Salivary Carbohydrate-Deficient Transferrin in Alcohol- and Nicotine-Dependent Males
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Ethical Issues
2.3. Procedures
2.3.1. Data and Sample Collection
2.3.2. Analytical Methods
2.4. Statistical Analysis
3. Results
3.1. Descriptive Characteristics of Studied Groups
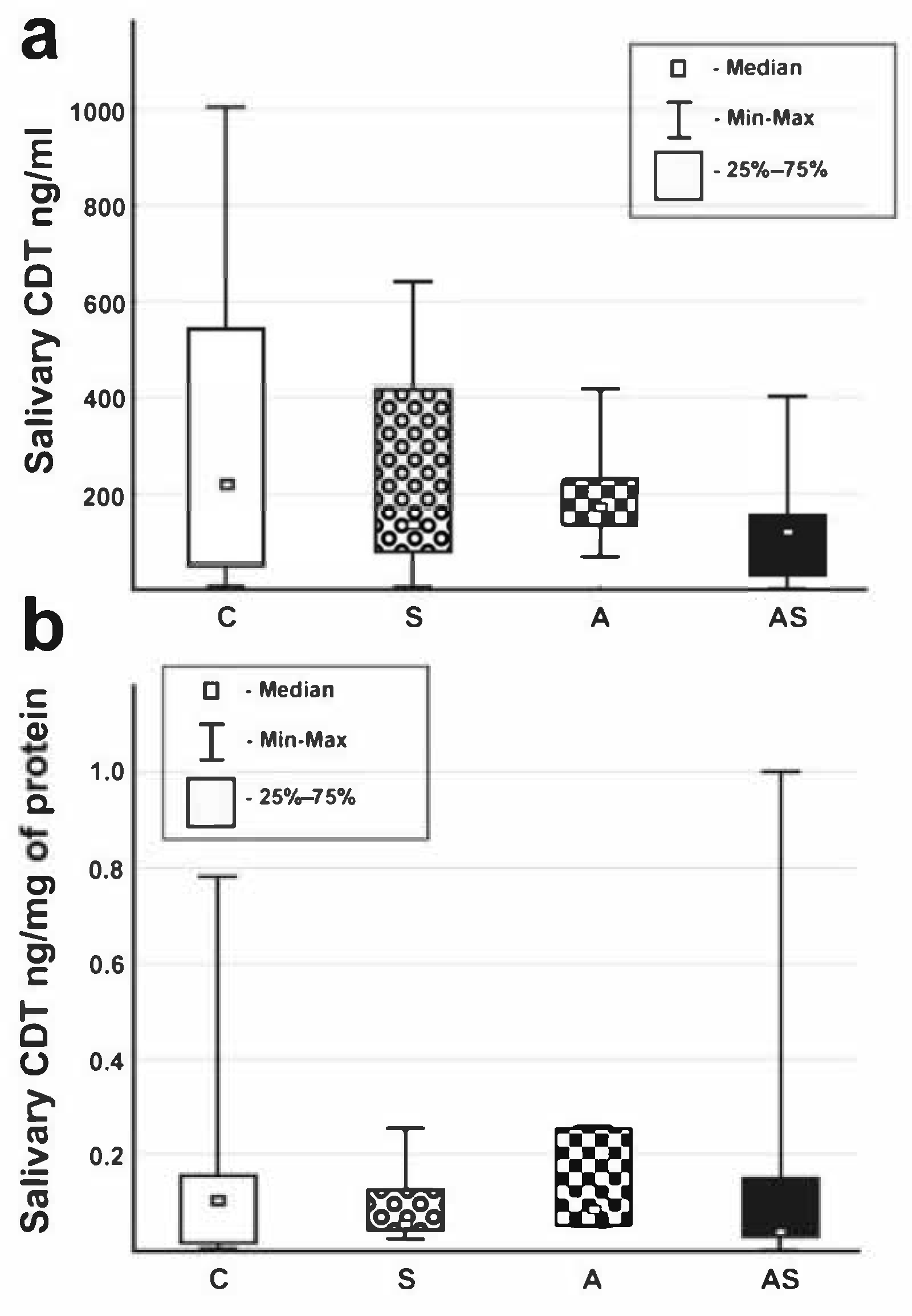
3.2. Salivary Proteins and CDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ames, S.C.; Stevens, S.R.; Werch, C.E.; Carlson, J.M.; Schroeder, D.R.; Kiros, G.E.; Kershaw, J.; Patten, C.A.; Ebbert, J.O.; Offord, K.P. The association of alcohol consumption with tobacco use in black and white college students. Subst. Use Misuse 2010, 45, 1230–1244. [Google Scholar] [CrossRef]
- Czaderny, K. Adolescent personality risk factors for tobacco smoking and alcohol misuse in adult men. Subst. Use Misuse 2020, 55, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Romberger, D.J.; Grant, K. Alcohol consumption and smoking status: The role of smoking cessation. Biomed. Pharm. 2004, 58, 77–83. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Szulc, A.; Zwierz, K. Binge drinking-induced subtle myocardial injury. Alcohol. Clin. Exp. Res. 2013, 37, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Waszkiewicz, N.; Chojnowska, S.; Zalewska, A.; Zwierz, K.; Szulc, A.; Szajda, S.D. Salivary hexosaminidase in smoking alcoholics with bad periodontal and dental states. Drug Alcohol Depend. 2013, 129, 33–40. [Google Scholar] [CrossRef]
- Klimiuk, A.; Maciejczyk, M.; Choromańska, M.; Fejfer, K.; Waszkiewicz, N.; Zalewska, A. Salivary redox biomarkers in different stages of dementia severity. J. Clin. Med. 2019, 8, 840. [Google Scholar] [CrossRef]
- Roi, A.; Roi, C.I.; Negruțiu, M.L.; Riviș, M.; Sinescu, C.; Rusu, L.-C. The challenges of OSCC diagnosis: Salivary Cytokines as potential biomarkers. J. Clin. Med. 2020, 9, 2866. [Google Scholar] [CrossRef] [PubMed]
- Samborska-Mazur, J.; Kostiukow, A.; Miechowicz, I.; Sikorska, D.; Rutkowski, R.; Wyganowska-Świątkowska, M.; Błochowiak, K. Salivary Cytokine profile as a possible predictor of autism spectrum disorder. J. Clin. Med. 2020, 9, 3101. [Google Scholar] [CrossRef] [PubMed]
- Waszkiewicz, N.; Kratz, E.M.; Chojnowska, S.; Zalewska, A.; Zwierz, K.; Szulc, A.; Szajda, S.D.; Nestsiarovich, A.; Kapitau, A.; Kępka, A.; et al. Long-term changes of salivary exoglycosidases and their applicability as chronic alcohol-drinking and dependence markers. World. J. Biol. Psychiatry 2019, 20, 64–75. [Google Scholar] [CrossRef]
- Waszkiewicz, N. Mentally sick or not-(Bio)markers of psychiatric disorders needed. J. Clin. Med. 2020, 9, 2375. [Google Scholar] [CrossRef] [PubMed]
- White, T. Drug testing at work: Issues and perspectives. Subst. Use Misuse 2003, 38, 1891–1902. [Google Scholar] [CrossRef]
- Shivashankara, A.R.; Johnny, C.; Malathi, M.; Arun Kumarm, K.; Avinashm, S.S.; Thomas, T. A correlative study on the aminotransferases and gamma glutamyl transferase in the saliva and serum of chronic alcoholics before and after alcohol deaddiction. J. Clin. Diagn. Res. 2011, 5, 512–515. [Google Scholar]
- Bendtsen, P.; Hultberg, J.; Carlsson, M.; Jones, A.W. Monitoring ethanol exposure in a clinical setting by analysis of blood, breath, saliva, and urine. Alcohol. Clin. Exp. Res. 1999, 23, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Tu, G.C.; Kapur, B.; Israel, Y. Characteristics of a new urine, serum, and saliva alcohol reagent strip. Alcohol. Clin. Exp. Res. 1992, 16, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Pönniö, M.; Alho, H.; Heinälä, P.; Nikkari, S.T.; Sillanaukee, P. Serum and saliva levels of sialic acid are elevated in alcoholics. Alcohol. Clin. Exp. Res. 1999, 23, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Heberlein, A.; Lenz, B.; Degner, D.; Kornhuber, J.; Hillemacher, T.; Bleich, S. Methanol levels in saliva—A non-invasive parameter that may be useful in detection of alcohol intoxication. Alcohol Alcohol. 2010, 45, 126–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kratz, E.M.; Waszkiewicz, N.; Kaluza, A.; Szajda, S.D.; Zalewska-Szajda, B.; Szulc, A.; Zwierz, K.; Ferens-Sieczkowska, M. Glycosylation changes in the salivary glycoproteins of alcohol-dependent patients: A pilot study. Alcohol Alcohol. 2014, 49, 23–30. [Google Scholar] [CrossRef][Green Version]
- Waszkiewicz, N.; Szajda, S.D.; Kępka, A.; Szulc, A.; Zwierz, K. Glycoconjugates in the detection of alcohol abuse. Biochem. Soc. Trans. 2011, 39, 365–369. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Szajda, S.D.; Zalewska, A.; Szulc, A.; Kępka, A.; Minarowska, A.; Wojewódzka-Żelezniakowicz, M.; Konarzewska, B.; Chojnowska, S.; Ladny, J.R.; et al. Alcohol abuse and glycoconjugate metabolism. Folia. Histochem. Cytobiol. 2012, 50, 1–11. [Google Scholar] [CrossRef][Green Version]
- Helander, A.; Wielders, J.; Anton, R.; Arndt, T.; Bianchi, V.; Deenmamode, J.; Jeppsson, J.O.; Whitfield, J.B.; Weykamp, C.; Schellenberg, F. International federation of clinical chemistry and laboratory medicine working group on standardisation of carbohydrate-deficient transferrin (IFCC WG-CDT). Reprint of standardisation and use of the alcohol biomarker carbohydrate-deficient transferrin (CDT). Clin. Chim. Acta 2017, 467, 15–20. [Google Scholar]
- Dawes, C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J. Dent. Res. 1987, 66, 648–653. [Google Scholar] [CrossRef]
- Navazesh, M.; Christensen, C.; Brightman, V. Clinical criteria for the diagnosis of salivary gland hypofunction. J. Dent. Res. 1992, 71, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Almoznino, G.; Kessler Baruch, O.; Kedem, R.; Protter, N.E.; Shay, B.; Yavnai, N.; Zur, D.; Mijiritsky, E.; Abramovitz, I. SOS Teeth: First priority teeth with advanced caries and its associations with metabolic syndrome among a national representative sample of young and middle-aged adults. J. Clin. Med. 2020, 9, 3170. [Google Scholar] [CrossRef] [PubMed]
- Waszkiewicz, N.; Szajda, S.D.; Jankowska, A.; Zwierz, P.; Czernikiewicz, A.; Szulc, A.; Zwierz, K. The effect of acute ethanol intoxication on salivary proteins of innate and adaptive immunity. Alcohol. Clin. Exp. Res. 2008, 32, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Waszkiewicz, N.; Jelski, W.; Zalewska, A.; Szulc, A.; Szmitkowski, M.; Zwierz, K.; Szajda, S.D. Salivary alcohol dehydrogenase in non-smoking and smoking alcohol-dependent persons. Alcohol 2014, 48, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Waszkiewicz, N.; Chojnowska, S.; Zalewska, A.; Zwierz, K.; Szulc, A.; Szajda, S.D. Salivary exoglycosidases as markers of alcohol dependence. Alcohol Alcohol. 2014, 49, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Waszkiewicz, N.; Galińska-Skok, B.; Zalewska, A.; Szajda, S.D.; Zwierz, K.; Więdłocha, M.; Szulc, A. Salivary immune proteins monitoring can help detection of binge and chronic alcohol drinkers: Preliminary findings. Drug. Alcohol. Depend. 2018, 183, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Winn, D.M. Tobacco use and oral disease. J. Dent. Educ. 2001, 65, 306–312. [Google Scholar] [CrossRef]
- Homann, N.; Tillonen, J.; Meurman, J.H.; Rintamäki, H.; Lindqvist, C.; Rautio, M.; Jousimies-Somer, H.; Salaspuro, M. Increased salivary acetaldehyde levels in heavy drinkers and smokers: A microbiological approach to oral cavity cancer. Carcinogenesis 2000, 21, 663–668. [Google Scholar] [CrossRef]
- Jones, A.W. Measuring ethanol in saliva with theQEDenzymatic test device: Comparison of results with blood- and breath-alcohol concentrations. J. Anal. Toxicol. 1995, 19, 169–174. [Google Scholar] [CrossRef]
- Ryder, M.I. The influence of smoking on host responses in periodontal infections. Periodontology 2007, 43, 267–277. [Google Scholar] [CrossRef]
- Barrio, P.; Gual, A.; Lligoña, A.; Teixidor, L.; Weinmann, W.; Yegles, M.; Wurst, F.M. Phosphatidylethanol for monitoring alcohol use in liver transplant candidates: An observational study. J. Clin. Med. 2020, 9, 3060. [Google Scholar] [CrossRef] [PubMed]
- Bomford, A.B.; Munro, H.N. Transferrin and its receptor: Their roles in cell function. Hepatology 1985, 5, 870–875. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Pdel, V.; Grégio, A.M.; Machado, M.A.; de Lima, A.A.; Azevedo, L.R. Saliva composition and functions: A comprehensive review. J. Contemp. Dent. Pract. 2008, 9, 72–80. [Google Scholar]
- Loo, L.A.; Yan, W.; Ramachandran, P.; Wong, D.T. Comparative human salivary and plasma Proteomes. J. Dent. Res. 2010, 89, 1016–1023. [Google Scholar] [CrossRef]
- Conigrave, K.M.; Degenhardt, L.J.; Whitfield, J.B.; Saunders, J.B.; Helander, A.; Tabakoff, B. WHO/ISBRA Study Group. CDT, GGT, and AST as markers of alcohol use: The WHO/ISBRA collaborative project. Alcohol. Clin. Exp. Res. 2002, 26, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Ino, T.; Ohta, M.; Otani, T.; Hanada, S.; Sakuraoka, A.; Matsumoto, A.; Ichiba, M.; Hara, M. Enzyme-linked immunosorbent assay of nicotine metabolites. Environ. Health. Prev. Med. 2010, 15, 211–216. [Google Scholar] [CrossRef]
- Goldblatt, D.; van Etten, L.; van Milligen, F.J.; Aalberse, R.C.; Turner, M.W. The Role of pH in modified ELISA procedures used for the estimation of functional antibody affinity. J. Immunol. Methods 1993, 166, 281–285. [Google Scholar] [CrossRef]
- Whitfield, J.B.; Fletcher, L.M.; Murphy, T.L.; Powell, L.W.; Halliday, J.; Heath, A.C.; Martin, N.G. Smoking, obesity, and hypertension alter the dose-response curve and test sensitivity of carbohydrate-deficient transferrin as a marker of alcohol intake. Clin. Chem. 1998, 44, 2480–2489. [Google Scholar] [CrossRef]
| Variable (Concentration) | C (Min–Max) {95% CI} n = 20 | S (Min–Max) {95% CI} n = 10 | A (Min–Max) {95% CI} n = 10 | AS (Min–Max) {95% CI} n = 15 | p |
|---|---|---|---|---|---|
| Age (years) | 41 (25–62) {6–48} | 40 (23–50) {35–47} | 47 (37–65) {35–60} | 46 (31–64) {41–54} | 0.115 |
| BMI | 26 (23–31) {25–27} | 28 (22–32) {25–29} | 24 (22–31) {21–30} | 24 (19–33) {11–19} | 0.878 |
| Alcohol dependence (years) | 0 | 0 | 7 (1–25) {1–24} | 25 (6–40) {16–29} | 0.059 |
| Last alcohol consumption (days) | 0 | 0 | 22 (3–360) {3–376} | 15 (3–700) {3–213} | 0.770 |
| Daily amount of the last alcohol consumption (g) | 0 | 0 | 200 (100–300) {112–287} | 200 (100–500) {190–326} | 0.387 |
| Nicotine dependence (years) | 0 | 10 (3–20) {5–16} | 0 | 25 (14–40) {21–32} | 0.000 |
| Daily number of smoked cigarettes | 0 | 10 (1–20) {3–17} | 0 | 20 (15–30) {18–23} | 0.006 |
| Salivary pH | 7.4 (6.4–7.9) {7.1–7.5} | 7.2 (5.6–7.8) {6.5–7.6} | 7.5 (7.3–8.1) {7.2–8.0} | 6.8 (5.9–8.4) {6.4–7.2} | 0.024 |
| Salivary protein content (mg/mL) | 2416 (1282–4998) {2144–3467} | 2643 (2276–5323) {2377–3871} | 1489 (907–3362) {1267–5107} | 2731 (404–5242) {1807–3446} | 0.474 |
| CDT (ng/mL) | 219 (8–1005) {166–452} | 137 (5–640) {78–375} | 174 (66–418) {39–370} | 122 (1–405) {61–176} | 0.351 |
| CDT (ng/mg of protein) | 0.103 (0.004–0.783) {0.141–0.043} | 0.053 (0.022–0.255) {0.026–0.142} | 0.090 (0.051–0.253) {0.130–0.399} | 0.039 (0.0009–1.002) {0.016–0.278} | 0.420 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waszkiewicz, N.; Pawłowicz, K.; Okuniewska, N.; Kwiatkowski, M.; Zalewski, D.; Wilczyńska, K.; Szulc, A.; Galińska-Skok, B.; Konarzewska, B.; Maciejczyk, M.; et al. Salivary Carbohydrate-Deficient Transferrin in Alcohol- and Nicotine-Dependent Males. J. Clin. Med. 2020, 9, 4054. https://doi.org/10.3390/jcm9124054
Waszkiewicz N, Pawłowicz K, Okuniewska N, Kwiatkowski M, Zalewski D, Wilczyńska K, Szulc A, Galińska-Skok B, Konarzewska B, Maciejczyk M, et al. Salivary Carbohydrate-Deficient Transferrin in Alcohol- and Nicotine-Dependent Males. Journal of Clinical Medicine. 2020; 9(12):4054. https://doi.org/10.3390/jcm9124054
Chicago/Turabian StyleWaszkiewicz, Napoleon, Katarzyna Pawłowicz, Natalia Okuniewska, Mikołaj Kwiatkowski, Daniel Zalewski, Karolina Wilczyńska, Agata Szulc, Beata Galińska-Skok, Beata Konarzewska, Mateusz Maciejczyk, and et al. 2020. "Salivary Carbohydrate-Deficient Transferrin in Alcohol- and Nicotine-Dependent Males" Journal of Clinical Medicine 9, no. 12: 4054. https://doi.org/10.3390/jcm9124054
APA StyleWaszkiewicz, N., Pawłowicz, K., Okuniewska, N., Kwiatkowski, M., Zalewski, D., Wilczyńska, K., Szulc, A., Galińska-Skok, B., Konarzewska, B., Maciejczyk, M., & Zalewska, A. (2020). Salivary Carbohydrate-Deficient Transferrin in Alcohol- and Nicotine-Dependent Males. Journal of Clinical Medicine, 9(12), 4054. https://doi.org/10.3390/jcm9124054










