The Tryptophan System in Cocaine-Induced Depression
Abstract
1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Clinical Assessments
2.3. Psychiatric Assessment
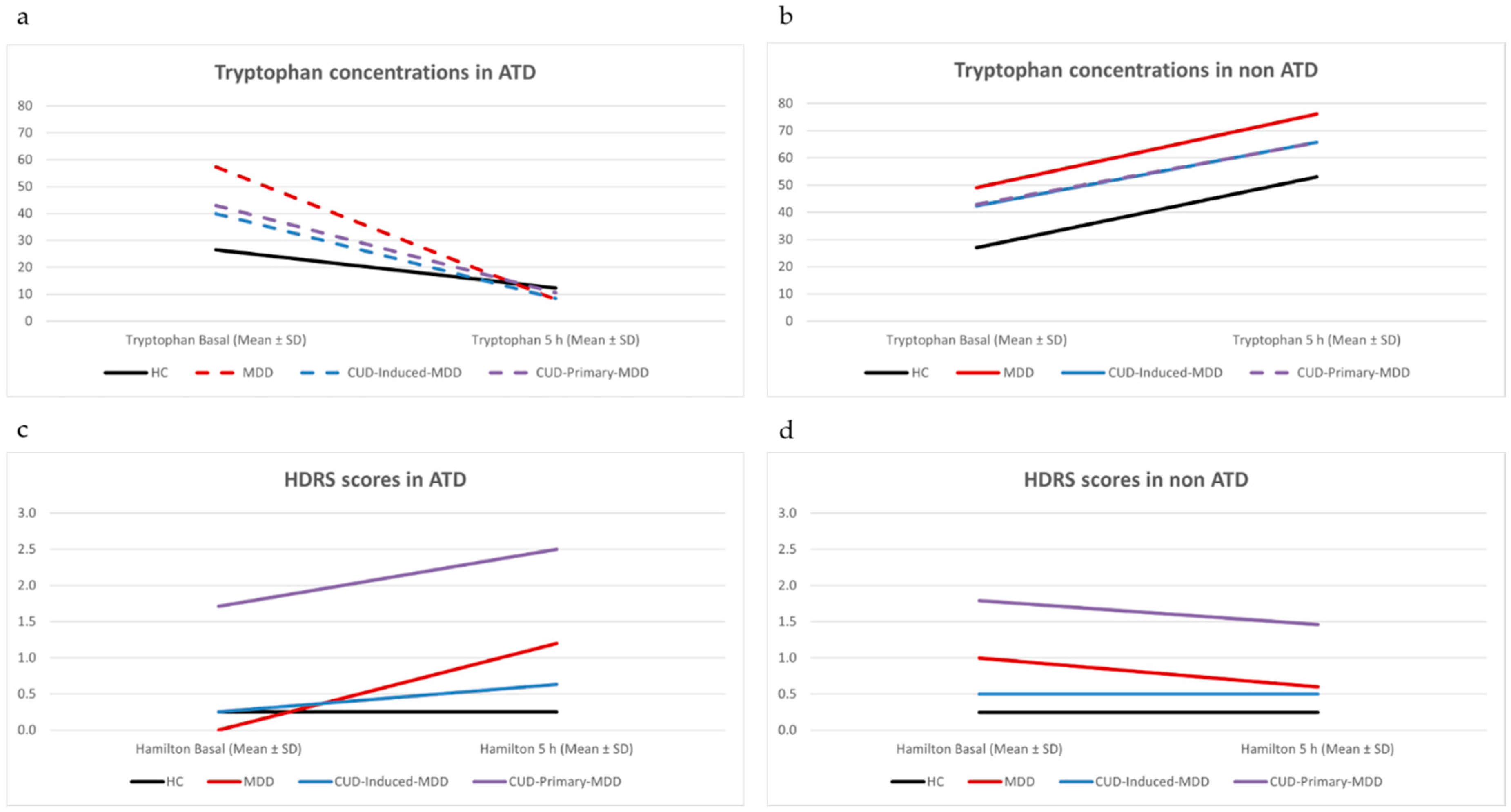
2.4. Acute Tryptophan Depletion Test (ATD)
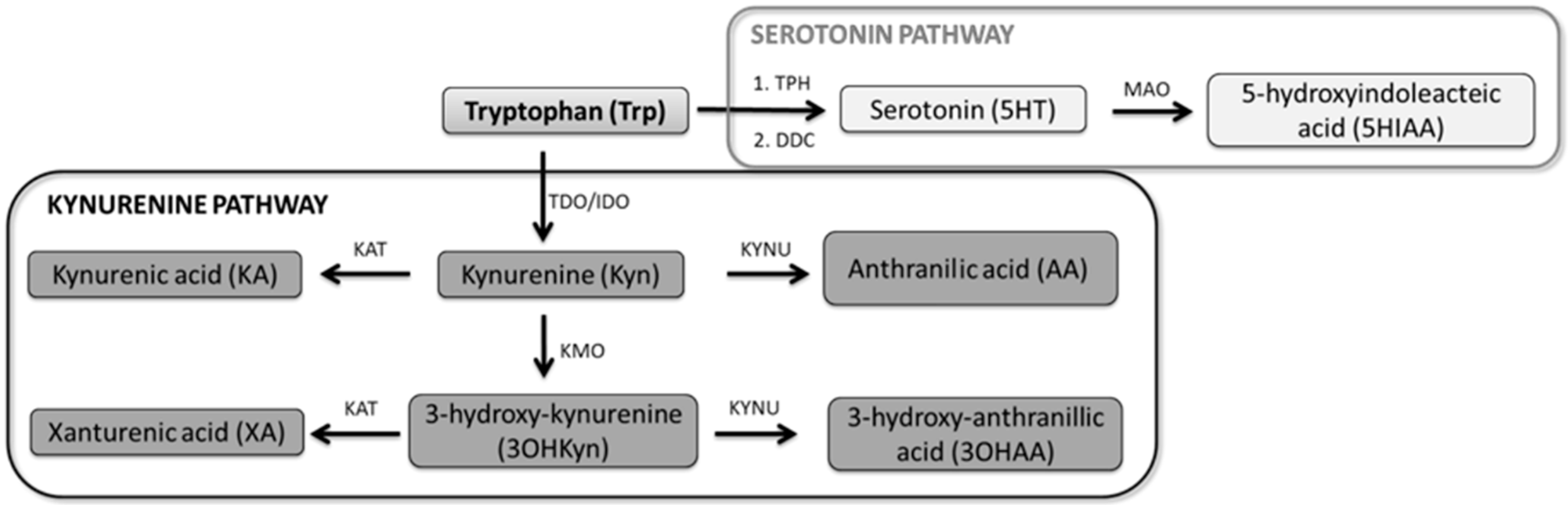
2.5. Selection of the Serotonin–Kynurenine Pathway Biomarkers
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Acute Tryptophan Depletion Test (ATD)
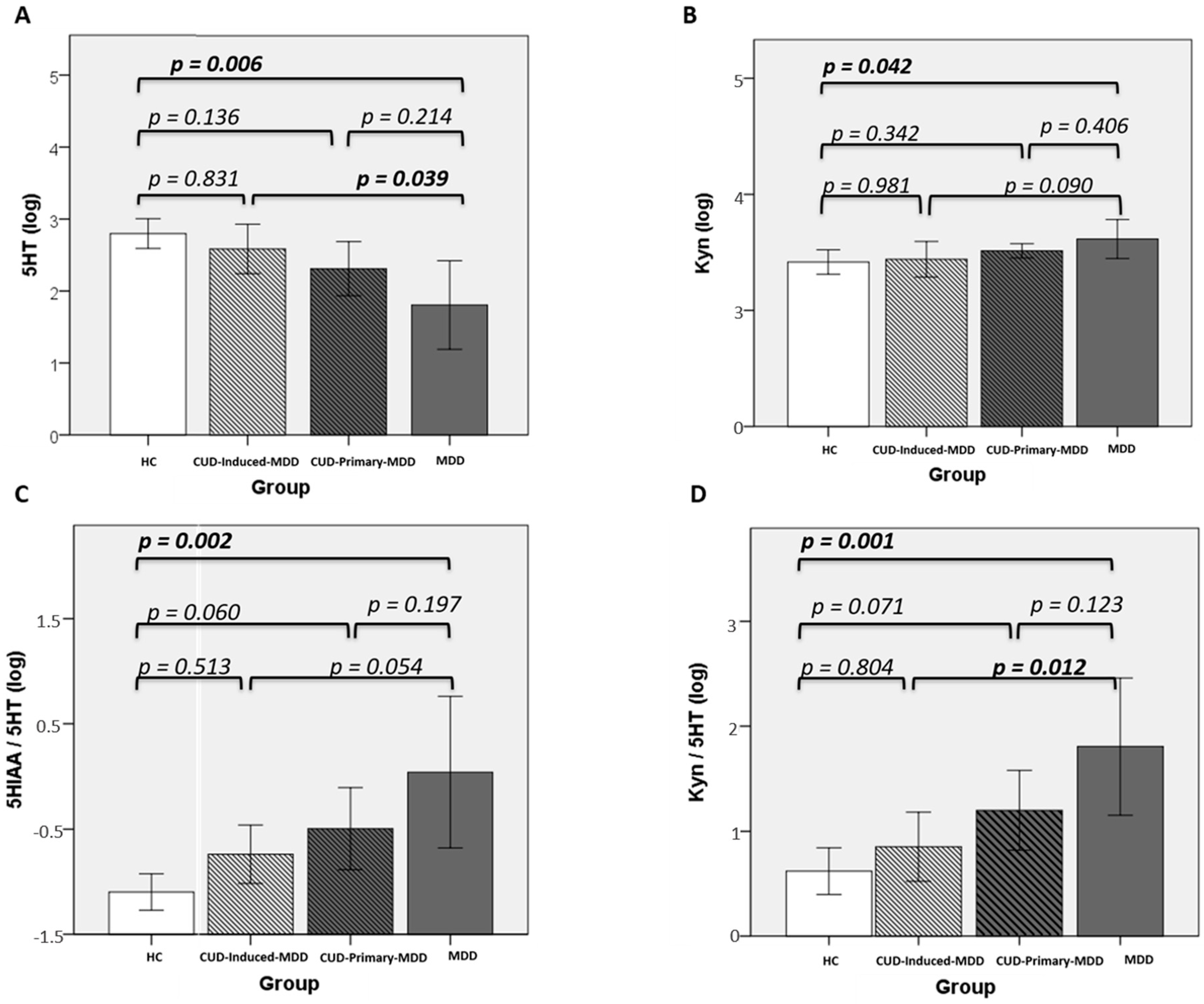
3.3. Kynurenine Pathway
3.3.1. Controls vs. MDD
3.3.2. Cocaine Use Disorder Groups
3.3.3. Overall Perspective
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pettinati, H.M.; O’Brien, C.P.; Dundon, W.D. Current Status of Co-Occurring Mood and Substance Use Disorders: A New Therapeutic Target. Am. J. Psychiatry 2013, 170, 23–30. [Google Scholar] [CrossRef]
- Alías-Ferri, M.; García-Marchena, N.; Mestre-Pintó, J.I.; Araos, P.; Vergara-Moragues, E.; Fonseca, F.; González-Saiz, F.; De Fonseca, F.R.; Torrens, M.; Neurodep Group. Cocaine and depressive disorders: When standard clinical diagnosis is insufficient. Adicciones 2020, 6, 1321. [Google Scholar] [CrossRef]
- Muñoz, J.T.; Farré, A.; Mestre-Pintó, J.; Szerman, N.; Torrens, M. Dual diagnosis in Depression: Treatment recommendations. Adicciones 2017, 30, 66–76. [Google Scholar] [CrossRef]
- Gómez-Coronado, N.; Sethi, R.; Bortolasci, C.C.; Arancini, L.; Berk, M.; Dodd, S. A review of the neurobiological underpinning of comorbid substance use and mood disorders. J. Affect. Disord. 2018, 241, 388–401. [Google Scholar] [CrossRef]
- Müller, C.P.; Homberg, J.R. The role of serotonin in drug use and addiction. Behav. Brain Res. 2015, 277, 146–192. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Carmassi, C.; Mucci, F.; Marazziti, D. Depression, Serotonin and Tryptophan. Curr. Pharm. Des. 2016, 22, 949–954. [Google Scholar] [CrossRef]
- Delgado, P.L.; Charney, D.S.; Price, L.H.; Aghajanian, G.K.; Landis, H.; Heninger, G.R. Serotonin Function and the Mechanism of Antidepressant Action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch. Gen. Psychiatry 1990, 47, 411–418. [Google Scholar] [CrossRef]
- Toker, L.; Amar, S.; Bersudsky, Y.; Benjamin, J.; Klein, E. The biology of tryptophan depletion and mood disorders. Isr. J. Psychiatry Relat. Sci. 2010, 47, 46–55. [Google Scholar]
- Young, S.N. Acute tryptophan depletion in humans: A review of theoretical, practical and ethical aspects. J. Psychiatry Neurosci. 2013, 38, 294–305. [Google Scholar] [CrossRef]
- Ruhé, H.G.; Mason, N.S.; Schene, A.H. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Mol. Psychiatry 2007, 12, 331–359. [Google Scholar] [CrossRef]
- Oxenkrug, G.F. Tryptophan kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: The serotonin hypothesis revisited 40 years later. Isr. J. Psychiatry Relat. Sci. 2010, 47, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ogyu, K.; Kubo, K.; Noda, Y.; Iwata, Y.; Tsugawa, S.; Omura, Y.; Wada, M.; Tarumi, R.; Plitman, E.; Moriguchi, S.; et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 90, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. Role of Kynurenine Metabolism Pathway Activation in Major Depressive Disorders. Curr. Top. Behav. Neurosci. 2017, 31, 249–267. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Elovainio, M.; Hurme, M.; Jokela, M.; Pulkki-Råback, L.; Kivimäki, M.; Hintsanen, M.; Hintsa, T.; Lehtimäki, T.; Viikari, J.; Raitakari, O.T.; et al. Indoleamine 2,3-Dioxygenase Activation and Depressive Symptoms: Results from the Young Finns Study. Psychosom. Med. 2012, 74, 675–681. [Google Scholar] [CrossRef]
- Barreto, F.S.; Filho, A.J.C.; De Araújo, M.C.; De Moraes, M.O.; De Moraes, M.E.; Maes, M.; De Lucena, D.F.; Macedo, D.S. Tryptophan catabolites along the indoleamine 2,3-dioxygenase pathway as a biological link between depression and cancer. Behav. Pharmacol. 2018, 29, 165–180. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Szemraj, J.; Bliźniewska, K.; Maes, M.; Berk, M.; Su, K.-P.; Gałecki, P. An immune gate of depression – Early neuroimmune development in the formation of the underlying depressive disorder. Pharmacol. Rep. 2019, 71, 1299–1307. [Google Scholar] [CrossRef]
- Maes, M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 664–675. [Google Scholar] [CrossRef]
- Roman, M.; Irwin, M.R. Novel neuroimmunologic therapeutics in depression: A clinical perspective on what we know so far. Brain Behav. Immun. 2020, 83, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Vengeliene, V.; Cannella, N.; Takahashi, T.T.; Spanagel, R. Metabolic shift of the kynurenine pathway impairs alcohol and cocaine seeking and relapse. Psychopharmacology 2016, 233, 3449–3459. [Google Scholar] [CrossRef] [PubMed]
- Quak, J.; Doornbos, B.; Roest, A.M.; Duivis, H.E.; Vogelzangs, N.; Nolen, W.A.; Penninx, B.W.; Kema, I.P.; De Jonge, P. Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms? Psychoneuroendocrinology 2014, 45, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Sublette, M.E.; Galfalvy, H.C.; Fuchs, D.; Lapidus, M.; Grunebaum, M.F.; Oquendo, M.A.; Mann, J.J.; Postolache, T.T. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav. Immun. 2011, 25, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.F. Serotonin-Kynurenine Hypothesis of Depression: Historical Overview and Recent Developments. Curr. Drug Targets 2013, 14, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Jansen, K.; Titus, S.; Carvalho, A.F.; Gabbay, V.; Quevedo, J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. J. Psychiatr. Res. 2015, 68, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Fidahic, M.; Kadic, A.J.; Radic, M.; Puljak, L. Celecoxib for rheumatoid arthritis. Cochrane Database Syst. Rev. 2017, 6, CD012095. [Google Scholar] [CrossRef]
- Puljak, L.; Marin, A.; Vrdoljak, D.; Markotic, F.; Utrobicic, A.; Tugwell, P. Celecoxib for osteoarthritis. Cochrane Database Syst. Rev. 2017, 5, CD009865. [Google Scholar] [CrossRef]
- Krause, D.; Myint, A.-M.; Schuett, C.; Musil, R.; Dehning, S.; Cerovecki, A.; Riedel, M.; Arolt, V.; Schwarz, M.J.; Müller, N. High Kynurenine (a Tryptophan Metabolite) Predicts Remission in Patients with Major Depression to Add-on Treatment with Celecoxib. Front. Psychiatry 2017, 8, 16. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Liu, W.; Zheng, W.; Wang, C.; Zhan, Y.; Lan, X.; Zhang, B.; Zhang, C.; Xiang, Y.-T. Predictors of response to repeated ketamine infusions in depression with suicidal ideation: An ROC curve analysis. J. Affect. Disord. 2020, 264, 263–271. [Google Scholar] [CrossRef]
- Torrens, M.; Fonseca, F.; Mateu, G.; Farré, M. Efficacy of antidepressants in substance use disorders with and without comorbid depression: A systematic review and meta-analysis. Drug Alcohol Depend. 2005, 78, 1–22. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision (DSM-IV-TR); American Psychiatric Association: Washington, DC, USA, 2000; ISBN 0890423342. [Google Scholar]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Bobes, J.; Bulbena, A.; Luque, A.; Dal-Ré, R.; Ballesteros, J.; Ibarra, N.; Grupo de Validacion en Espanol de Escalas Psicometricas. A comparative psychometric study of the Spanish versions with 6, 17, and 21 items of the Hamilton Depression Rating Scale. Med. Clin. 2003, 120, 693–700. [Google Scholar] [CrossRef]
- Torrens, M.; Serrano, D.; Astals, M.; Pérez-Domínguez, G.; Martín-Santos, R. Diagnosing Comorbid Psychiatric Disorders in Substance Abusers: Validity of the Spanish Versions of the Psychiatric Research Interview for Substance and Mental Disorders and the Structured Clinical Interview for DSM-IV. Am. J. Psychiatry 2004, 161, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.S.; Samet, S.; Nunes, E.; Meydan, J.; Matseoane, K.; Waxman, R. Diagnosis of Comorbid Psychiatric Disorders in Substance Users Assessed With the Psychiatric Research Interview for Substance and Mental Disorders for DSM-IV. Am. J. Psychiatry 2006, 163, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Marcos, J.; Renau, N.; Valverde, O.; Aznar-Laín, G.; Gracia-Rubio, I.; Gonzalez-Sepulveda, M.; Pérez-Jurado, L.A.; Ventura, R.; Segura, J.; Pozo, Ó.J. Targeting tryptophan and tyrosine metabolism by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1434, 91–101. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 2012, 6, 241–252. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Revised Edition; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Satel, S.L.; Krystal, J.; Delgado, P.L.; Kosten, T.R.; Charney, D.S. Tryptophan depletion and attenuation of cue-induced craving for cocaine. Am. J. Psychiatry 1995, 152, 778–783. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J.; Telang, F.; Logan, J.; Jayne, M.; Ma, Y.; Pradhan, K.; Wong, C.; Swanson, J.M. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage 2010, 49, 2536–2543. [Google Scholar] [CrossRef]
- Knott, V.; Bisserbe, J.-C.; Eshah, D.; Thompson, A.; Bowers, H.; Blais, C.; Eilivitsky, V. The moderating influence of nicotine and smoking on resting-state mood and EEG changes in remitted depressed patients during tryptophan depletion. Biol. Psychol. 2013, 94, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.; Mestre-Pintó, J.-I.; Álvaro-Bartolomé, M.; Martínez-Sanvisens, D.; Farré, M.; García-Fuster, M.J.; García-Sevilla, J.A.; Torrens, M.; Fonseca, F.; Mateus, J.; et al. A Biomarker to Differentiate between Primary and Cocaine-Induced Major Depression in Cocaine Use Disorder: The Role of Platelet IRAS/Nischarin (I1-Imidazoline Receptor). Front. Psychiatry 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Gryz, M.; Lehner, M.; Wisłowska-Stanek, A.; Płaźnik, A. Dopaminergic system activity under stress condition—seeking individual differences, preclinical studies. Psychiatr. Pol. 2018, 52, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Kim, H.-C.; Oh, S.; Lee, Y.-M.; Hu, Z.; Oh, K.-W. Cocaine- and Amphetamine-Regulated Transcript (CART) Peptide Plays Critical Role in Psychostimulant-Induced Depression. Biomol. Ther. 2018, 26, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Pedraz, M.; Martin, A.I.; García-Marchena, N.; Araos, P.; Serrano, A.; Romero-Sanchiz, P.; Suárez, J.; Castilla-Ortega, E.; Barrios, V.; Campos-Cloute, R.; et al. Plasma Concentrations of BDNF and IGF-1 in Abstinent Cocaine Users with High Prevalence of Substance Use Disorders: Relationship to Psychiatric Comorbidity. PLoS ONE 2015, 10, e0118610. [Google Scholar] [CrossRef] [PubMed]
- Cléry-Melin, M.-L.; Jollant, F.; Gorwood, P. Reward systems and cognitions in Major Depressive Disorder. CNS Spectr. 2019, 24, 64–77. [Google Scholar] [CrossRef]
- Thomas, M.J.; Kalivas, P.W.; Shaham, Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br. J. Pharmacol. 2008, 154, 327–342. [Google Scholar] [CrossRef]
- Neupane, S.P.; Lien, L.; Martinez, P.; Hestad, K.; Bramness, J.G. The Relationship of Alcohol Use Disorders and Depressive Symptoms to Tryptophan Metabolism: Cross-Sectional Data from a Nepalese Alcohol Treatment Sample. Alcohol. Clin. Exp. Res. 2015, 39, 514–521. [Google Scholar] [CrossRef]
- Farré, A.; Tirado-Muñoz, J.; Torrens, M. Dual Depression: A Sex Perspective. Addict. Disord. Treat. 2017, 16, 180–186. [Google Scholar] [CrossRef]
- Labaka, A.; Goñi-Balentziaga, O.; Lebeña, A.; Pérez-Tejada, J. Biological Sex Differences in Depression: A Systematic Review. Biol. Res. Nurs. 2018, 20, 383–392. [Google Scholar] [CrossRef]
- Rehm, J.; Shield, K.D. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr. Psychiatry Rep. 2019, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.L.; Uezato, A.; Newell, J.M.; Frazier, E. Major depression and comorbid substance use disorders. Curr. Opin. Psychiatry 2008, 21, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Samet, S.; Fenton, M.C.; Nunes, E.; Greenstein, E.; Aharonovich, E.; Hasin, D.S. Effects of independent and substance-induced major depressive disorder on remission and relapse of alcohol, cocaine and heroin dependence. Addiction 2013, 108, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Magidson, J.; Wang, S.; Lejuez, C.W.; Iza, M.; Blanco, C. Prospective study of substance-induced and independent major depressive disorder among individuals with substance use disorders in a nationally representative sample. Depress. Anxiety 2013, 30, 538–545. [Google Scholar] [CrossRef]
- Foulds, J.A.; Adamson, S.J.; Boden, J.M.; Williman, J.A.; Mulder, R.T. Depression in patients with alcohol use disorders: Systematic review and meta-analysis of outcomes for independent and substance-induced disorders. J. Affect. Disord. 2015, 185, 47–59. [Google Scholar] [CrossRef]
| HC | CUD-Induced-MDD | CUD-Primary-MDD | MDD | |
|---|---|---|---|---|
| n = 8 | n = 8 | n = 14 | n = 5 | |
| Sex (Male n) | 5 (50) | 6 (75) | 12 (85.7) | 5 (100) |
| Age (Mean ± SD) | 32.13 ± 3.94 | 38 ± 12.17 | 44.21 ± 8.26 | 43.80 ± 13.9 |
| Civil status (% Single) | 62.5 | 62.5 | 35.7 | 40 |
| Work status (% Employed) | 25 | 37.5 | 50 | 40 |
| Depression (MDD) | ||||
| Age of onset first primary-MDD (Mean ± SD) | NA | NA | 34.29 ± 10.56 | 36.4 ± 11.97 |
| Age of onset first induced-MDD (Mean ± SD) | NA | 34.01 ± 12.47 | NA | NA |
| Number of episodes (Mean ± SD) | NA | 6.63 ± 8.23 | 2.08 ± 1.04 | 1.6 ± 0.89 |
| Months since last episode (Mean ± SD) | NA | 22.75 ± 31.23 | 36.29 ± 55 | 34.4 ± 40.9 |
| Family history of depression (%) | NA | 37.5 | 71.4 | 80 |
| Current antidepressant treatment (%) | NA | 37.5 | 57.1 | 100 |
| Age of onset CUD | NA | 26.75 ± 10.29 | 31.43 ± 7.39 | NA |
| Age of cocaine problematic use | NA | 26.38 ± 9.61 | 31.21 ± 7.43 | NA |
| Maximum cocaine abstinence period (Months) | NA | 19.50 ± 18.42 | 37.77 ± 54.17 | NA |
| No. 1st and 2nd degree relatives with CUD | 0 | 0 | 0.64 ± 0.93 | 0 |
| Nicotine use disorder (%) | 0 | 75 | 78.6 | 0 |
| HC | CUD-Induced-MDD | CUD-Primary-MDD | MDD | |
|---|---|---|---|---|
| n = 8 | n = 8 | n = 14 | n = 5 | |
| Kyn | 2716 ± 847 | 2971 ± 1171 | 3358 ± 894 | 4293 ± 1424 |
| KA | 247 ± 95 | 272 ± 135 | 304 ± 128 | 370 ± 205 |
| AA | 64 ± 23 | 79 ± 61 | 54 ± 32 | 32 ± 12 |
| 5-HT | 729 ± 456 | 512 ± 332 | 521 ± 584 | 107 ± 127 |
| 5-HIAA | 52 ± 14 | 87 ± 76 | 68 ± 21 | 75 ± 15 |
| Kyn/Trp | 0.016 ± 0.004 | 0.019 ± 0.006 | 0.021 ± 0.006 | 0.027 ± 0.015 |
| KA/Kyn | 0.093 ± 0.028 | 0.091 ± 0.026 | 0.094 ± 0.037 | 0.084 ± 0.021 |
| AA/Kyn | 0.026 ± 0.014 | 0.031 ± 0.023 | 0.016 ± 0.009 | 0.008 ± 0.004 |
| KA/AA | 4.2 ± 1.6 | 6.8 ± 7.3 | 9.4 ± 10.2 | 13.8 ± 9.4 |
| Kyn/5-HIAA | 56 ± 22 | 43 ± 17 | 58 ± 37 | 60 ± 16 |
| 5-HT/Trp | 0.0042 ± 0.0022 | 0.0032 ± 0.0018 | 0.0031 ± 0.0033 | 0.0008 ± 0.0003 |
| 5-HIAA /5-HT | 0.088 ± 0.038 | 0.26 ± 0.29 | 0.91 ± 1.12 | 2.08 ± 2.67 |
| 5-HT/Kyn | 0.286 ± 0.188 | 0.183 ± 0.114 | 0.164 ± 0.184 | 0.027 ± 0.032 |
| 5-HIAA/Trp | 0.00031 ± 0.00009 | 0.00053 ± 0.00038 | 0.00044 ± 0.00015 | 0.00043 ± 0.00014 |
| Kyn/5-HT | 4.8 ± 2.5 | 11 ± 15 | 44 ± 55 | 112 ± 136 |
| CUD-Induced-MDD (n = 8) | CUD-Primary-MDD (n = 14) | MDD (n = 5) | ||
|---|---|---|---|---|
| 5-HT | HC (n = 8) | 0.54 | 0.38 | 1.67 ** |
| CUD-Induced-MDD | — | 0.02 | 1.47 * | |
| CUD-Primary-MDD | — | — | 0.80 | |
| Kyn | HC | 0.25 | 0.73 | 1.44 * |
| CUD-Induced-MDD | — | 0.39 | 1.04 | |
| CUD-Primary-MDD | — | — | 0.90 | |
| 5-HIAA/5-HT | HC | 0.83 | 0.91 | 1.24 ** |
| CUD-Induced-MDD | — | 0.71 | 1.12 | |
| CUD-Primary-MDD | — | — | 0.72 | |
| Kyn/5-HT | HC | 0.58 | 0.88 | 1.31 ** |
| CUD-Induced-MDD | — | 0.73 | 1.22 ** | |
| CUD-Primary-MDD | — | — | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, F.; Mestre-Pintó, J.-I.; Gómez-Gómez, À.; Martinez-Sanvisens, D.; Rodríguez-Minguela, R.; Papaseit, E.; Pérez-Mañá, C.; Langohr, K.; Valverde, O.; Pozo, Ó.J.; et al. The Tryptophan System in Cocaine-Induced Depression. J. Clin. Med. 2020, 9, 4103. https://doi.org/10.3390/jcm9124103
Fonseca F, Mestre-Pintó J-I, Gómez-Gómez À, Martinez-Sanvisens D, Rodríguez-Minguela R, Papaseit E, Pérez-Mañá C, Langohr K, Valverde O, Pozo ÓJ, et al. The Tryptophan System in Cocaine-Induced Depression. Journal of Clinical Medicine. 2020; 9(12):4103. https://doi.org/10.3390/jcm9124103
Chicago/Turabian StyleFonseca, Francina, Joan-Ignasi Mestre-Pintó, Àlex Gómez-Gómez, Diana Martinez-Sanvisens, Rocío Rodríguez-Minguela, Esther Papaseit, Clara Pérez-Mañá, Klaus Langohr, Olga Valverde, Óscar J. Pozo, and et al. 2020. "The Tryptophan System in Cocaine-Induced Depression" Journal of Clinical Medicine 9, no. 12: 4103. https://doi.org/10.3390/jcm9124103
APA StyleFonseca, F., Mestre-Pintó, J.-I., Gómez-Gómez, À., Martinez-Sanvisens, D., Rodríguez-Minguela, R., Papaseit, E., Pérez-Mañá, C., Langohr, K., Valverde, O., Pozo, Ó. J., Farré, M., Torrens, M., & on behalf of NEURODEP GROUP. (2020). The Tryptophan System in Cocaine-Induced Depression. Journal of Clinical Medicine, 9(12), 4103. https://doi.org/10.3390/jcm9124103







