Abstract
Donor-specific anti-human leukocyte antigen antibodies (DSA) are controversially discussed in the context of liver transplantation (LT). We investigated the relationship between the presence of DSA and the outcome after LT. All the LTs performed at our center between 1 January 2008 and 31 December 2015 were examined. Recipients < 18 years, living donor-, combined, high-urgency-, and re-transplantations were excluded. Out of 510 LTs, 113 DSA-positive cases were propensity score-matched with DSA-negative cases based on the components of the Balance of Risk score. One-, three-, and five-year survival after LT were 74.3% in DSA-positive vs. 84.8% (p = 0.053) in DSA-negative recipients, 71.8% vs. 71.5% (p = 0.821), and 69.3% vs. 64.9% (p = 0.818), respectively. Rejection therapy was more often applied to DSA-positive recipients (n = 77 (68.1%) vs. 37 (32.7%) in the control group, p < 0.001). At one year after LT, 9.7% of DSA-positive patients died due to sepsis compared to 1.8% in the DSA-negative group (p = 0.046). The remaining causes of death were comparable in both groups (cardiovascular 6.2% vs. 8.0%; p = 0.692; hepatic 3.5% vs. 2.7%, p = 0.788; malignancy 3.5% vs. 2.7%, p = 0.788). DSA seem to have an indirect effect on the outcome of adult LTs, impacting decision-making in post-transplant immunosuppression and rejection therapies and ultimately increasing mortality due to infectious complications.
1. Introduction
The clinical relevance of donor-specific anti-human leukocyte antigen antibodies (DSA) in liver transplantation (LT) has been the basis for many controversial discussions. In kidney transplantation, the negative effects of preformed and de novo DSA on patient and graft survival have been well demonstrated [1,2]. Furthermore, the presence of DSA in other solid organ transplantations, such as of the lung [3], heart [4], or pancreas [5], has been reported to be associated with inferior graft outcomes. For many years, liver grafts have been considered less vulnerable to DSA due to the graft size, dual blood supply, and the patient’s own immunological activity [6]. Since the first observations of antibody-mediated rejections (ABMR) in LT 30 years ago [7], especially recent data have led to a new perception of DSA in the context of LT. In 2016, the Banff Working Group provided a first approach on standardized (histopathological) ABMR criteria [8] and new laboratory techniques, such as the Luminex® assay, helping to achieve a more precise antibody detection, specification, and quantification [9,10]. Additionally, pathologic conditions such as T-cell-mediated rejections (TCMR) and infections can lead to an upregulation of tissue human leukocyte antigen (HLA) expression and make the liver graft more susceptible to ABMR [6,11,12].
Recent findings indicate an association between DSA and early/chronic rejections and graft injury [13,14,15,16,17,18] However, the data regarding the impact of DSA on patient and graft survival after LT are less clear [13,14,15,16,18,19,20,21,22,23].
Consequently, there is still a need for data to clarify the effects of DSA’s presence on LT outcomes. The aim of this study was to investigate the impact of DSA on patient and graft survival by means of a matched case-control analysis and to identify risk factors for inferior patient and graft outcomes.
2. Patients and Methods
2.1. Patient Recruitment and Study Design
Since January 2008, DSA were prospectively assessed by the Luminex® assay in all patients waitlisted for LT and post-LT. All patients undergoing deceased organ donor LT at the Department of Surgery, Campus Charité Mitte and Campus Virchow-Klinikum, Berlin, Germany, from 1 January 2008 to 31 December 2015, were examined, with follow-up ceasing on 1 January 2018. Combined liver-kidney, multi-visceral, high-urgency [24], and re-transplantations or patients who were under the age of 18 years at the time of LT were excluded from the analysis (Figure 1).
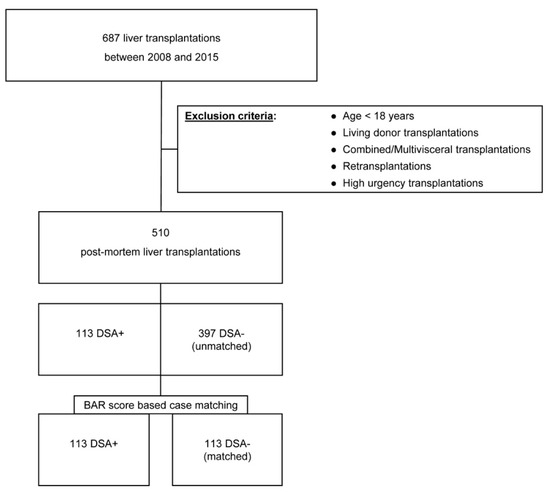
Figure 1.
Visualization of the patient selection and matching process.
The study cohort was divided into two groups (DSA-positive and DSA-negative patients) and compared regarding their demographic variables and transplant outcomes. A 1:1 propensity score matching of DSA-positive and DSA-negative individuals based on the components of the BAR (Balance of Risk) score was performed and the groups were compared. The primary endpoints were patient and graft survival regarding the presence of DSA. Secondary endpoints were the appearance of any rejection and cause of death. The study was approved by the institutional ethics committee (ID: EA4/061/17).
2.2. Data Collection and Definition of Patient and Graft Survival
Electronic records of recipient information were obtained from a prospectively collected hospital database (SAP® SE, Walldorf, Germany). Anonymous donor data were acquired from the Eurotransplant Network Information System (ENIS).
Cold ischemia time (CIT), warm ischemia time (WIT), patient survival, and graft survival were defined according to the United Network for Organ Sharing (UNOS) criteria [25,26].
2.3. Antibody Screening
The detection and specification of anti-HLA antibodies were performed using LABScreen® Mixed and Single Antigen Beads (OneLambda, West Hills, CA, USA), respectively, according to the authors’ previously published work [9]. Samples were measured on a Luminex® 200 (Luminex®, Austin, TX, USA) and analyzed using the HLA Fusion software (OneLambda). Donor-HLA-typing for HLA-A, -B, -C, -DRB1, and -DQB1 was acquired from ENIS and matched with the antibody specificities of the recipient to define the DSA. Organ recipients were routinely screened for DSA before LT listing and on the day of LT to detect preformed DSA. Postoperatively, de novo DSA screening was performed on a weekly basis until the discharge from the ICU and at the request of the treating surgeon. During routine check-ups, DSA detection was performed in patients with graft function impairment or for the follow-up of previously detected DSA. Preformed DSA were defined as antibodies present before or at the time of LT. De novo DSA were defined as newly detected antibodies after LT or antibodies against epitopes that were not present before LT in patients with preformed DSA. Data were normalized to negative control serum and all the DSA exceeding a mean fluorescence intensity (MFI) of 1000 were considered positive.
2.4. Postoperative Management
Liver function parameters were monitored daily during the entire perioperative stay and routinely examined after 3, 6, 12, 18, 24, 36, and 60 months. Postoperative care and immunosuppression (IS) regimens were provided according to a standardized protocol. In the case of LTs due to autoimmune liver disease (AILD), antibody induction with antithymocyte globulin (ATG) or IL-2 receptor antagonists was applied. The standard IS consisted of prednisolone and tacrolimus. No adaptation of IS has been made in the case of preformed DSA. In AILD recipients, the IS was extended by mycophenolic acid. Initial tacrolimus levels were set at 6–8 ng/mL in standard LTs and 8–10 ng/mL in LTs due to AILD and tapered within the first 6 months to maintenance levels of 4–6 ng/mL in all patients. Steroids were tapered off for most patients within 2 months after LT. Patients who underwent LT due to AILD were maintained on a double therapy with tacrolimus and mycophenolate.
2.5. Rejection Diagnosis and Treatment
Biopsies were performed in case of suspected rejection and routinely at 1, 3, and 5 years post LT. Histological diagnosis and the grading of allograft rejections was classified according to the Banff criteria of 2000 and later its update of 2016 [8,27]. Rejection therapy was initiated in the case of histologically proven rejection or in patients with a suspected rejection due to a significant increase in liver function parameters and after the exclusion of other causes. Initial rejection therapy consisted of steroids and increased doses of the standard IS. In ABMR, the initial immunosuppressive therapy was augmented by plasmapheresis (PP), intravenous immunoglobulins (IVIGs), and occasionally rituximab. Refractory TCMR were treated with ATG. Cases receiving rejection treatment with equivocal histological findings or without a bioptical verification of rejection were retrospectively defined as ABMR or TCMR treated “on clinical suspicion” depending on the therapy received.
2.6. Survival Stratification Model and Outcome
The BAR score achieves the most accurate outcome stratification in terms of patient survival compared to all known scores and was used for the outcome stratification and matching process [28,29,30]. The BAR score and MELD were calculated according to the UNOS and Eurotransplant formulas [28,30] and the donor risk index (DRI) according to the published formula by Feng et al. [31].
2.7. Statistical Analyses
Statistical analyses were carried out using IBM SPSS Statistics, version 25 (IBM Corporation, Armonk, NY, USA), and GraphPad Prism, version 6.01 (GraphPad Software, Inc., San Diego, CA, USA). Categorical data are presented as frequencies and percentages and were compared by Pearson’s chi-squared test. Continuous data are presented as median and interquartile range and compared by the non-parametric Mann–Whitney U test. The propensity score method with a logistic model was used to match cases and controls. The matching process included the following components of the BAR score: MELD score, donor age, recipient age, pretransplant mechanical, ventilated or organ-perfusion support, and CIT. Retransplantation was an exclusion criterion for the analysis and was not embedded in the matching process.
Patient and organ survival were analyzed by the Kaplan–Meier method and the log-rank test to compare groups. A two-sided p-value of <0.05 was considered statistically significant.
3. Results
3.1. Study Population
During the observation period, a total of 687 LTs were performed. The final analysis comprised 510 patients. Clinical data were available for all patients. DSA were identified in 113 LT recipients (22.2%). The remaining 397 patients (77.8%) were DSA-negative. Within the DSA-positive LT recipients, 49 (43.4%) individuals showed preformed, 55 (48.6%) de novo, and 9 (8%) individuals were tested positive for both preformed and de novo DSA. The median time to the first detection of de novo DSA was 13 days (9.5–20). Class II DSA were present in 50.9% (n = 28) of the patients with de novo and 10.2% of those with preformed DSA (n = 5, p < 0.001) (data listed in Supplementary Table S1).
The median follow-up was 58 (29–86) months. In the unmatched analysis, the median recipient age at LT was 54 (48–61) years in the DSA-positive group and 57 (51–62) years in the DSA-negative group (p = 0.073). The main indication of LT in both groups DSA-positive vs. DSA-negative was alcoholic cirrhosis (n = 34 (30.1%) vs. n = 188 (47.4%); p = 0.001), followed by hepatocellular carcinoma (n = 32 (28.3%), vs. n = 157 (39.5%), p = 0.029). The median BAR score at LT significantly differed between the DSA-positive and the DSA-negative group (8 (3–13) vs. 6 (3–9), p = 0.013). There was no significant difference in the MELD score between both groups (16 (9–22) in DSA-positive vs. 15 (10–20) in DSA-negative recipients, p = 0.377; Table 1). Out of all DSA-negative patients, 28% (n = 111) showed a female-to-male (F-M) donor-to-recipient mismatch. The DSA-positive group consisted of 17.7% (n = 20, p = 0.028) F-M mismatches.

Table 1.
Epidemiological, clinical, and operative data of patients who underwent LT from deceased donors between 1 January 2008, and 31 December 2015, sorted by DSA-positive versus all DSA-negative recipients (unmatched).
BAR Score Group Matching
Out of the 397 DSA-negative individuals, 113 patients were matched 1:1 with 113 DSA-positive individuals (Table 2). Median follow-up was 59 (25–101) months.

Table 2.
Epidemiological, clinical, and operative data of patients who underwent LT from deceased donors between 1 January 2008 and 31 December 2015 sorted by DSA-positive (DSA+) versus matched DSA-negative recipients (matched DSA-).
In both groups, the main indication for LT remained alcoholic cirrhosis (DSA-positive: n = 34 (30.1%) vs. 56 (49.6%) DSA-negative, p = 0.003) and hepatocellular carcinoma (DSA-positive n = 32 (28.3%) vs. 45 (39.8%) DSA-negative, p = 0.068). Organ recipients with PSC/PBC/AIH and NASH as underlying disease were more frequently represented in the DSA-positive group. No significant differences were observed between the DSA-positive and DSA-negative group regarding the BAR score (8 (3–13) vs. 7 (4–13), p = 0.619) and the distribution of its single components (MELD at LT: 16 (9–22) vs. 16 (10–29), p = 0.426; necessity of pretransplant mechanical, ventilated or organ-perfusion support: n = 6 (5.4%) vs. 4 (3.6%), p = 0.322; recipient age: 54 (48–61) vs. 57 (51–62) years, p = 0.061; and donor age: 56 (44–69) vs. 59 (45–70) years, p = 0.301). The number of high labMELD (> 35) patients was comparable in both groups (n = 20 (17.7%) vs. 16 (14.2%), p = 0.686).
DSA-positive recipients showed a shorter CIT and WIT compared to the DSA-negative recipients (CIT: 9.7 (8–11) vs. 10.2 (9–12) hours, p = 0.021, and WIT: 43 (38–50) vs. 48 (40–56) minutes; p = 0.012).
No differences in the overall courses of liver function parameters were observed between the groups, apart from only three particular lab tests being statistically significant at distinct points in time (Gamma-glutamyl transferase: on POD 7: DSA-group 245 (134–435) vs. 206 (118–308) in the control group, p = 0.047; Albumin on POD 7: 2.9 (2.7–3.1) vs. 3.0 (2.8–3.3), p = 0.010; and alkaline phosphatase 12 months after LT: 106 (81–202) vs. 97 (75–129), p = 0.043; Figure 2).
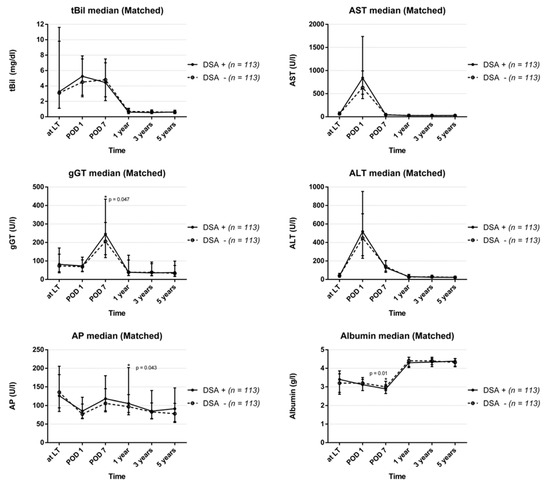
Figure 2.
Course of routine laboratory parameters after liver transplantation divided into DSA-positive and DSA-negative recipients (total bilirubin: tBil; alanine aminotransferase: ALT; serum aspartate aminotransferase: AST; gamma-glutamyl transferase: gGT; albumin: ALB; alkaline phosphatase: AP).
The median length of stay (38 (28–62) vs. 32 (23–49) days; p = 0.012) and median time spent on ICU after LT (13 (7–32) vs. 9 (6–15) days; p = 0.008) were both significantly longer in the DSA-positive patients. No significant difference was observed with regard to the F-M donor-to-recipient mismatch between the groups (DSA-positive group n = 20 (17.7%) vs. n = 30 (26.6%) in the DSA-negative group, p = 0.109).
3.2. Patient and Graft Survival
3.2.1. DSA-Positive vs. All DSA-Negative LT Recipients
The overall patient and graft survival is shown in Figure 3a. In the unmatched analysis at one year, the patient survival after LT was significantly lower in DSA-positive compared to DSA-negative allograft recipients (74.4% vs. 86.3%; p = 0.003). No significant differences in patient survival were observed at three (71.8% vs. 76.9%; p = 0.156) and five years after LT (69.3% vs. 71.8%; p = 0.400). The graft survival of DSA-positive individuals was inferior at one year after LT compared to DSA-negative individuals (73.4% vs. 80.2%; p = 0.167), with no significant difference at three (68.9% vs. 70.8%; p = 0.636) and five years (66.6% vs. 64.7%; p = 0.636) after LT.
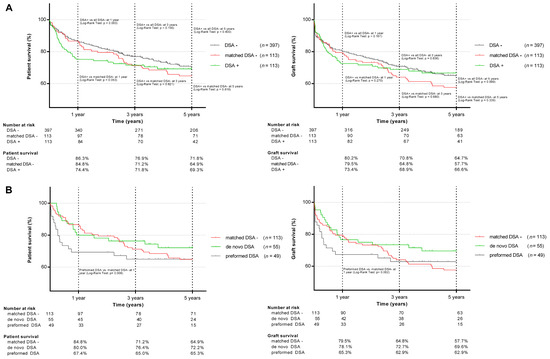
Figure 3.
Patient and graft survival in DSA-positive, DSA-negative, matched DSA-negative (A), and preformed and de novo DSA (B) liver transplant recipients.
3.2.2. DSA-Positive vs. Matched DSA-Negative LT Recipients
In the matched analysis, the one-year patient survival after LT was 74.4% in the DSA-positive vs. 84.8% (p = 0.053) in the DSA-negative cohort, 71.8% vs. 71.2% (p = 0.821) at three years and 69.3% vs. 64.9% (p = 0.818) at five years, respectively. Infections and sepsis were the major causes of death in the DSA-positive allograft recipients and significantly more frequent than in the matched DSA-negative cohort (n = 11 (9.7%) vs. n = 2 (1.8%); p = 0.046). Hospital-acquired pneumonia was the main cause of sepsis (n = 7 in DSA-positive; and n = 2 in DSA-negative patients). All the infection-related deaths occurred within the first 6 months after LT. The distribution of the remaining causes of death was comparable in both groups (cardiovascular 6.2% vs. 8.0%; p = 0.692; hepatic 3.5% vs. 2.7%, p = 0.788; malignancy 3.5% vs. 2.7%, p = 0.788). DSA-positive individuals showed an inferior one-year graft survival compared to the matched DSA-negative ones (73.4% vs. 79.5%; p = 0.270), with a non-significant reciprocal trend at five years (66.5% vs. 57.7%; p = 0.339). Retransplantations were necessary in 6 (5.3%) DSA-positive and 11 (9.7%) DSA-negative LT recipients (p = 0.207).
Significantly more patients were female (48.7%) in the DSA-positive group than in the matched DSA-negative control (29.2%, p = 0.003). The proportion was higher in LT recipients with preformed DSA (65.3% women).
3.2.3. DSA Subgroup Analysis
Recipients with preformed DSA showed an inferior one-year patient (67.4% vs. 84.8%; p = 0.008) and graft survival (65.3% vs. 79.5%; p = 0.062) compared to the matched DSA-negative individuals, with sepsis as the leading cause of death (preformed DSA: n = 7 (6.2%) vs. n = 2 (1.8%); p = 0.031; Table 3). The lower survival rate in the first year was even more pronounced in patients with preformed DSA and a MFImax of ≥5000 (n= 19, 63.2% vs. 84.8%, p = 0.022). No significant differences in mortality or graft loss were observed between individuals with de novo and matched DSA-negative individuals at any time point (Figure 3b), regardless of the MFI level.

Table 3.
Reasons for one-year graft loss and mortality in preformed DSA, de novo DSA, and matched DSA-negative (matched DSA-) LT organ recipients a.
3.3. Rejections and Rejection Treatment
Within the first year after LT, biopsies were performed in 72 DSA-positive (63.7%) and 127 DSA-negative patients (32.0%, p < 0.001). Rejection therapies were more frequently conducted in the DSA-positive (n = 77, 68.1%) than in the DSA-negative group (n = 135, 34.0%, p < 0.001; Table 4). In the matched cohorts, ABMR were histologically confirmed in 2 (1.8%) DSA-positive cases and no DSA-negative cases (p = 0.155). ABMR treatment was applied in 41 (36.3%) DSA-positive and in 2 (1.8%, p < 0.001) DSA-negative LT recipients. TCMR were histologically confirmed in 59 (52.2%) DSA-positive and 34 (30.1%, p < 0.001) DSA-negative recipients. In total, 71 (62.8%) DSA-positive and 37 (32.7%, p < 0.001) DSA-negative individuals received TCMR therapy. The most common rejection therapy was a combination of steroids and increase in the standard IS dose (DSA-positive group: n = 70 (61.9%) vs. 37 (32.7%) DSA-negative group, p < 0.001). The overall number of treated ABMR and TCMR was comparable in the LT recipients with preformed and de novo DSA (Table 5). Patients with de novo DSA were more often treated for biopsy-proven TCMR (n = 35, 63.6%) than patients with preformed DSA (n = 19, 38.8%, p = 0.011) and without DSA (n = 34, 30.1%, p < 0.001). Six DSA-positive (5.3%) and 3 DSA-negative patients (2.7%, p = 0.307) were treated for TCMR on clinical suspicion without histological verification. In two (1.8%) of these DSA-positive patients, rejection therapy was additionally augmented by PP/IVIG due to suspected refractory rejection and the presence of preformed DSA. In one DSA-positive patient (0.9%) with preformed DSA, PP/IVIG therapy was applied without biopsy.

Table 4.
Comparison of rejections and rejection treatments in LT organ recipients within the first postoperative year sorted by DSA-positive (DSA+) versus DSA-negative (DSA-) and matched DSA-negative (matched DSA-) recipients.

Table 5.
Comparison of rejections and rejection treatments in LT organ recipients within the first postoperative year sorted by patients with de novo DSA versus preformed DSA and matched DSA-negative (matched DSA) recipients.
Patients with preformed DSA received the first rejection therapy significantly earlier compared to patients with de novo DSA (6 (5–16) vs. 11.5 (7–18) days; p = 0.024) and DSA-negative patients (9 (7–23) days, p = 0.003).
4. Discussion
This study demonstrates that especially preformed DSA are associated with an impaired (one-year) patient survival after LT, and sepsis-related mortality needs to be considered as a major cause. No remarkable adverse effects on graft survival were observed. Our data thereby indicate that DSA presence may have an indirect effect on the outcome in adult LTs by interfering with the diagnosis of rejections and the decision-making in post-transplant IS and rejection therapies.
The incidence of DSA (22.2%) in our study is within the range of the published data. We observed 9.6% preformed and 10.8% de novo DSA in our cohort, which is comparable with previous reports (preformed: 4.7–22.2% [13,19,32]; de novo: 8–19.9% [16,19,21,22]). Similar to other authors, the DSA MFI threshold of our laboratory is >1000 [14,18,22]. This cut-off value lies within the lower range of published thresholds (e.g., Musat et al. 300 [15], Koch et al. ≥ 1500 [20], Taner et al. ≥ 2000 [32], Kaneku et al. ≥ 5000 [21]). The distribution of antibody- classes in preformed DSA (Supplementary Table S1) showed a predominance of class I-DSA. However, the proportion of antibody classes in preformed DSA varies in previous reports, and at this point we cannot provide an adequate explanation for this finding. Out of 184 patients with preformed DSA, O’Leary et al. reported n = 84 (45.7%) class I-, and n = 50 (27.2%) class II-DSA [13]. As reported by Tamura et al. [33], 6/8 (75%) patients with preformed DSA presented solely class II-, and none presented with class I-DSA only. Vandevoorde et al. [19] reported 14 patients with preformed DSA, with n = 3 (21.4%) having class I- and n = 5 (35.7%) having class II-DSA alone.
As there is no clear evidence on the effects of DSA in LT, we planned our analysis under consideration of potential pitfalls. First, we eliminated confounding risk factors for poor transplant outcome, such as high-urgency, multivisceral, and retransplantations [34,35,36] to achieve more homogeneous recipient groups in terms of demographic and clinical parameters. In a second step, we performed a 1:1 propensity score matching and assigned for each DSA-positive LT recipient a DSA-negative control with a similar predicted survival by the means of the BAR score and its individual components. For our analysis, we used the BAR score, as it achieves the highest accuracy in predicting the outcome after LT compared to other score systems such as MELD, D-MELD (donor age multiplied by recipient MELD), and DRI [28,29,37]. It consists of six items and includes key LT survival predictors as donor, recipient, and graft factors and is therefore very convenient in daily use. While some scores such as the DRM (donor to recipient model, 13 items) and the SOFT (survival outcome following liver transplant; 18 items) seem to outperform the BAR score in terms of the long-term survival [38], the BAR score has the best composition of usability (number of items) and accuracy and was therefore used for our analysis [28].
We observed two known sex-related effects in the analysis. First, the DSA-positive group consisted of significantly more women (48.7%) than the matched DSA-negative control (29.2%). This observation was even more evident in LT recipients with preformed DSA (65.3% women). The result is in accordance with the published data [13,15,32] and explained by previous pregnancies as the main trigger for anti-HLA antibodies prior to solid organ transplantation [39,40,41,42].
The second observed sex-related effect refers to the lower rate of LTs due to alcoholic cirrhosis in the DSA-positive group. The main indication for LT in men is still alcoholic liver cirrhosis [43,44], and no publication so far had linked alcohol consumption in LT recipients with lower HLA antibody development. Hence, this result is more likely to reflect an epidemiological reality than an immunological origin.
Female donor-to-male recipient mismatch is another possible risk factor for graft loss [45]. However, in our analysis F-M donor-to-recipient mismatches seem not to be relevant factors in graft and patient survival. Female-to-male mismatch was more present in the DSA-negative cohort, which showed better organ and patient survival rates. Second, within the F-M-mismatched patients, only those with preformed DSA showed a significantly lower patient (p = 0.004) and graft survival (p = 0.001) compared to matched DSA-negative recipients after one year.
While we observed a higher rate of autoimmune liver diseases (AILD) and cryptogenic cirrhosis or NASH in the DSA-positive cohort, this was not found to have a negative impact on the outcome. In fact, in some studies AILD and cryptogenic cirrhosis/NASH have been shown to achieve a favorable graft and patient survival [46,47,48]. This also applied for the AILD patients in our study cohort (patient survival at one and five years: both 85.7%). DSA-positive AILD patients showed a non-significant inferior patient survival compared to the DSA-negative AILD patients at one and five years (both 76.9% vs. 100%, p = 0.156).
DSA-positive LT recipients showed significantly shorter CIT and WIT compared with the matched DSA-negative control. An increase in these parameters is associated with a longer postoperative stay [49] and inferior transplant outcomes [34,50,51]. In our observation cohort, however, the DSA-positive patients showed a significantly longer ICU- and postoperative stay and worse short-term survival compared to DSA-negative LT recipients. The longer hospital stay in our cohort was mostly related to a prolonged rejection treatment in DSA-positive patients. No relevant differences in liver function parameters were observed between the groups, though three parameters differed at distinct time points without clinical correlates. Possible explanations for this result may be that DSA have no direct effect on liver function parameters, or the effects of DSA presence cannot be measured by the means of such routine liver function tests. In both situations, the results support the theory of some authors that the commonly used laboratory markers of the liver function are of limited predictive value in the interpretation of the course and prognosis of liver grafts [52,53].
With regard to patient and organ survival, we observed a significant difference in the one-year patient survival between the DSA-positive group and the unmatched DSA-negative control (p = 0.003). After the group matching, this effect was less pronounced and marginally missed the significance threshold (p = 0.053). However, a subgroup analysis revealed a significant correlation between the detection of preformed DSA and inferior patient survival after LT (p = 0.008). Regarding the 3-year and 5-year patient and graft survival, no significant differences were observed, regardless of the matching. Similarly, O’Leary et al. showed an association between preformed DSA and patient death [13] and, more recently, Tamura et al. [33] observed an inferior 90-day survival in living-donor LT recipients with preformed DSA. In accordance to O’Leary et al. [13], patients with high MFI (≥ 5000) preformed DSA showed an even more pronounced inferior survival compared to matched DSA-negative individuals (p = 0.022).
Additionally, Kaneku et al. have described the compromising effect of de novo DSA on patient and graft survival in the first year after LT [21]. In contrast to these results, the one-year survival of LT recipients with de novo DSA showed comparable outcomes to the matched DSA-negative control in our study. In turn, some authors could not confirm a correlation between DSA and inferior outcomes after LT [19,20]. These differences in study results may be caused by heterogeneous patient cohorts (analyzing first LTs, re-LTs and living donor LTs together), varying sample sizes, the use of different DSA detection methods, as well as the lack of a uniform definition for ABMR in LT.
Herein, this study is among those with the largest number of cases analyzed and the most recent observation period. Furthermore, all the DSA detections were performed on the Luminex® platform, which allows highly accurate DSA determination. Over the last decade, this method has shown its great value in antibody assessment compared to older techniques, such as the complement-dependent cytotoxicity test (CDC) or ELISA. Not only does it provide a higher sensitivity and specificity, but also a more detailed HLA antibody specification [9,54]. This has led to a broader understanding of DSA pathophysiology and immunohistology.
With respect to patient survival within the first year after LT, our findings strongly support the validity of the previously published results regarding the impact of DSA in LT. The rate of suspected rejections and performed biopsies, and especially histologically confirmed TCMR, was higher in the DSA-positive cohort than in the DSA-negative control group.
Furthermore, a remarkable finding of this study is that DSA patients have been treated significantly more often for (suspected) rejections. Especially in cases with the unclear or steroid-resistant deterioration of liver function and equivocal or missing biopsies, rejection therapy was augmented on clinical suspicion in the presence of DSA. In addition, patients with preformed DSA were treated in a highly vulnerable post-LT phase (6 days vs. 9 days in the DSA-negative cohort; p = 0.003). Another major finding of this study is that sepsis was observed significantly more often in DSA-positive LT-recipients and was the main cause of death in this cohort (p = 0.046). Koch et al. recently described sepsis as a relevant cause of death in DSA-positive LT recipients [20]. All our 11 DSA-positive patients who succumbed to death due to sepsis received cytoreductive therapy consisting of either IVIGs, PP, ATG, Rituximab, or the combination of two or more.
This result is of utmost importance in the controversial discussion on the role of DSA in LT. We observed that the initiation or augmentation of rejection therapies in DSA-positive LT recipients with unclear delayed/impaired liver function may result in increased mortality due to infectious complications. This effect seems most pronounced in the first year after LT, particularly due to the increased vulnerability of LT recipients in this phase after transplantation, whereas the mid-term and long-term results were comparable for DSA-negative LT recipients.
Certainly, our study has limitations. First, the single-center, observational, retrospective study design has limitations that we are well aware of, so that our findings cannot be immediately extrapolated to all LTs as we excluded also high-urgency and re-transplantations. Nonetheless, this design ensured a relatively low heterogeneity regarding the clinical interpretation and therapeutic management of DSA detection. Second, the used MFI threshold of >1000 was at the lower end of previous publications. However, no international MFI threshold standard has been defined so far, and the MFIs in our analysis were considerably above the detection limit (4332 (1470–7336)).
Third, the used BAR score for matching is not yet an established and validated method. Despite the high predictive power of the BAR score regarding survival in LT, such matching can also cause a selection bias and may also explain the observation of reciprocal results in the long-term course of the examined matched collectives. Given the study’s retrospective design, the therapeutic decision-making for rejection treatment is hard to reconstruct: some rejection treatment decisions were performed on a “ex juvantibus” basis and rejection protocols also changed throughout the follow-up. Additionally, histological samples taken before 2017 were rarely stained for C4d and no reevaluation according to the Banff 2016 criteria was performed. Although a reevaluation of the histological samples may have provided deeper understanding of the pathology of DSA in LT, it would have no impact on the past decision making. An important point is that the fear of DSA, but not actual ABMR, led to more robust immunosuppression treatments, which caused increased rates of sepsis-related death. Due to methodological issues at the time period analyzed, it was not possible to provide further information about complement-binding DSA or DSA subclasses. We decided not to include data on HLA classes and subclasses in the analysis, as a further breakdown would only increase the probability of a type I error and dilute the focus of the manuscript.
On the other hand, important insights into the role of DSA in LT were gained. While the immunological component for inferior short-term outcome after LT delivered by DSA cannot be excluded, our observation implies that immunosuppressive (over-) treatment notably increased morbidity due to infectious complications. Apparently, the trigger to initiate or increase rejection therapies in DSA-positive patients with equivocal histological findings and/or unclear deterioration of liver function is dramatically low due to a lack of clear diagnostic and therapeutic guidelines. Thus, the interpretation of DSA in LT should be considered very carefully.
We therefore propose to warily investigate suspected ABMR histologically before the initiation or augmentation of rejection treatment. Future steps need to be the development of a common consensus regarding MFI thresholds and simplified ABMR guidelines to better identify patients who may benefit from DSA detection and ABMR treatment in the context of LT.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/12/3986/s1: Table S1: DSA characteristics of 113 DSA-positive patients who underwent LT from deceased donors between 1 January 2008, and 31 December 2015.
Author Contributions
Conceptualization, T.D., S.Ü., and R.Ö.; methodology, T.D., S.Ü., R.Ö.; validation, T.D. and S.Ü.; formal analysis, T.D., S.Ü., and M.J.; investigation, T.D. and S.Ü.; resources, T.D., S.Ü. and N.L.; data curation, T.D., S.Ü., P.V.R., L.W., and N.L.; writing—original draft preparation, T.D. and S.Ü.; writing—review and editing, R.Ö., N.L., M.J.; supervision, R.Ö., N.L., D.E., F.T., C.D., M.B., S.C., S.G.-K., W.S., M.S., P.R., and J.P.; project administration, T.D. and R.Ö. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité—Universitätsmedizin Berlin. We gratefully thank Helena Ganterer and Jantje Black for proof-reading and editing the final manuscript. Any remaining errors are the responsibility of the authors.
Conflicts of Interest
The authors of this manuscript have no conflict of interest to disclose. The authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Nankivell, B.J.; Kuypers, D.R. Diagnosis and prevention of chronic kidney allograft loss. Lancet 2011, 378, 1428–1437. [Google Scholar] [CrossRef]
- Worthington, J.E.; Martin, S.; Al-Husseini, D.M.; Dyer, P.A.; Johnson, R.W. Posttransplantation production of donor HLA-specific antibodies as a predictor of renal transplant outcome. Transplantation 2003, 75, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.J.; Glanville, A.R.; Aboyoun, C.; Belperio, J.; Benden, C.; Berry, G.J.; Hachem, R.; Hayes, D., Jr.; Neil, D.; Reinsmoen, N.L.; et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2016, 35, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Hodges, A.M.; Lyster, H.; McDermott, A.; Rice, A.J.; Smith, J.D.; Rose, M.L.; Banner, N.R. Late antibody-mediated rejection after heart transplantation following the development of de novo donor-specific human leukocyte antigen antibody. Transplantation 2012, 93, 650–656. [Google Scholar] [CrossRef]
- de Kort, H.; Mallat, M.J.; van Kooten, C.; de Heer, E.; Brand-Schaaf, S.H.; van der Wal, A.M.; Roufosse, C.; Roelen, D.L.; Bruijn, J.A.; Claas, F.H.; et al. Diagnosis of early pancreas graft failure via antibody-mediated rejection: Single-center experience with 256 pancreas transplantations. Am. J. Transplant. 2014, 14, 936–942. [Google Scholar] [CrossRef]
- Del Bello, A.; Congy-Jolivet, N.; Danjoux, M.; Muscari, F.; Kamar, N. Donor-specific antibodies and liver transplantation. Hum. Immunol. 2016, 77, 1063–1070. [Google Scholar] [CrossRef]
- Demetris, A.J.; Jaffe, R.; Tzakis, A.; Ramsey, G.; Todo, S.; Belle, S.; Esquivel, C.; Shapiro, R.; Markus, B.; Mroczek, E.; et al. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. Am. J. Pathol. 1988, 132, 489–502. [Google Scholar]
- Demetris, A.J.; Bellamy, C.; Hubscher, S.G.; O’Leary, J.; Randhawa, P.S.; Feng, S.; Neil, D.; Colvin, R.B.; McCaughan, G.; Fung, J.J.; et al. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am. J. Transplant. 2016, 16, 2816–2835. [Google Scholar] [CrossRef]
- Lachmann, N.; Todorova, K.; Schulze, H.; Schonemann, C. Luminex((R)) and its applications for solid organ transplantation, hematopoietic stem cell transplantation, and transfusion. Transfus. Med. Hemother. 2013, 40, 182–189. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Demetris, A.J.; Friedman, L.S.; Gebel, H.M.; Halloran, P.F.; Kirk, A.D.; Knechtle, S.J.; McDiarmid, S.V.; Shaked, A.; Terasaki, P.I.; et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am. J. Transplant. 2014, 14, 779–787. [Google Scholar] [CrossRef]
- Steinhoff, G.; Wonigeit, K.; Pichlmayr, R. Analysis of sequential changes in major histocompatibility complex expression in human liver grafts after transplantation. Transplantation 1988, 45, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Takacs, L.; Szende, B.; Monostori, E.; Rot, A.; Lapis, K.; Szecseny, A.; Ando, I. Expression of HLA-DR antigens on bileduct cells of rejected liver transplant. Lancet 1983, 2, 1500. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Kaneku, H.; Jennings, L.W.; Banuelos, N.; Susskind, B.M.; Terasaki, P.I.; Klintmalm, G.B. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Liver Transplant. 2013, 19, 973–980. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.G.; Kaneku, H.; Demetris, A.J.; Marr, J.D.; Shiller, S.M.; Susskind, B.M.; Tillery, G.W.; Terasaki, P.I.; Klintmalm, G.B. Antibody-mediated rejection as a contributor to previously unexplained early liver allograft loss. Liver Transplant. 2014, 20, 218–227. [Google Scholar] [CrossRef]
- Musat, A.I.; Pigott, C.M.; Ellis, T.M.; Agni, R.M.; Leverson, G.E.; Powell, A.J.; Richards, K.R.; D’Alessandro, A.M.; Lucey, M.R. Pretransplant donor-specific anti-HLA antibodies as predictors of early allograft rejection in ABO-compatible liver transplantation. Liver Transplant. 2013, 19, 1132–1141. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Kaneku, H.; Banuelos, N.; Jennings, L.W.; Klintmalm, G.B.; Terasaki, P.I. Impact of IgG3 subclass and C1q-fixing donor-specific HLA alloantibodies on rejection and survival in liver transplantation. Am. J. Transplant. 2015, 15, 1003–1013. [Google Scholar] [CrossRef]
- Kaneku, H.; O’Leary, J.G.; Taniguchi, M.; Susskind, B.M.; Terasaki, P.I.; Klintmalm, G.B. Donor-specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with chronic rejection and graft loss after liver transplantation. Liver Transplant. 2012, 18, 984–992. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Kaneku, H.; Susskind, B.M.; Jennings, L.W.; Neri, M.A.; Davis, G.L.; Klintmalm, G.B.; Terasaki, P.I. High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection Postliver transplant. Am. J. Transplant. 2011, 11, 1868–1876. [Google Scholar] [CrossRef]
- Vandevoorde, K.; Ducreux, S.; Bosch, A.; Guillaud, O.; Hervieu, V.; Chambon-Augoyard, C.; Poinsot, D.; Andre, P.; Scoazec, J.Y.; Robinson, P.; et al. Prevalence, risk factors, and impact of donor-specific alloantibodies after adult liver transplantation. Liver Transplant. 2018. [Google Scholar] [CrossRef]
- Koch, M.; Marget, M.; Sterneck, M.; Fischer, L.; Thude, H.; Nashan, B. Limited impact of pre-existing donor specific HLA-antibodies (DSA) on long term allograft survival after first adult liver transplantation. Hum. Immunol. 2018, 79, 545–549. [Google Scholar] [CrossRef]
- Kaneku, H.; O’Leary, J.G.; Banuelos, N.; Jennings, L.W.; Susskind, B.M.; Klintmalm, G.B.; Terasaki, P.I. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am. J. Transplant. 2013, 13, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, A.; Congy-Jolivet, N.; Danjoux, M.; Muscari, F.; Lavayssiere, L.; Esposito, L.; Cardeau-Desangles, I.; Guitard, J.; Dorr, G.; Milongo, D.; et al. De novo donor-specific anti-HLA antibodies mediated rejection in liver-transplant patients. Transpl. Int. 2015, 28, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, A.; Congy-Jolivet, N.; Muscari, F.; Lavayssiere, L.; Esposito, L.; Cardeau-Desangles, I.; Guitard, J.; Dorr, G.; Suc, B.; Duffas, J.P.; et al. Prevalence, incidence and risk factors for donor-specific anti-HLA antibodies in maintenance liver transplant patients. Am. J. Transplant. 2014, 14, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Eurotransplant Foundation International. Eurotransplant Manual Version 5.15, Chapter 5—ET Liver Allocation System (ELAS); Eurotransplant Foundation International: Leiden, The Netherlands, 2019. [Google Scholar]
- Leppke, S.; Leighton, T.; Zaun, D.; Chen, S.C.; Skeans, M.; Israni, A.K.; Snyder, J.J.; Kasiske, B.L. Scientific Registry of Transplant Recipients: Collecting, analyzing, and reporting data on transplantation in the United States. Transplant. Rev. (Orlando) 2013, 27, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.M.; Sullivan, B.E. Regulatory oversight in transplantation: There and back again. JAMA Surg. 2013, 148, 997–998. [Google Scholar] [CrossRef] [PubMed]
- Demetris, A.; Adams, D.; Bellamy, C.; Blakolmer, K.; Clouston, A.; Dhillon, A.P.; Fung, J.; Gouw, A.; Gustafsson, B.; Haga, H.; et al. Update of the International Banff Schema for Liver Allograft Rejection: Working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. Hepatology 2000, 31, 792–799. [Google Scholar] [CrossRef]
- Dutkowski, P.; Oberkofler, C.E.; Slankamenac, K.; Puhan, M.A.; Schadde, E.; Mullhaupt, B.; Geier, A.; Clavien, P.A. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann. Surg. 2011, 254, 745–753. [Google Scholar] [CrossRef]
- Dutkowski, P.; Oberkofler, C.E.; Bechir, M.; Mullhaupt, B.; Geier, A.; Raptis, D.A.; Clavien, P.A. The model for end-stage liver disease allocation system for liver transplantation saves lives, but increases morbidity and cost: A prospective outcome analysis. Liver Transplant. 2011, 17, 674–684. [Google Scholar] [CrossRef]
- Wiesner, R.; Edwards, E.; Freeman, R.; Harper, A.; Kim, R.; Kamath, P.; Kremers, W.; Lake, J.; Howard, T.; Merion, R.M.; et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003, 124, 91–96. [Google Scholar] [CrossRef]
- Feng, S.; Goodrich, N.P.; Bragg-Gresham, J.L.; Dykstra, D.M.; Punch, J.D.; DebRoy, M.A.; Greenstein, S.M.; Merion, R.M. Characteristics associated with liver graft failure: The concept of a donor risk index. Am. J. Transplant. 2006, 6, 783–790. [Google Scholar] [CrossRef]
- Taner, T.; Gandhi, M.J.; Sanderson, S.O.; Poterucha, C.R.; De Goey, S.R.; Stegall, M.D.; Heimbach, J.K. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am. J. Transplant. 2012, 12, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Tohyama, T.; Watanabe, J.; Nakamura, T.; Ueno, Y.; Inoue, H.; Honjo, M.; Sakamoto, K.; Takai, A.; Ogawa, K.; et al. Preformed donor-specific antibodies are associated with 90-day mortality in living-donor liver transplantation. Hepatol. Res. 2019, 49, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Strasberg, S.M.; Howard, T.K.; Molmenti, E.P.; Hertl, M. Selecting the donor liver: Risk factors for poor function after orthotopic liver transplantation. Hepatology 1994, 20 Pt 1, 829–838. [Google Scholar] [CrossRef]
- Brandsaeter, B.; Hockerstedt, K.; Friman, S.; Ericzon, B.G.; Kirkegaard, P.; Isoniemi, H.; Olausson, M.; Broome, U.; Schmidt, L.; Foss, A.; et al. Fulminant hepatic failure: Outcome after listing for highly urgent liver transplantation-12 years experience in the nordic countries. Liver Transplant. 2002, 8, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Robles, S.; Russo, M.J.; Halazun, K.J.; Woodland, D.C.; Witkowski, P.; Ratner, L.E.; Hardy, M.A. The combined organ effect: Protection against rejection? Ann. Surg. 2008, 248, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Linecker, M.; Kron, P.; Gyori, G.; De Oliveira, M.L.; Mullhaupt, B.; Clavien, P.A.; Dutkowski, P. Risk Assessment in High- and Low-MELD Liver Transplantation. Am. J. Transplant. 2017, 17, 1050–1063. [Google Scholar] [CrossRef]
- de Boer, J.D.; Putter, H.; Blok, J.J.; Alwayn, I.P.J.; van Hoek, B.; Braat, A.E. Predictive Capacity of Risk Models in Liver Transplantation. Transplant. Direct 2019, 5, e457. [Google Scholar] [CrossRef]
- Del Bello, A.; Congy-Jolivet, N.; Audry, B.; Antoine, C.; Esposito, L.; Hebral, A.L.; Kamar, N. Impact of transplant accessibility for sensitized patients by avoiding unacceptable antigens. Liver Transplant. 2017, 23, 880–886. [Google Scholar] [CrossRef]
- Dumortier, J.; Dedic, T.; Erard-Poinsot, D.; Rivet, C.; Guillaud, O.; Chambon-Augoyard, C.; Bosch, A.; Lachaux, A.; Couchonnal, E.; Thaunat, O.; et al. Pregnancy and donor-specific HLA-antibody-mediated rejection after liver transplantation: “Liaisons dangereuses”? Transpl. Immunol. 2019, 54, 47–51. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Coutance, G.; Salem, J.E.; Ouldamar, S.; Lebreton, G.; Combes, A.; Amour, J.; Laali, M.; Leprince, P.; Varnous, S. Effect of recipient gender and donor-specific antibodies on antibody-mediated rejection after heart transplantation. Am. J. Transplant. 2019, 19, 1160–1167. [Google Scholar] [CrossRef]
- Higgins, R.; Lowe, D.; Daga, S.; Hathaway, M.; Williams, C.; Lam, F.T.; Kashi, H.; Tan, L.C.; Imray, C.; Fletcher, S.; et al. Pregnancy-induced HLA antibodies respond more vigorously after renal transplantation than antibodies induced by prior transplantation. Hum. Immunol. 2015, 76, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.; Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Skeans, M.A.; Noreen, S.M.; Foutz, J.; Miller, E.; Snyder, J.J.; et al. OPTN/SRTR 2018 Annual Data Report: Liver. Am. J. Transplant. 2020, 20, 193–299. [Google Scholar] [CrossRef] [PubMed]
- Marroni, C.A.; Fleck, A.M., Jr.; Fernandes, S.A.; Galant, L.H.; Mucenic, M.; de Mattos Meine, M.H.; Mariante-Neto, G.; Brandao, A.B.M. Liver transplantation and alcoholic liver disease: History, controversies, and considerations. World J. Gastroenterol. 2018, 24, 2785–2805. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Giovanardi, F.; Melandro, F.; Larghi Laureiro, Z.; Merli, M.; Lattanzi, B.; Hassan, R.; Rossi, M.; Mennini, G. Donor-to-recipient gender match in liver transplantation: A systematic review and meta-analysis. World J. Gastroenterol. 2018, 24, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Karam, V.; Delvart, V.; O’Grady, J.; Mirza, D.; Klempnauer, J.; Castaing, D.; Neuhaus, P.; Jamieson, N.; Salizzoni, M.; et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J. Hepatol. 2012, 57, 675–688. [Google Scholar] [CrossRef]
- Ilyas, J.A.; O’Mahony, C.A.; Vierling, J.M. Liver transplantation in autoimmune liver diseases. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Thuluvath, P.J.; Hanish, S.; Savva, Y. Liver Transplantation in Cryptogenic Cirrhosis: Outcome Comparisons Between NASH, Alcoholic, and AIH Cirrhosis. Transplantation 2018, 102, 656–663. [Google Scholar] [CrossRef]
- Pan, E.T.; Yoeli, D.; Galvan, N.T.N.; Kueht, M.L.; Cotton, R.T.; O’Mahony, C.A.; Goss, J.A.; Rana, A. Cold ischemia time is an important risk factor for post-liver transplant prolonged length of stay. Liver Transplant. 2018, 24, 762–768. [Google Scholar] [CrossRef]
- Mathur, A.K.; Heimbach, J.; Steffick, D.E.; Sonnenday, C.J.; Goodrich, N.P.; Merion, R.M. Donation after cardiac death liver transplantation: Predictors of outcome. Am. J. Transplant. 2010, 10, 2512–2519. [Google Scholar] [CrossRef]
- Jochmans, I.; Fieuws, S.; Tieken, I.; Samuel, U.; Pirenne, J. The Impact of Implantation Time During Liver Transplantation on Outcome: A Eurotransplant Cohort Study. Transplant. Direct 2018, 4, e356. [Google Scholar] [CrossRef]
- Jara, M.; Dziodzio, T.; Malinowski, M.; Luttgert, K.; Nikolov, R.; Ritschl, P.V.; Ollinger, R.; Pratschke, J.; Stockmann, M. Prospective Assessment of Liver Function by an Enzymatic Liver Function Test to Estimate Short-Term Survival in Patients with Liver Cirrhosis. Dig. Dis. Sci. 2019, 64, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.; Malinowski, M.; Luttgert, K.; Schott, E.; Neuhaus, P.; Stockmann, M. Prognostic value of enzymatic liver function for the estimation of short-term survival of liver transplant candidates: A prospective study with the LiMAx test. Transpl. Int. 2015, 28, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Zachary, A.A.; Vega, R.M.; Lucas, D.P.; Leffell, M.S. HLA antibody detection and characterization by solid phase immunoassays: Methods and pitfalls. Methods Mol. Biol. 2012, 882, 289–308. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).