CannabinEYEds: The Endocannabinoid System as a Regulator of the Ocular Surface Nociception, Inflammatory Response, Neovascularization and Wound Healing
Abstract
1. Introduction
2. Materials and Methods
3. ECS in the Ocular Surface: An Overview
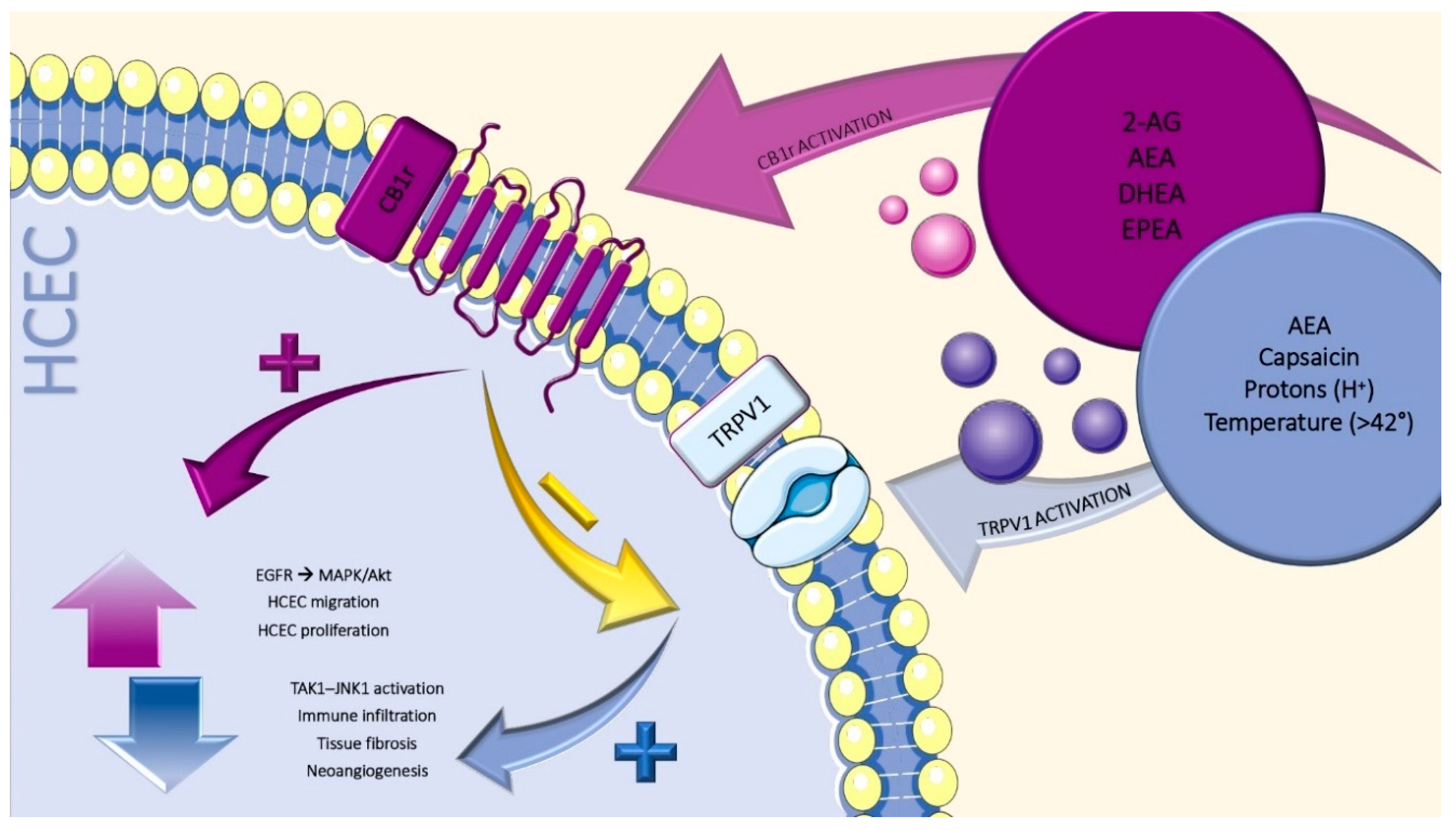
4. Cannabinoid Receptors
5. Non-Cannabinoid Receptors
6. Transient Receptors Potential Vanilloid-1
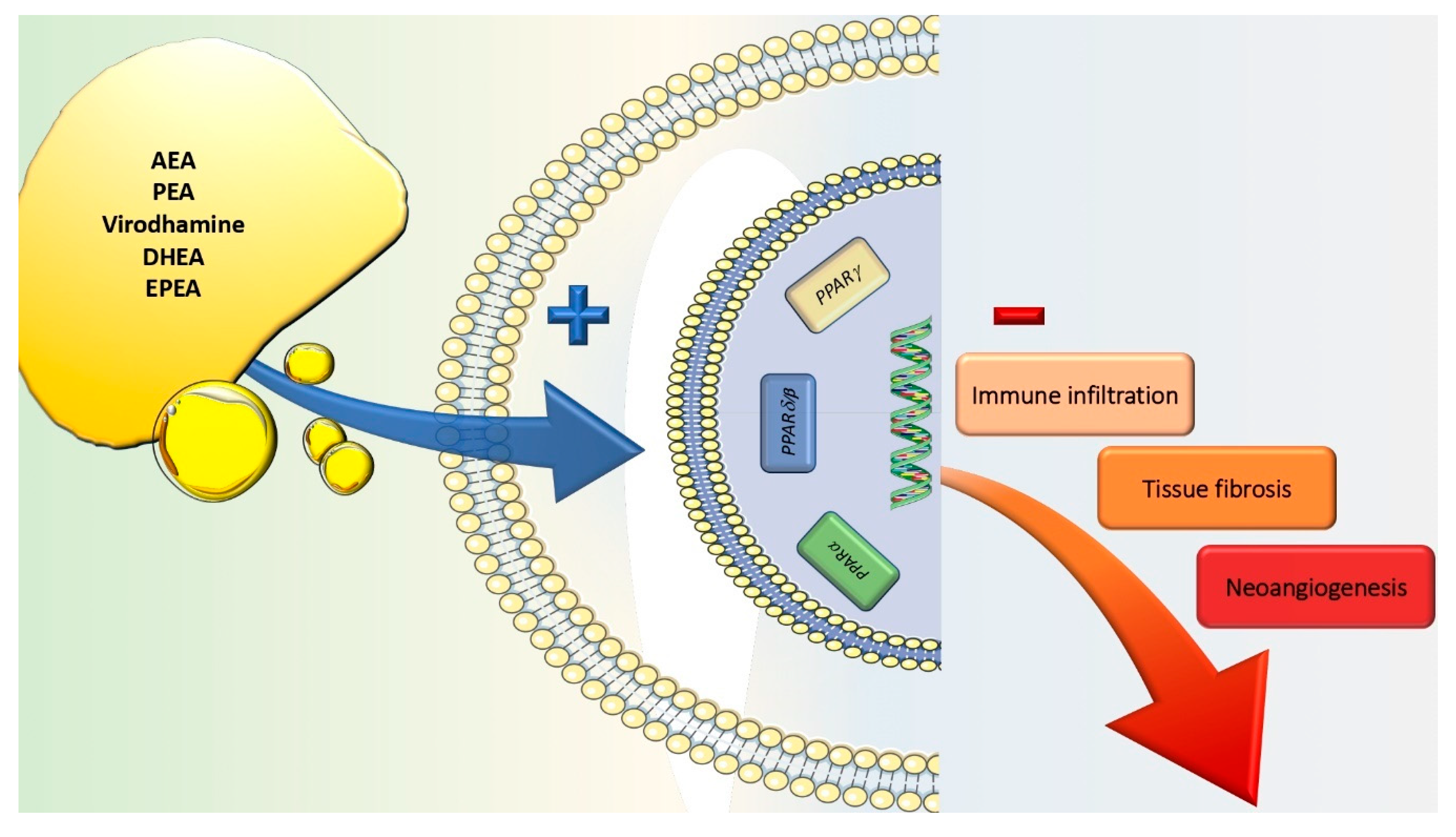
7. Peroxisome Proliferator-Activated Receptors
8. Endogenous Cannabinoid
9. ECS Modulation in Dry Eye Syndrome
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [PubMed]
- Wong, H.L.; Poon, S.H.L.; Bu, Y.; Lo, A.C.Y.; Jhanji, V.; Chan, Y.K.; Shih, K.C. A Systematic Review on Cornea Epithelial-Stromal Homeostasis. Ophthalmic Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB(1) and CB(2). Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Gaoni, Y. A Total Synthesis of Dl-Delta-1-Tetrahydrocannabinol, the Active Constituent of Hashish. J. Am. Chem. Soc. 1965, 87, 3273–3275. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, X.; Zhang, J.; Chen, C. Inhibition of COX-2 expression by endocannabinoid 2-arachidonoylglycerol is mediated via PPAR-gamma. Br. J. Pharmacol. 2011, 163, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Grim, T.W.; Ghosh, S.; Hsu, K.L.; Cravatt, B.F.; Kinsey, S.G.; Lichtman, A.H. Combined inhibition of FAAH and COX produces enhanced anti-allodynic effects in mouse neuropathic and inflammatory pain models. Pharmacol. Biochem. Behav. 2014, 124, 405–411. [Google Scholar] [CrossRef]
- Nakajima, Y.; Furuichi, Y.; Biswas, K.K.; Hashiguchi, T.; Kawahara, K.; Yamaji, K.; Uchimura, T.; Izumi, Y.; Maruyama, I. Endocannabinoid, anandamide in gingival tissue regulates the periodontal inflammation through NF-kappaB pathway inhibition. FEBS Lett. 2006, 580, 613–619. [Google Scholar] [CrossRef]
- Sancho, R.; Calzado, M.A.; Di Marzo, V.; Appendino, G.; Munoz, E. Anandamide inhibits nuclear factor-kappaB activation through a cannabinoid receptor-independent pathway. Mol. Pharmacol. 2003, 63, 429–438. [Google Scholar] [CrossRef]
- Schwarz, H.; Blanco, F.J.; Lotz, M. Anadamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J. Neuroimmunol. 1994, 55, 107–115. [Google Scholar] [CrossRef]
- Bilfinger, T.V.; Salzet, M.; Fimiani, C.; Deutsch, D.G.; Tramu, G.; Stefano, G.B. Pharmacological evidence for anandamide amidase in human cardiac and vascular tissues. Int. J. Cardiol 1998, 64 (Suppl. 1), S15–S22. [Google Scholar] [CrossRef]
- Stefano, G.B.; Bilfinger, T.V.; Rialas, C.M.; Deutsch, D.G. 2-arachidonyl-glycerol stimulates nitric oxide release from human immune and vascular tissues and invertebrate immunocytes by cannabinoid receptor 1. Pharmacol. Res. 2000, 42, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Liu, Y.; Goligorsky, M.S. Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human monocytes. J. Biol. Chem. 1996, 271, 19238–19242. [Google Scholar] [CrossRef] [PubMed]
- Lafreniere, J.D.; Kelly, M.E.M. Potential for endocannabinoid system modulation in ocular pain and inflammation: Filling the gaps in current pharmacological options | Neuronal Signaling | Portland Press. Neuronal Signal. 2018, 2, NS20170144. [Google Scholar] [CrossRef] [PubMed]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R. Endocannabinoid System in Health and Disease: Current Situation and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 3549. [Google Scholar] [CrossRef]
- Mechoulam, R.; Hanus, L.O.; Pertwee, R.; Howlett, A.C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell Neurosci. 2016, 10, 294. [Google Scholar] [CrossRef]
- Mackie, K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb. Exp. Pharmacol. 2005. [Google Scholar] [CrossRef]
- Sugiura, T.; Waku, K. 2-Arachidonoylglycerol and the cannabinoid receptors. Chem. Phys. Lipids 2000, 108, 89–106. [Google Scholar] [CrossRef]
- Berdyshev, E.V. Cannabinoid receptors and the regulation of immune response. Chem. Phys. Lipids 2000, 108, 169–190. [Google Scholar] [CrossRef]
- Straiker, A.J.; Maguire, G.; Mackie, K.; Lindsey, J. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2442–2448. [Google Scholar]
- Iribarne, M.; Torbidoni, V.; Julian, K.; Prestifilippo, J.P.; Sinha, D.; Rettori, V.; Berra, A.; Suburo, A.M. Cannabinoid receptors in conjunctival epithelium: Identification and functional properties. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4535–4544. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulou, M.; Pagoulatos, D.; Nterma, P.; Pharmakakis, N. Immunolocalization of cannabinoid receptor type 1 and CB2 cannabinoid receptors, and transient receptor potential vanilloid channels in pterygium. Mol. Med. Rep. 2017, 16, 5285–5293. [Google Scholar] [CrossRef] [PubMed]
- Suburo, A.M.; Sgroi, M.; Cubilla, M.A.; Iribarne, M.; Berra, A. Cannabinoid Receptors and Corneal Epithelium Repair. Investig. Ophthalmol. Vis. Sci. 2009, 50, 6927. [Google Scholar]
- Murataeva, N.; Miller, S.; Dhopeshwarkar, A.; Leishman, E.; Daily, L.; Taylor, X.; Morton, B.; Lashmet, M.; Bradshaw, H.; Hillard, C.J.; et al. Cannabinoid CB2R receptors are upregulated with corneal injury and regulate the course of corneal wound healing. Exp. Eye Res. 2019, 182, 74–84. [Google Scholar] [CrossRef]
- Murataeva, N.; Li, S.; Oehler, O.; Miller, S.; Dhopeshwarkar, A.; Hu, S.S.J.; Bonanno, J.A.; Bradshaw, H.; Mackie, K.; McHugh, D.; et al. Cannabinoid-Induced Chemotaxis in Bovine Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3304–3313. [Google Scholar] [CrossRef]
- Pisanti, S.; Picardi, P.; Prota, L.; Proto, M.C.; Laezza, C.; McGuire, P.G.; Morbidelli, L.; Gazzerro, P.; Ziche, M.; Das, A.; et al. Genetic and pharmacologic inactivation of cannabinoid CB1 receptor inhibits angiogenesis. Blood 2011, 117, 5541–5550. [Google Scholar] [CrossRef]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.M.; Kulkarni, P.M.; Straiker, A.J.; Thakur, G.A.; Kelly, M.E.M. Allosteric Cannabinoid Receptor 1 (CB1) Ligands Reduce Ocular Pain and Inflammation. Molecules 2020, 25, 417. [Google Scholar] [CrossRef]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.M.; Toguri, J.T.; Caldwell, M.D.; Kelly, M.E.M. The Cannabinoids Delta(8)THC, CBD, and HU-308 Act via Distinct Receptors to Reduce Corneal Pain and Inflammation. Cannabis Cannabinoid Res. 2018, 3, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bereiter, D.A.; Bereiter, D.F.; Hirata, H. Topical cannabinoid agonist, WIN55,212-2, reduces cornea-evoked trigeminal brainstem activity in the rat. Pain 2002, 99, 547–556. [Google Scholar] [CrossRef]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient Receptor Potential (TRP) Channels. Subcell. Biochem. 2018, 87, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 2003, 140, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, H.; Wang, Z.; Varadaraj, K.; Kumari, S.S.; Mergler, S.; Okada, Y.; Saika, S.; Kingsley, P.J.; Marnett, L.J.; et al. Cannabinoid receptor 1 suppresses transient receptor potential vanilloid 1-induced inflammatory responses to corneal injury. Cell Signal. 2013, 25, 501–511. [Google Scholar] [CrossRef][Green Version]
- Okada, Y.; Reinach, P.S.; Shirai, K.; Kitano-Izutani, A.; Miyajima, M.; Yamanaka, O.; Sumioka, T.; Saika, S. Transient Receptor Potential Channels and Corneal Stromal Inflammation. Cornea 2015, 34 (Suppl. 11), S136–S141. [Google Scholar] [CrossRef]
- Mergler, S.; Valtink, M.; Takayoshi, S.; Okada, Y.; Miyajima, M.; Saika, S.; Reinach, P.S. Temperature-sensitive transient receptor potential channels in corneal tissue layers and cells. Ophthalmic Res. 2014, 52, 151–159. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, Z.; Yang, H.; Zhang, F.; Reinach, P.S. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 485–493. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, H.; Wang, Z.; Mergler, S.; Liu, H.; Kawakita, T.; Tachado, S.D.; Pan, Z.; Capo-Aponte, J.E.; Pleyer, U.; et al. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J. Cell Physiol. 2007, 213, 730–739. [Google Scholar] [CrossRef]
- Nidegawa-Saitoh, Y.; Sumioka, T.; Okada, Y.; Reinach, P.S.; Flanders, K.C.; Liu, C.Y.; Yamanaka, O.; Kao, W.W.Y.; Saika, S. Impaired healing of cornea incision injury in a TRPV1-deficient mouse. Cell Tissue Res. 2018, 374, 329–338. [Google Scholar] [CrossRef]
- Okada, Y.; Reinach, P.S.; Shirai, K.; Kitano, A.; Kao, W.W.Y.; Flanders, K.C.; Miyajima, M.; Liu, H.; Zhang, J.; Saika, S. TRPV1 Involvement in Inflammatory Tissue Fibrosis in Mice. Am. J. Pathol. 2011, 178, 2654–2664. [Google Scholar] [CrossRef]
- Tomoyose, K.; Okada, Y.; Sumioka, T.; Miyajima, M.; Flanders, K.C.; Shirai, K.; Morii, T.; Reinach, P.S.; Yamanaka, O.; Saika, S. Suppression of In Vivo Neovascularization by the Loss of TRPV1 in Mouse Cornea. J. Ophthalmol. 2015, 2015, 706404. [Google Scholar] [CrossRef] [PubMed]
- Sibaev, A.; Massa, F.; Yuce, B.; Marsicano, G.; Lehr, H.A.; Lutz, B.; Goke, B.; Allescher, H.D.; Storr, M. CB1 and TRPV1 receptors mediate protective effects on colonic electrophysiological properties in mice. J. Mol. Med. 2006, 84, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.; Santha, P.; Paule, C.C.; Nagy, I. Cannabinoid 1 receptor activation inhibits transient receptor potential vanilloid type 1 receptor-mediated cationic influx into rat cultured primary sensory neurons. Neuroscience 2009, 162, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Z.; Capo-Aponte, J.E.; Zhang, F.; Pan, Z.; Reinach, P.S. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp. Eye Res. 2010, 91, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Barish, G.D.; Wang, Y.X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARgamma signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Chinetti, G.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors and inflammation: From basic science to clinical applications. Int. J. Obes. Relat. Metab. Disord. 2003, 27 (Suppl. 3), S41–S45. [Google Scholar] [CrossRef]
- Daynes, R.A.; Jones, D.C. Emerging roles of PPARs in inflammation and immunity. Nat. Rev. Immunol. 2002, 2, 748–759. [Google Scholar] [CrossRef]
- Fezza, F.; Bari, M.; Florio, R.; Talamonti, E.; Feole, M.; Maccarrone, M. Endocannabinoids, related compounds and their metabolic routes. Molecules 2014, 19, 17078–17106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.; Yang, L.; Li, M.; Li, B.; Wang, W.; Sheng, M. Decreased PPAR-gamma expression in the conjunctiva and increased expression of TNF-alpha and IL-1beta in the conjunctiva and tear fluid of dry eye mice. Mol. Med. Rep. 2014, 9, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Sarayba, M.A.; Li, L.; Tungsiripat, T.; Liu, N.H.; Sweet, P.M.; Patel, A.J.; Osann, K.E.; Chittiboyina, A.; Benson, S.C.; Pershadsingh, H.A.; et al. Inhibition of corneal neovascularization by a peroxisome proliferator-activated receptor-gamma ligand. Exp. Eye Res. 2005, 80, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Uchiyama, M.; Arima, T.; Nagasaka, S.; Igarashi, T.; Shimizu, A.; Takahashi, H. PPARalpha Agonist Suppresses Inflammation after Corneal Alkali Burn by Suppressing Proinflammatory Cytokines, MCP-1, and Nuclear Translocation of NF-kappaB. Molecules 2018, 24, 114. [Google Scholar] [CrossRef] [PubMed]
- Beauregard, C.; Brandt, P.C. Peroxisome proliferator-activated receptor agonists inhibit interleukin-1beta-mediated nitric oxide production in cultured lacrimal gland acinar cells. J. Ocul. Pharmacol. Ther. 2003, 19, 579–587. [Google Scholar] [CrossRef]
- Mu, P.Y.; Chu, C.C.; Yu, D.; Shao, Y.; Zhao, S.Z. PPARgamma: The dominant regulator among PPARs in dry eye lacrimal gland and diabetic lacrimal gland. Int. J. Ophthalmol. 2020, 13, 860–869. [Google Scholar] [CrossRef]
- Tobita, Y.; Arima, T.; Nakano, Y.; Uchiyama, M.; Shimizu, A.; Takahashi, H. Peroxisome Proliferator-Activated Receptor Beta/Delta Agonist Suppresses Inflammation and Promotes Neovascularization. Int. J. Mol. Sci. 2020, 21, 5296. [Google Scholar] [CrossRef]
- Yamanaka, O.; Miyazaki, K.; Kitano, A.; Saika, S.; Nakajima, Y.; Ikeda, K. Suppression of injury-induced conjunctiva scarring by peroxisome proliferator-activated receptor gamma gene transfer in mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 187–193. [Google Scholar] [CrossRef][Green Version]
- Saika, S.; Yamanaka, O.; Okada, Y.; Miyamoto, T.; Kitano, A.; Flanders, K.C.; Ohnishi, Y.; Nakajima, Y.; Kao, W.W.; Ikeda, K. Effect of overexpression of PPARgamma on the healing process of corneal alkali burn in mice. Am. J. Physiol. Cell Physiol. 2007, 293, C75–C86. [Google Scholar] [CrossRef]
- Uchiyama, M.; Shimizu, A.; Masuda, Y.; Nagasaka, S.; Fukuda, Y.; Takahashi, H. An ophthalmic solution of a peroxisome proliferator-activated receptor gamma agonist prevents corneal inflammation in a rat alkali burn model. Mol. Vis. 2013, 19, 2135–2150. [Google Scholar]
- Arima, T.; Uchiyama, M.; Nakano, Y.; Nagasaka, S.; Kang, D.; Shimizu, A.; Takahashi, H. Peroxisome proliferator-activated receptor alpha agonist suppresses neovascularization by reducing both vascular endothelial growth factor and angiopoietin-2 in corneal alkali burn. Sci. Rep. 2017, 7, 17763. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kobayashi, Y.; Oka, S.; Waku, K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot. Essent. Fatty Acids 2002, 66, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Dainese, E.; Oddi, S. Intracellular trafficking of anandamide: New concepts for signaling. Trends Biochem. Sci. 2010, 35, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Zoerner, A.A.; Gutzki, F.M.; Batkai, S.; May, M.; Rakers, C.; Engeli, S.; Jordan, J.; Tsikas, D. Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: A comprehensive review from an analytical and biological perspective. Biochim. Biophys. Acta 2011, 1811, 706–723. [Google Scholar] [CrossRef]
- Navarini, L.; Afeltra, A.; Afflitto, G.; Margiotta, D.P.E. Polyunsaturated fatty acids: Any role in rheumatoid arthritis? Lipids Health Dis. 2017, 16, 197. [Google Scholar] [CrossRef]
- Gomez, E.A.; Colas, R.A.; Souza, P.R.; Hands, R.; Lewis, M.J.; Bessant, C.; Pitzalis, C.; Dalli, J. Blood pro-resolving mediators are linked with synovial pathology and are predictive of DMARD responsiveness in rheumatoid arthritis. Nat. Commun. 2020, 11, 5420. [Google Scholar] [CrossRef]
- Chen, J.; Matias, I.; Dinh, T.; Lu, T.; Venezia, S.; Nieves, A.; Woodward, D.F.; Di Marzo, V. Finding of endocannabinoids in human eye tissues: Implications for glaucoma. Biochem. Biophys. Res. Commun. 2005, 330, 1062–1067. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Yang, W.; Coassin, M.; Micera, A.; Antonini, M.; Piccinni, F.; De Piano, M.; Kohler, I.; Harms, A.C.; Hankemeier, T.; et al. Signaling lipids as diagnostic biomarkers for ocular surface cicatrizing conjunctivitis. J. Mol. Med. 2020, 98, 751–760. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]
- Bereiter, D.A.; Rahman, M.; Thompson, R.; Stephenson, P.; Saito, H. TRPV1 and TRPM8 Channels and Nocifensive Behavior in a Rat Model for Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3739–3746. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Roberti, G.; Mashaghi, A.; Abud, T.B.; Pavese, D.; Bonini, S. Use of Topical Cannabinomimetic Palmitoylethanolamide in Ocular Surface Disease Associated with Antiglaucoma Medications. J. Ocul. Pharmacol. Ther. 2017, 33, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Cairns, E.A.; Toguri, J.T.; Porter, R.F.; Szczesniak, A.M.; Kelly, M.E. Seeing over the horizon—Targeting the endocannabinoid system for the treatment of ocular disease. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Green, K.; Bigger, J.F.; Kim, K.; Bowman, K. Cannabinoid penetration and chronic effects in the eye. Exp. Eye Res. 1977, 24, 197–205. [Google Scholar] [CrossRef]
- Polat, N.; Cumurcu, B.; Cumurcu, T.; Tuncer, I. Corneal endothelial changes in long-term cannabinoid users. Cutan. Ocul. Toxicol. 2018, 37, 19–23. [Google Scholar] [CrossRef]
- Toguri, J.T.; Lehmann, C.; Laprairie, R.B.; Szczesniak, A.M.; Zhou, J.; Denovan-Wright, E.M.; Kelly, M.E. Anti-inflammatory effects of cannabinoid CB(2) receptor activation in endotoxin-induced uveitis. Br. J. Pharmacol. 2014, 171, 1448–1461. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiello, F.; Gallo Afflitto, G.; Li, J.-P.O.; Martucci, A.; Cesareo, M.; Nucci, C. CannabinEYEds: The Endocannabinoid System as a Regulator of the Ocular Surface Nociception, Inflammatory Response, Neovascularization and Wound Healing. J. Clin. Med. 2020, 9, 4036. https://doi.org/10.3390/jcm9124036
Aiello F, Gallo Afflitto G, Li J-PO, Martucci A, Cesareo M, Nucci C. CannabinEYEds: The Endocannabinoid System as a Regulator of the Ocular Surface Nociception, Inflammatory Response, Neovascularization and Wound Healing. Journal of Clinical Medicine. 2020; 9(12):4036. https://doi.org/10.3390/jcm9124036
Chicago/Turabian StyleAiello, Francesco, Gabriele Gallo Afflitto, Ji-Peng Olivia Li, Alessio Martucci, Massimo Cesareo, and Carlo Nucci. 2020. "CannabinEYEds: The Endocannabinoid System as a Regulator of the Ocular Surface Nociception, Inflammatory Response, Neovascularization and Wound Healing" Journal of Clinical Medicine 9, no. 12: 4036. https://doi.org/10.3390/jcm9124036
APA StyleAiello, F., Gallo Afflitto, G., Li, J.-P. O., Martucci, A., Cesareo, M., & Nucci, C. (2020). CannabinEYEds: The Endocannabinoid System as a Regulator of the Ocular Surface Nociception, Inflammatory Response, Neovascularization and Wound Healing. Journal of Clinical Medicine, 9(12), 4036. https://doi.org/10.3390/jcm9124036




