Effects of Prone Ventilation on Oxygenation, Inflammation, and Lung Infiltrates in COVID-19 Related Acute Respiratory Distress Syndrome: A Retrospective Cohort Study
Abstract
1. Introduction
2. Experimental Section
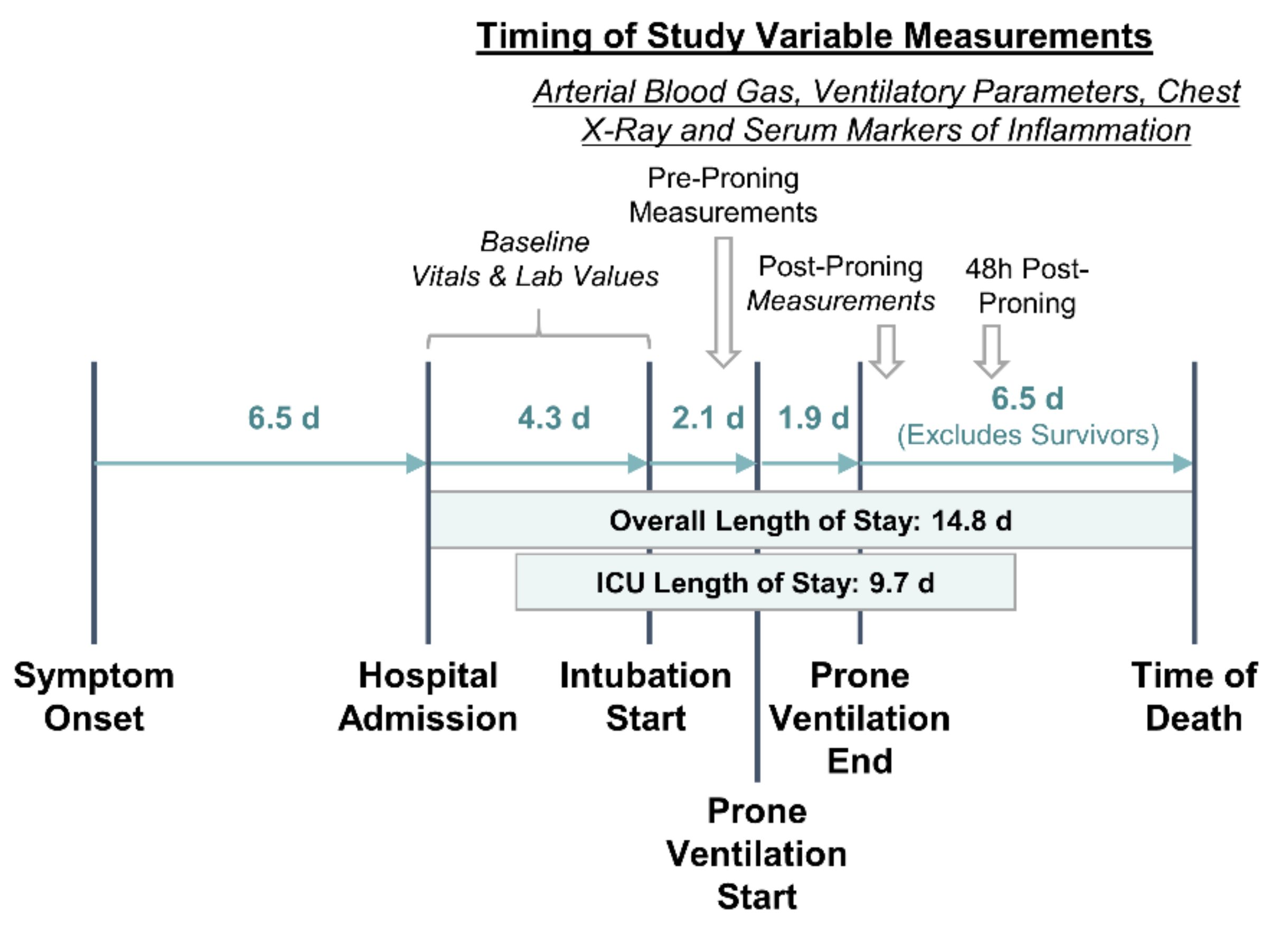
2.1. Study Design and Patient Selection
2.2. Outcomes
2.3. Data Collection and Definitions
2.4. Radiograph Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Cohort Identification and Grouping by Living Status
3.2. Patient Characteristics before Intubation and Prone Positioning
3.3. Patient Response to Prone Ventilation
3.4. Patients with Sustained Improvement in PaO2/FiO2
3.5. Evaluation of Lung Infiltrates on Chest Radiographs
3.6. Pre-Proning Characteristics of Patients with Sustained Improvement in PaO2/FiO2
3.7. Proning Effects on Serological Markers of Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Lung Segment | Pre-Proning | Post-Proning | 48 h Post-Proning | p-Value (Post–Pre) | p-Value (48 h Post–Pre) |
|---|---|---|---|---|---|
| All Cases | |||||
| All Lung Segments | 6.1 | 6.5 | 6.0 | 0.49 | 0.77 |
| Lower Lung Zones | 2.7 | 2.8 | 2.4 | 0.52 | 0.39 |
| Middle Lung Zones | 2.1 | 2.4 | 2.3 | 0.22 | 0.62 |
| Upper Lung Zones | 1.3 | 1.3 | 1.4 | 0.91 | 0.66 |
| Living | |||||
| All Lung Segments | 7.3 | 6.8 | 6.0 | 0.50 | 0.28 |
| Lower Lung Zones | 3.5 | 2.8 | 2.5 | 0.33 | 0.06 |
| Middle Lung Zones | 2.5 | 2.3 | 2.3 | 0.79 | 0.56 |
| Upper Lung Zones | 1.3 | 1.7 | 1.2 | 0.16 | 0.79 |
| Deceased | |||||
| All Lung Segments | 5.6 | 6.0 | 6.4 | 0.16 | 0.61 |
| Lower Lung Zones | 2.3 | 2.8 | 2.4 | 0.07 | 0.71 |
| Middle Lung Zones | 2.0 | 2.4 | 2.2 | 0.08 | 0.45 |
| Upper Lung Zones | 1.3 | 1.2 | 1.4 | 0.71 | 0.71 |
Appendix B
| Characteristic | Same or Decline (n = 12) | 48 h Improvement (n = 11) | Total (n = 23) |
|---|---|---|---|
| Demographics | |||
| Age, Median (Range) | 58.5 (31, 65) | 55 (25, 75) | 57 (25, 75) |
| Sex, Female (%) | 3 (25) | 5 (45) | 8 (34.8) |
| BMI, Median (Range), kg/m2 | 30.9 (22.4, 45.0) | 30.8 (22.5, 39.8) | 30.8 (22.4, 45.0) |
| Race/Ethnicity, Count (% Distribution) | - | - | - |
| African American | 7 (58.3) | 5 (45.5) | 12 (52.2) |
| Hispanic | 4 (33.3) | 5 (45.5) | 9 (39.1) |
| American Indian | 0 | 1 (9.1) | 1 (4.3) |
| Asian | 1 (8.3) | 0 | 1 (4.3) |
| Symptoms and Comorbidities at Admission | |||
| Symptoms (%) | - | - | - |
| Dyspnea | 10 (83.3) | 11 (100) | 21 (91.3) |
| Fever | 10 (83.3) | 8 (72.7) | 18 (78.3) |
| Cough | 9 (75.0) | 6 (54.5) | 15 (65.2) |
| Weakness | 12 (100) | 10 (90.9) | 22 (95.7) |
| Diarrhea | 2 (16.7) | 0 | 2 (8.7) |
| † Comorbidities (%) | - | - | - |
| Diabetes Mellitus | 4 (33.3) | 5 (45.5) | 9 (39.1%) |
| Hypertension | 4 (33.3) | 7 (63.6) | 11 (47.8%) |
| Congestive Heart Failure | 0 | 1 (9.1) | 1 (4.3%) |
| Clinical Values before Intubation, Median (Range) | |||
| Vitals | - | - | - |
| Blood Pressure—Systolic (mmHg) | 125 (109, 167) | 124 (110, 153) | 124 (109, 167) |
| Blood Pressure—Diastolic (mmHg) | 75.5 (57, 89) | 68 (49, 93) | 72 (49, 93) |
| Heart Rate (Beats/min) | 97 (66, 121) | 105 (53, 125) | 102 (53, 125) |
| Respiratory Rate (Breaths/min) | 26 (20, 35) | 26 (21, 34) | 26 (20, 35) |
| Temperature (°F) | 99.8 (97, 102.6) | 99.2 (97.8, 101.4) | 99.7 (97, 102.6) |
| O2% Saturation | 93 (87, 98) | 92 (87, 100) | 92 (87, 100) |
| Lab Values | - | - | - |
| Glucose (mg/dL) | 136 (103, 354) | 136 (101, 196) | 136 (101, 354) |
| Sodium (mEQ/L) | 142 (135, 158) | 143 (138, 151) | 142 (135, 158) |
| Blood Urea Nitrogen (mg/dL) | 30.5 (13, 104) | 20 (13, 60) | 28 (13, 104) |
| * Creatinine (IU/L) | 1.32 (0.72, 4.68) | 0.79 (0.44, 2.7) | 0.97 (0.44, 4.68) |
| Lactate (mg/dL) | 1.9 (0.7, 6.7) | 1.8 (0.7, 2.6) | 1.8 (0.7, 6.7) |
| Troponin (ng/mL) | 0.091 (0.015, 0.71) | 0.081 (0.015, 0.289) | 0.081 (0.015, 0.71) |
| LDH (U/L) | 991 (538, 1757) | 733 (535, 1875) | 958 (535, 1875) |
| * AST (IU/L) | 84 (54, 342) | 55 (24, 144) | 73 (24, 342) |
| ALT (IU/L) | 78 (25, 163) | 44 (12, 111) | 72 (12, 163) |
| Alkaline Phosphatase (IU/L) | 106 (51, 201) | 125 (77, 294) | 117 (51, 294) |
| Procalcitonin (ng/mL) | 2.51 (0.16, 200) | 0.71 (0.41, 2.6) | 0.92 (0.16, 200) |
| C-Reactive Protein (mg/L) | 19.4 (1.25, 32.6) | 8.65 (0.34, 34) | 11.1 (0.34, 34) |
| D-Dimer (ng/mL) | 10.85 (0.96, 35.78) | 24.08 (1.8, 35.78) | 13.82 (0.96, 35.78) |
| Fibrinogen (mg/dL) | 513 (165, 679) | 269 (100, 653) | 483 (100, 679) |
| * Ferritin (μg/L) | 1863 (572, 5509) | 992 (298, 2198) | 1285 (298, 5509) |
| Total Bilirubin (mg/dL) | 0.5 (0.2, 1.8) | 0.7 (0.3, 0.9) | 0.5 (0.2, 1.8) |
| Sedimentation Rate (mm/h) | 42.5 (7, 107) | 17 (5, 100) | 39 (5, 107) |
| White Blood Cell Count (1000/mm3) | 11.25 (6.1, 21) | 17.1 (7, 31.7) | 13.3 (6.1, 31.7) |
| Lymphocyte Percent | 5 (2, 11) | 4 (3, 15) | 5 (2, 15) |
| ABG and Ventilatory Parameters, Supine, Median (Range) | |||
| PAO2 (mmHg) | 79 (48, 190) | 66 (35, 152) | 73 (35, 190) |
| FiO2 (%) | 100 (50, 100) | 100 (80, 100) | 100 (50, 100) |
| P/F Ratio (mmHg) | 79 (48, 190) | 66 (35, 152) | 76 (35, 190) |
| PEEP (cm H2O) | 15 (10, 20) | 15 (10, 20) | 15 (10, 20) |
| Respiratory Rate | 23 (16, 30) | 24 (20, 26) | 24 (16, 30) |
| Tidal Volume/IBW (mL/kg) | 450 (380, 500) | 450 (380, 500) | 450 (380, 500) |
| Pronation Timeline and Length of Stay (In Days) | |||
| Time to Intubation, Median (Range) | - | - | - |
| * From Admission | 3 (0, 5) | 5 (3, 15) | 3 (0, 15) |
| From Symptom Onset | 7 (3, 14) | 7 (2, 14) | 7 (2, 14) |
| Time to Pronation, Median (Range) | - | - | - |
| * From Admission | 4 (2, 10) | 8 (3, 18) | 5 (2, 18) |
| From 1st Symptom Appearance | 11 (7, 17) | 13 (8, 25) | 12 (7, 25) |
| From Intubation | 2 (0, 7) | 1 (0, 13) | 1 (0, 13) |
| Days Proned | 1 (1, 6) | 2 (1, 3) | 1 (1, 6) |
| Time to Death After Pronation | 3 (25.0) | 3 (27.3) | 3 (26.1) |
Appendix C. Prone Ventilation Protocol
References
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensiv. Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef]
- Marini, J.J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329–2330. [Google Scholar] [CrossRef]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- McGuinness, G.; Zhan, C.; Rosenberg, N.; Azour, L.; Wickstrom, M.; Mason, D.M.; Thomas, K.M.; Moore, W.H. High Incidence of Barotrauma in Patients with COVID-19 Infection on Invasive Mechanical Ventilation. Radiology 2020, 202352. [Google Scholar] [CrossRef]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.; Fan, E.; Baudouin, S.V. New UK guidelines for the management of adult patients with ARDS. Thorax 2019, 74, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Lai-Fook, S.J.; Rodarte, J.R. Pleural pressure distribution and its relationship to lung volume and interstitial pressure. J. Appl. Physiol. 1991, 70, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, E.; Mead, J. Statics of the respiratory system. In Handbook of Physiology; Macklem, P., Mead, J., Eds.; American Physiologic Society: Bethesda, MD, USA, 1986; p. 387. [Google Scholar]
- Malbouisson, L.M.; Busch, C.J.; Puybasset, L.; Lu, Q.; Cluzel, P.; Rouby, J.-J. Role of the Heart in the Loss of Aeration Characterizing Lower Lobes in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2000, 161, 2005–2012. [Google Scholar] [CrossRef]
- Wiener, C.M.; McKenna, W.J.; Myers, M.J.; Lavender, J.P.; Hughes, J.M.B. Left Lower Lobe Ventilation Is Reduced in Patients with Cardiomegaly in the Supine But Not the Prone Position. Am. Rev. Respir. Dis. 1990, 141, 150–155. [Google Scholar] [CrossRef]
- Nyrén, S.; Mure, M.; Jacobsson, H.; Larsson, S.A.; Lindahl, S.G. Pulmonary perfusion is more uniform in the prone than in the supine position: Scintigraphy in healthy humans. J. Appl. Physiol. 1999, 86, 1135–1141. [Google Scholar] [CrossRef]
- Guérin, C.; Reignier, J.; Richard, J.-C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Scholten, E.L.; Beitler, J.R.; Prisk, G.K.; Malhotra, A. Treatment of ARDS with Prone Positioning. Chest 2017, 151, 215–224. [Google Scholar] [CrossRef]
- Albert, R.K.; Keniston, A.; Baboi, L.; Ayzac, L.; Guérin, C.; Proseva Investigators. Prone Position-induced Improvement in Gas Exchange Does Not Predict Improved Survival in the Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2014, 189, 494–496. [Google Scholar] [CrossRef]
- Gattinoni, L.; Taccone, P.; Carlesso, E.; Marini, J.J. Prone Position in Acute Respiratory Distress Syndrome. Rationale, Indications, and Limits. Am. J. Respir. Crit. Care Med. 2013, 188, 1286–1293. [Google Scholar] [CrossRef]
- Aoyama, H.; Uchida, K.; Aoyama, K.; Pechlivanoglou, P.; Englesakis, M.; Yamada, Y.; Fan, E. Assessment of Therapeutic Interventions and Lung Protective Ventilation in Patients With Moderate to Severe Acute Respiratory Distress Syndrome: A Systematic Review and Network Meta-analysis. JAMA Netw. Open 2019, 2, e198116. [Google Scholar] [CrossRef] [PubMed]
- Beitler, J.R.; Shaefi, S.; Montesi, S.B.; Devlin, A.; Loring, S.H.; Talmor, D.; Malhotra, A. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: A meta-analysis. Intensiv. Care Med. 2014, 40, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, R.; Noble, D.W.; Sudlow, A. Prone position for acute respiratory failure in adults. Cochrane Database Syst. Rev. 2015, CD008095. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, H.J.; Yoo, K.H.; Park, Y.B.; Kim, S.W.; Lee, S.J.; Kim, E.K.; Kim, J.H.; Kim, Y.H.; Moon, J.-Y.; et al. The efficacy and safety of prone positioning in adults patients with acute respiratory distress syndrome: A meta-analysis of randomized controlled trials. J. Thorac. Dis. 2015, 7, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Ziehr, D.R.; Alladina, J.; Petri, C.R.; Maley, J.H.; Moskowitz, A.; Medoff, B.D.; Hibbert, K.A.; Thompson, B.T.; Hardin, C.C. Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study. Am. J. Respir. Crit. Care Med. 2020, 201, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Chen, L.; Lu, C.; Zhang, W.; Xia, J.-A.; Sklar, M.C.; Du, B.; Brochard, L.; Qiu, H. Lung Recruitability in COVID-19-associated Acute Respiratory Distress Syndrome: A Single-Center Observational Study. Am. J. Respir. Crit. Care Med. 2020, 201, 1294–1297. [Google Scholar] [CrossRef]
- Borghesi, A.; Maroldi, R. COVID-19 outbreak in Italy: Experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol. Med. 2020, 125, 509–513. [Google Scholar] [CrossRef]
- Wasilewski, P.G.; Mruk, B.; Mazur, S.; Półtorak-Szymczak, G.; Sklinda, K.; Walecki, J. COVID-19 severity scoring systems in radiological imaging—A review. Pol. J. Radiol. 2020, 85, e361. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Mittermaier, M.; Pickerodt, P.; Kurth, F.; De Jarcy, L.B.; Uhrig, A.; Garcia, C.; Machleidt, F.; Pergantis, P.; Weber, S.; Li, Y.; et al. Evaluation of PEEP and prone positioning in early COVID-19 ARDS. EClinicalMedicine 2020, 28, 100579. [Google Scholar] [CrossRef]
- Gattinoni, L.; Busana, M.; Giosa, L.; Macrì, M.M.; Quintel, M. Prone Positioning in Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pesenti, A.; Carlesso, E. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure: Impact and clinical fallout through the following 20 years. Intensiv. Care Med. 2013, 39, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Priolet, B.; Tempelhoff, G.; Millet, J.M.M.; Cannamela, A.; Carton, M.J.; Condamine, S.; Ducreux, J.C.; Driencourt, J.B. Ventilation assistée en décubitus ventral: Évaluation tomodensitométrique de son efficacité dans le traitement des condensations pulmonaires. Réanimation Urgences 1993, 2, 81–85. [Google Scholar] [CrossRef]
- Richter, T.; Bellani, G.; Scott Harris, R.; Vidal Melo, M.F.; Winkler, T.; Venegas, J.G.; Musch, G. Effect of Prone Position on Regional Shunt, Aeration, and Perfusion in Experimental Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2005, 172, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J.; Gattinoni, L. Time Course of Evolving Ventilator-Induced Lung Injury: The “Shrinking Baby Lung”. Crit. Care Med. 2020, 48, 1203–1209. [Google Scholar] [CrossRef]
- Gozes, O.; Frid-Adar, M.; Greenspan, H.; Browning, P.D.; Zhang, H.; Ji, W.; Bernheim, A.; Siegel, E. Rapid AI Development Cycle for the Coronavirus (COVID-19) Pandemic: Initial Results for Automated Detection & Patient Monitoring using Deep Learning CT Image Analysis. arXiv 2020, arXiv:2003.05037. [Google Scholar]
- Wang, S.; Kang, B.; Ma, J.; Zeng, X.; Xiao, M.; Guo, J.; Cai, M.; Yang, J.; Li, Y.; Meng, X.; et al. A deep learning algorithm using CT images to screen for Corona Virus Disease (COVID-19). medRxiv 2020. [Google Scholar] [CrossRef]
- Bae, J.; Kapse, S.; Singh, G.; Phatak, T.; Green, J.; Madan, N.; Prasanna, P. Predicting Mechanical Ventilation Requirement and Mortality in COVID-19 using Radiomics and Deep Learning on Chest Radiographs: A Multi-Institutional Study. arXiv 2020, arXiv:2007.08028, arXiv:2007.08028. [Google Scholar]
- Ouyang, X.; Huo, J.; Xia, L.; Shan, F.; Liu, J.; Mo, Z.; Yan, F.; Ding, Z.; Yang, Q.; Song, B.; et al. Dual-Sampling Attention Network for Diagnosis of COVID-19 From Community Acquired Pneumonia. IEEE Trans. Med. Imaging 2020, 39, 2595–2605. [Google Scholar] [CrossRef]
- Akatsuka, M.; Tatsumi, H.; Yama, N.; Masuda, Y. Therapeutic Evaluation of Computed Tomography Findings for Efficacy of Prone Ventilation in Acute Respiratory Distress Syndrome Patients with Abdominal Surgery. J. Crit. Care Med. 2020, 6, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Galiatsou, E.; Kostanti, E.; Svarna, E.; Kitsakos, A.; Koulouras, V.; Efremidis, S.C.; Nakos, G. Prone Position Augments Recruitment and Prevents Alveolar Overinflation in Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2006, 174, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Beitler, J.R.; Brochard, L.; Calfee, C.S.; Ferguson, N.D.; Slutsky, A.S.; Brodie, D. COVID-19-associated acute respiratory distress syndrome: Is a different approach to management warranted? Lancet Respir. Med. 2020, 8, 816–821. [Google Scholar] [CrossRef]
- Caputo, N.D.; Strayer, R.J.; Levitan, R. Early Self-Proning in Awake, Non-intubated Patients in the Emergency Department: A Single ED’s Experience during the COVID-19 Pandemic. Acad. Emerg. Med. 2020, 27, 375–378. [Google Scholar] [CrossRef]
- Elharrar, X.; Trigui, Y.; Dols, A.-M.; Touchon, F.; Martinez, S.; Prud’Homme, E.; Papazian, L. Use of Prone Positioning in Nonintubated Patients With COVID-19 and Hypoxemic Acute Respiratory Failure. JAMA 2020, 323, 2336–2338. [Google Scholar] [CrossRef]
- Sun, Q.; Qiu, H.; Huang, M.; Yang, Y. Lower mortality of COVID-19 by early recognition and intervention: Experience from Jiangsu Province. Ann. Intensiv. Care 2020, 10, 33. [Google Scholar] [CrossRef]
- Sartini, C.; Tresoldi, M.; Scarpellini, P.; Tettamanti, A.; Carcò, F.; Landoni, G.; Zangrillo, A. Respiratory Parameters in Patients With COVID-19 After Using Noninvasive Ventilation in the Prone Position Outside the Intensive Care Unit. JAMA 2020, 323, 2338–2340. [Google Scholar] [CrossRef]
- Thompson, A.E.; Ranard, B.L.; Wei, Y.; Jelic, S. Prone Positioning in Awake, Nonintubated Patients with COVID-19 Hypoxemic Respiratory Failure. JAMA Intern. Med. 2020. [Google Scholar] [CrossRef]
- Mrozek, S.; Jabaudon, M.; Jaber, S.; Paugam-Burtz, C.; Lefrant, J.-Y.; Rouby, J.-J.; Asehnoune, K.; Allaouchiche, B.; Baldesi, O.; Leone, M.; et al. Elevated Plasma Levels of sRAGE Are Associated With Nonfocal CT-Based Lung Imaging in Patients with ARDS: A Prospective Multicenter Study. Chest 2016, 150, 998–1007. [Google Scholar] [CrossRef]
- Chan, M.-C.; Hsu, J.-Y.; Liu, H.-H.; Lee, Y.-L.; Pong, S.-C.; Chang, L.-Y.; Kuo, B.I.-T.; Wu, C.-L. Effects of Prone Position on Inflammatory Markers in Patients with ARDS Due to Community-acquired Pneumonia. J. Formos. Med Assoc. 2007, 106, 708–716. [Google Scholar] [CrossRef]
- Papazian, L.; Paladini, M.-H.; Bregeon, F.; Thirion, X.; Durieux, O.; Gainnier, M.; Huiart, L.; Agostini, S.; Auffray, J.-P. Can the Tomographic Aspect Characteristics of Patients Presenting with Acute Respiratory Distress Syndrome Predict Improvement in Oxygenation-related Response to the Prone Position? Anesthesiology 2002, 97, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X. Acute respiratory failure in COVID-19: Is it “typical” ARDS? Crit. Care 2020, 24, 198. [Google Scholar] [CrossRef] [PubMed]





| Characteristic | Deceased (n = 17) | Living (n = 6) | Total (n = 23) |
|---|---|---|---|
| Demographics | |||
| Age, Median (Range) | 57 (25, 75) | 56 (40, 63) | 57 (25, 75) |
| Sex, Female (%) | 6 (35.3) | 2 (33.3) | 8 (34.8) |
| BMI, Median (Range), kg/m2 | 30 (23, 42) | 36 (22, 45) | 31 (22, 45) |
| Race/Ethnicity, Count (% Distribution) | |||
| African American | 9 (52.9) | 3 (50.0) | 12 (52.2) |
| Hispanic | 7 (41.2) | 2 (33.3) | 9 (39.1) |
| American Indian | - | 1 (16.7) | 1 (4.3) |
| Asian | 1 (5.9) | - | 1 (4.3) |
| Symptoms and Comorbidities at Admission | |||
| Symptoms (%) | |||
| Dyspnea | 15 (88.2) | 6 (100.0) | 21 (91.3) |
| Fever | 14 (82.4) | 4 (66.7) | 18 (78.3) |
| Cough | 12 (70.6) | 3 (50.0) | 15 (65.2) |
| Weakness | 16 (94.1) | 6 (100.0) | 22 (95.7) |
| Diarrhea | 1 (5.9) | 1 (16.7) | 2 (8.7) |
| † Comorbidities (%) | |||
| Diabetes Mellitus | 7 (41.2%) | 2 (33.3%) | 9 (39.1%) |
| Hypertension | 8 (47.1%) | 3 (50.0%) | 11 (47.8%) |
| Congestive Heart Failure | 1 (5.9%) | 0 (0%) | 1 (4.3%) |
| Clinical Values before Prone Ventilation, Median (Range) | |||
| Vitals | |||
| Blood Pressure—Systolic (mmHg) | 125 (109, 153) | 119.5 (110, 167) | 124 (109, 167) |
| Blood Pressure—Diastolic (mmHg) | 75 (49, 93) | 62.5 (52, 87) | 72 (49, 93) |
| Heart Rate (Beats/min) | 105 (66, 125) | 96.5 (53, 118) | 102 (53, 125) |
| Respiratory Rate (Breaths/min) | 28 (20, 34) | 25 (21, 35) | 26 (20, 35) |
| Temperature (°F) | 99.7 (97, 102.6) | 99.5 (98, 100.3) | 99.7 (97, 102.6) |
| O2% Saturation | 92 (87, 100) | 92 (88, 98) | 92 (87, 100) |
| Lab Values | |||
| Blood Urea Nitrogen (mg/dL) | 28 (13, 104) | 26 (14, 32) | 28 (13, 104) |
| Creatinine (IU/L) | 0.91 (0.54, 4.68) | 1.47 (0.44, 2.2) | 0.97 (0.44, 4.68) |
| Lactate (mg/dL) | 1.8 (0.7, 6.7) | 1.8 (1.2, 4.9) | 1.8 (0.7, 6.7) |
| Troponin (ng/mL) | 0.12 (0.015, 0.71) | 0.055 (0.015, 0.28) | 0.081 (0.015, 0.71) |
| LDH (U/L) | 958 (535, 1875) | 888.5 (621, 1757) | 958 (535, 1875) |
| Procalcitonin (ng/mL) | 0.92 (0.16, 200) | 0.78 (0.41, 24.81) | 0.92 (0.16, 200) |
| C-Reactive Protein (mg/L) | 9.52 (1.46, 34) | 14.6 (0.34, 24.1) | 11.1 (0.34, 34) |
| D-Dimer (ng/mL) | 13.8 (0.96, 35.78) | 23.2 (1.8, 35.78) | 13.8 (0.96, 35.78) |
| Fibrinogen (mg/dL) | 502 (100, 679) | 290 (165, 572) | 483 (100, 679) |
| Ferritin (μg/L) | 1151 (298, 5509) | 1308 (992, 2198) | 1285 (298, 5509) |
| Sedimentation Rate (mm/h) | 40 (5, 107) | 26 (7, 72) | 39 (5, 107) |
| White Blood Cell Count (1000/mm3) | 13.6 (6.1, 31.7) | 11.2 (7.1, 26.8) | 13.3 (6.1, 31.7) |
| Lymphocyte Percent | 0.05 (0.02, 0.15) | 0.04 (0.03, 0.08) | 0.05 (0.02, 0.15) |
| ABG and Ventilatory Parameters, Supine, Median (Range) | |||
| PaO2 (mmHg) | 66.0 (35, 190) | 77.5 (66, 138) | 73.0 (35, 190) |
| FiO2 (%) | 100 (50, 100) | 100 (100, 100) | 100 (50, 100) |
| PaO2/FiO2 (mmHg) | 76.0 (35, 190) | 77.5 (66, 138) | 76.0 (35, 190) |
| PEEP (cm H2O) | 15.0 (10, 20) | 15.0 (10, 20) | 15.0 (10, 20) |
| Respiratory Rate | 24 (16, 30) | 24 (20, 26) | 24 (16, 30) |
| Tidal Volume/IBW (mL/kg) | 7.0 (5.8, 8.3) | 7.4 (5.3, 8.0) | 7.0 (5.3, 8.3) |
| Pronation Timeline and Length of Stay (In Days) | |||
| Time to Intubation, Median (Range) | |||
| From Admission | 3.0 (0, 15) | 3.5 (0, 5) | 3.0 (0, 15) |
| From Symptom Onset | 7.0 (2, 14) | 7.0 (5, 14) | 7.0 (2, 14) |
| Time to Pronation, Median (Range) | |||
| From Admission | 5.0 (2, 18) | 4.5 (2, 17) | 5.0 (2, 18) |
| From 1st Symptom Appearance | 11.0 (7, 25) | 13.0 (9, 24) | 12.0 (7, 25) |
| From Intubation | 1.0 (0, 7) | 2.0 (0, 13) | 1.0 (0, 13) |
| Days Proned | 1 (1, 4) | 1.5 (1, 6) | 1 (1, 6) |
| Time to Death After Pronation | 7 (2, 16) | - | 7 (2, 16) |
| Characteristic | Pre-Proning | Post-Proning | Δ * (Post−Pre) | p-Value † |
|---|---|---|---|---|
| Arterial Blood Gas and Ventilatory Markers, Mean Values | ||||
| ‡ Respiratory Rate (bpm) | 27.2 | 23.6 | (3.6) | 0.006 |
| ‡, § Tidal Volume/IBW (mL/kg) | 7.1 | 7.0 | (0.1) | 0.109 |
| Patient Scores, Mean Values | ||||
| SOFA | 4.78 | 3.65 | (1.13) | 4 × 10−4 |
| SAPS | 32.57 | 29.91 | (2.65) | 4 × 10−5 |
| Inflammatory Markers, Mean Values | ||||
| LDH | 986 | 840 | (146) | 0.03 |
| Procalcitonin | 12.5 | 1.61 | (2.29) | 0.36 |
| C-Reactive Protein | 13.8 | 8.51 | (5.46) | 0.04 |
| D-Dimer | 19.5 | 22.1 | 2.65 | 0.71 |
| Ferritin | 1672 | 1195 | (490) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khullar, R.; Shah, S.; Singh, G.; Bae, J.; Gattu, R.; Jain, S.; Green, J.; Anandarangam, T.; Cohen, M.; Madan, N.; et al. Effects of Prone Ventilation on Oxygenation, Inflammation, and Lung Infiltrates in COVID-19 Related Acute Respiratory Distress Syndrome: A Retrospective Cohort Study. J. Clin. Med. 2020, 9, 4129. https://doi.org/10.3390/jcm9124129
Khullar R, Shah S, Singh G, Bae J, Gattu R, Jain S, Green J, Anandarangam T, Cohen M, Madan N, et al. Effects of Prone Ventilation on Oxygenation, Inflammation, and Lung Infiltrates in COVID-19 Related Acute Respiratory Distress Syndrome: A Retrospective Cohort Study. Journal of Clinical Medicine. 2020; 9(12):4129. https://doi.org/10.3390/jcm9124129
Chicago/Turabian StyleKhullar, Rohit, Shrey Shah, Gagandeep Singh, Joseph Bae, Rishabh Gattu, Shubham Jain, Jeremy Green, Thiruvengadam Anandarangam, Marc Cohen, Nikhil Madan, and et al. 2020. "Effects of Prone Ventilation on Oxygenation, Inflammation, and Lung Infiltrates in COVID-19 Related Acute Respiratory Distress Syndrome: A Retrospective Cohort Study" Journal of Clinical Medicine 9, no. 12: 4129. https://doi.org/10.3390/jcm9124129
APA StyleKhullar, R., Shah, S., Singh, G., Bae, J., Gattu, R., Jain, S., Green, J., Anandarangam, T., Cohen, M., Madan, N., & Prasanna, P. (2020). Effects of Prone Ventilation on Oxygenation, Inflammation, and Lung Infiltrates in COVID-19 Related Acute Respiratory Distress Syndrome: A Retrospective Cohort Study. Journal of Clinical Medicine, 9(12), 4129. https://doi.org/10.3390/jcm9124129





