Eklund, E.E.; Strongin, R.; Messerlian, G. Multiple biomarker algorithms to predict epithelial ovarian cancer in women with a pelvic mass: Can additional makers improve performance? Gynecol. Oncol. 2019, 154, 150–155. [Google Scholar] [CrossRef]
- Yanaranop, M.; Tiyayon, J.; Siricharoenthai, S.; Nakrangsee, S.; Thinkhamrop, B. Rajavithi-ovarian cancer predictive score (R-OPS): A new scoring system for predicting ovarian malignancy in women presenting with a pelvic mass. Gynecol. Oncol. 2016, 141, 479–484. [Google Scholar] [CrossRef]
- Ueland, F.R.; DeSimone, C.P.; Seamon, L.G.; Miller, R.A.; Goodrich, S.; Podzielinski, I.; Sokoll, L.; Smith, A.; van Nagell, J.R.; Zhang, Z. Effectiveness of a Multivariate Index Assay in the Preoperative Assessment of Ovarian Tumors. Obstet. Gynecol. 2011, 117, 1289–1297. [Google Scholar] [CrossRef]
- Muka, T.; Oliver-Williams, C.; Kunutsor, S.; Laven, J.S.E.; Fauser, B.C.J.M.; Chowdhury, R.; Kavousi, M.; Franco, O.H. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol. 2016, 1, 767–776. [Google Scholar] [CrossRef]
- Al Musalhi, K.; Al Kindi, M.; Al Aisary, F.; Ramadhan, F.; Al Rawahi, T.; Al Hatali, K.; Mula-Abed, W.-A. Evaluation of HE4, CA-125, Risk of Ovarian Malignancy Algorithm (ROMA) and Risk of Malignancy Index (RMI) in the Preoperative Assessment of Patients with Adnexal Mass. Oman Med. J. 2016, 31, 336–344. [Google Scholar] [CrossRef]
- Dochez, V.; Randet, M.; Renaudeau, C.; Dimet, J.; Le Thuaut, A.; Winer, N.; Thubert, T.; Vaucel, E.; Caillon, H.; Ducarme, G. Efficacy of HE4, CA125, Risk of Malignancy Index and Risk of Ovarian Malignancy Index to Detect Ovarian Cancer in Women with Presumed Benign Ovarian Tumours: A Prospective, Multicentre Trial. J. Clin. Med. 2019, 8, 1784. [Google Scholar] [CrossRef] [PubMed]
- Bolstad, N.; Oijordsbakken, M.; Nustad, K.; Bjerner, J. Human epididymis protein 4 reference limits and natural variation in a nordic reference population. Tumor Biol. 2012, 33, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Jabre-Raughley, M.; Brown, A.K.; Robison, K.M.; Miller, M.C.; Allard, W.J.; Kurman, R.J.; Bast, R.C.; Skates, S.J. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am. J. Obstet. Gynecol. 2010, 203, 228.e1–228.e6. [Google Scholar] [CrossRef] [PubMed]
| Pre-M n | Post-M n | All n (%) | ||
|---|---|---|---|---|
| Benign | ||||
| age (mean) | 38.76 | 63.6 | 50.76 | |
| Histology n | ||||
| Serous | 21 | 76 | 97 (21.8) | |
| Mucinous | 26 | 33 | 59 (13.3) | |
| Endometrioma | 53 | 7 | 60 (13.5) | |
| Simple | 64 | 52 | 116 (26.1) | |
| Stromal | 3 | 15 | 18 (4.0) | |
| Inflammation | 6 | 8 | 14 (3.1) | |
| Teratoma | 47 | 11 | 58 (13.0) | |
| Myoma | 10 | 13 | 23 (5.2) | |
| Total n (%) | 230 | 214 | 445 (69.8) | |
| Borderline | ||||
| age (mean) | 38.2 | 63.86 | 55.58 | |
| Histology n | Serous | 5 | 13 | 18 (58.1) |
| Mucinous | 5 | 7 | 12 (38.7) | |
| Stromal | 1 | 1 (3.2) | ||
| Total n (%) | 10 | 21 | 31 (4.9) | |
| Malignant | ||||
| age (mean) | 44.26 | 66.46 | 62.67 | |
| Histology n | EOC | |||
| Serous | 12 | 85 | 97 (71.9) | |
| Mucinous | 4 | 8 | 12 (8.9) | |
| Endometrioid | 4 | 11 | 15 (11.1) | |
| Clearcell | 3 | 2 | 5 (3.7) | |
| Carcinosarcoma | 3 | 3 (2.2) | ||
| Undifferentiated | 2 | 2 (1.5) | ||
| Total n (%) | 23 | 112 | 135 (21.2) | |
| Non-epithelial OC | 1 | 1 | 2 (0.3) | |
| Metastasis | 7 | 18 | 25 (3.9) | |
| Type I/II | I | 9 | 27 | 36 |
| II | 14 | 85 | 99 | |
| Total n (%) | 23 | 112 | 135 | |
| FIGO | I | 6 | 30 | 36 |
| II | 3 | 13 | 16 | |
| III | 12 | 56 | 68 | |
| IV | 2 | 13 | 15 | |
| Total n (%) | 23 | 112 | 135 (21.2) | |
| Group (n) | Model | p-Value | ROC | SN % (75% SP) | SP % (Target SN) | |
|---|---|---|---|---|---|---|
| AUC | 95% CI | |||||
| Benign (445) vs. EOC (135) | RMI3 (cut-off > 200) | <0.001 | 0.95 | 0.93–0.97 | 97 | 84 |
| GOT 1 (RMI + HE4) | 0.95 | 0.93–0.98 | 96 | 86 | ||
| Benign (445) vs. EOC (135) | CA125 (cut-off > 35 U/mL) | <0.001 | 0.92 | 0.89–0.94 | 88 | 68 |
| GOT 2 (CA125 + HE4) | 0.94 | 0.92–0.97 | 93 | 79 | ||
| Benign (230) vs. EOC (23) Pre-M | ROMA (cut-off ≥11.4%) | <0.001 | 0.93 | 0.86–1.00 | 87 | 81 |
| GOT 3 (ROMA + TVU) | 0.94 | 0.87–1.00 | 91 | 88 | ||
| Benign (215) vs. EOC (112) Post-M | ROMA (cut-off ≥ 29.9%) | <0.001 | 0.94 | 0.91–0.96 | 93 | 77 |
| GOT 3 (ROMA + TVU) | 0.94 | 0.91–0.96 | 94 | 80 | ||
| Group (n) | Model | FIGO I + II | FIGO III + IV | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ROC | SN% (75% SP) | SP% (Target SN) | ROC | SN% (75% SP) | SP% (Target SN) | ||||
| AUC | 95% CI | AUC | 95% CI | ||||||
| Benign (445) vs. EOC FIGO I + II (52)/FIGO III + IV (83) | RMI (cut-off < 200) | 0.90 | 0.85–0.94 | 94 | 84 | 0.98 | 0.97-0.99 | 99 | 84 |
| GOT-1 (RMI + HE4) | 0.90 | 0.86–0.95 | 92 | 86 | 0.98 | 0.97-1.00 | 99 | 90 | |
| Benign (445) vs. EOC FIGO I + II (52)/FIGO III + IV (83) | CA125 (cut-off > 35 U/mL) | 0.84 | 0.79–0.90 | 75 | 68 | 0.96 | 0.94-0.98 | 96 | 68 |
| GOT-2 (CA125 + HE4) | 0.88 | 0.82–0.93 | 85 | 74 | 0.98 | 0.96-1.00 | 99 | 81 | |
| Group (n) | Model | Type I | Type II | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ROC | SN% (75% SP) | SP% (Target SN) | ROC | SN% (75% SP) | SP% (Target SN) | ||||
| AUC | 95% CI | AUC | 95% CI | ||||||
| Benign (445) vs. Type I (36)/Type II (99) | RMI (cut-off <200) | 0.89 | 0.84–0.94 | 94 | 84 | 0.97 | 0.95–0.98 | 98 | 84 |
| GOT-1 (RMI + HE4) | 0.90 | 0.84–0.95 | 94 | 85 | 0.97 | 0.95–0.99 | 97 | 89 | |
| Benign (445) vs. Type I (36)/Type II (99) | CA125 (cut-off >35 U/mL) | 0.85 | 0.79–0.91 | 75 | 68 | 0.94 | 0.91–0.97 | 93 | 68 |
| GOT-2 (CA125 + HE4) | 0.87 | 0.82–0.93 | 83 | 80 | 0.96 | 0.94–0.99 | 96 | 71 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lycke, M.; Ulfenborg, B.; Kristjansdottir, B.; Sundfeldt, K. Increased Diagnostic Accuracy of Adnexal Tumors with A Combination of Established Algorithms and Biomarkers. J. Clin. Med. 2020, 9, 299. https://doi.org/10.3390/jcm9020299
Lycke M, Ulfenborg B, Kristjansdottir B, Sundfeldt K. Increased Diagnostic Accuracy of Adnexal Tumors with A Combination of Established Algorithms and Biomarkers. Journal of Clinical Medicine. 2020; 9(2):299. https://doi.org/10.3390/jcm9020299
Chicago/Turabian StyleLycke, Maria, Benjamin Ulfenborg, Björg Kristjansdottir, and Karin Sundfeldt. 2020. "Increased Diagnostic Accuracy of Adnexal Tumors with A Combination of Established Algorithms and Biomarkers" Journal of Clinical Medicine 9, no. 2: 299. https://doi.org/10.3390/jcm9020299
APA StyleLycke, M., Ulfenborg, B., Kristjansdottir, B., & Sundfeldt, K. (2020). Increased Diagnostic Accuracy of Adnexal Tumors with A Combination of Established Algorithms and Biomarkers. Journal of Clinical Medicine, 9(2), 299. https://doi.org/10.3390/jcm9020299




