Relationship between Standard Uptake Values of Positron Emission Tomography/Computed Tomography and Salivary Metabolites in Oral Cancer: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Metabolomics Data
2.3. FDG PET/CT Protocol
2.4. Collection of SUVmax Data
2.5. Statistical Analyses
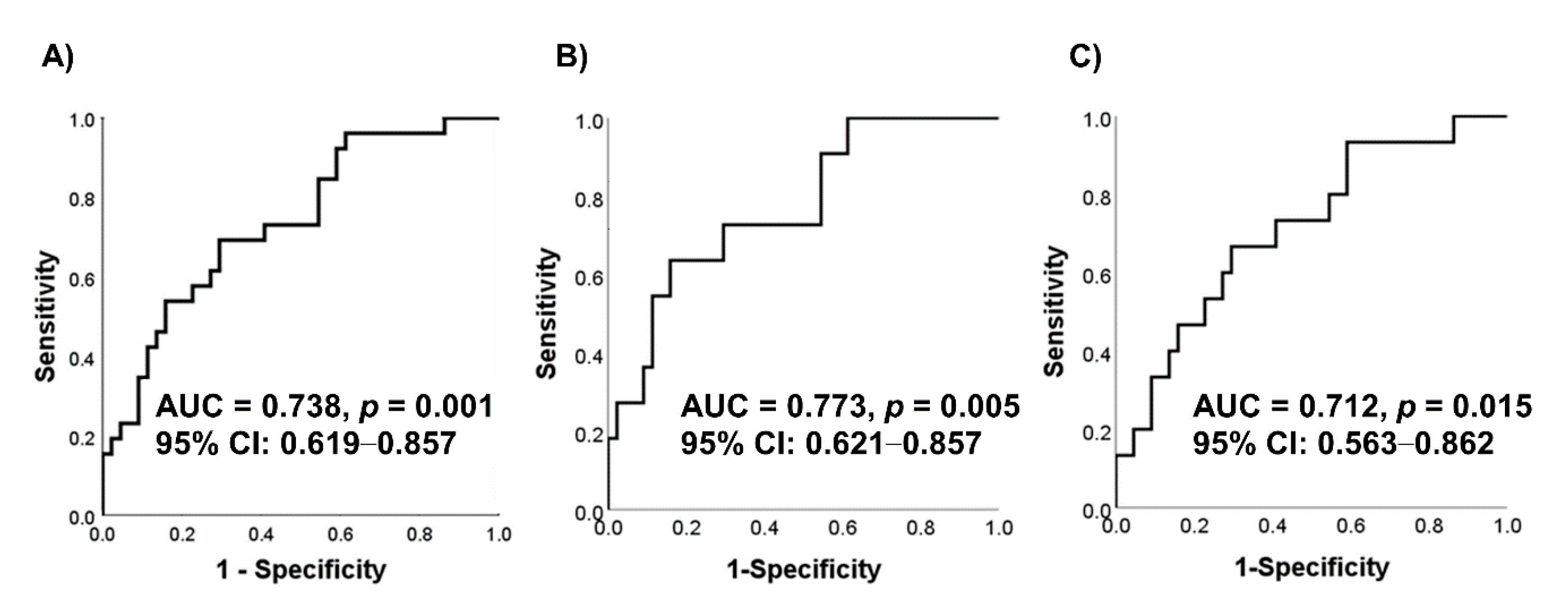
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pasha, M.A.; Marcus, C.; Fakhry, C.; Kang, H.; Kiess, A.P.; Subramaniam, R.M. FDG PET/CT for Management and Assessing Outcomes of Squamous Cell Cancer of the Oral Cavity. Am. J. Roentgenol. 2015, 205, W150–W161. [Google Scholar] [CrossRef]
- Pérez, M.G.S.; Bagan, J.; Jiménez-Soriano, Y.; Margaix, M.; Marzal, C. Utility of imaging techniques in the diagnosis of oral cancer. J. Cranio-Maxillofac. Surg. 2015, 43, 1880–1894. [Google Scholar] [CrossRef]
- Pentenero, M.; Cistaro, A.; Brusa, M.; Ferraris, M.M.; Pezzuto, C.; Carnino, R.; Colombini, E.; Valentini, M.C.; Giovanella, L.; Spriano, G.; et al. Accuracy of 18F-FDG-PET/CT for staging of oral squamous cell carcinoma. Head Neck 2008, 30, 1488–1496. [Google Scholar] [CrossRef]
- Schinagl, D.A.; Span, P.N.; Oyen, W.J.; Kaanders, J.H.A.M. Can FDG PET predict radiation treatment outcome in head and neck cancer? Results of a prospective study. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, V.; Fukui, M.B.; McCook, B.M. Role of 18FFDG PET/CT in the treatment of head and neck cancers: Principles, technique, normal distribution, and initial staging. AJR Am. J. Roentgenol. 2005, 184, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Sürücü, E.; Polack, B.D.; Demir, Y.; Durmusoglu, M.; Ekmekci, S.; Sarıoğlu, S.; Çelik, A.O.; Ada, E.; Ikiz, A.O.; Sarioglu, S. Dual-phase F-18 FDG PET-CT in staging and lymphoscintigraphy for detection of sentinel lymph nodes in oral cavity cancers. Clin. Imaging 2015, 39, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Badger, S.; Lynch, T. A potential diagnostic role of dual-phase 18F-FDG PET/CT scanning. Ulst. Med. J. 2014, 83, 52–54. [Google Scholar]
- Lee, J.-K.; Min, K.J.; A So, K.; Kim, S.; Hong, J.H. The effectiveness of dual-phase 18F-FDG PET/CT in the detection of epithelial ovarian carcinoma: A pilot study. J. Ovarian Res. 2014, 7, 15. [Google Scholar] [CrossRef][Green Version]
- Demir, Y.; Polack, B.D.; Karaman, C.; Ozdoğan, O.; Sürücü, E.; Ayhan, S.; Akkoçlu, A.; Ozdemir, N. The diagnostic role of dual-phase (18)F-FDG PET/CT in the characterization of solitary pulmonary nodules. Nucl. Med. Commun. 2014, 35, 260–267. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Shen, B.; Huang, T.; Sun, Y.; Jin, Z.; Li, X.-F. Revisit 18F-fluorodeoxyglucose oncology positron emission tomography: “Systems molecular imaging” of glucose metabolism. Oncotarget 2017, 8, 43536–43542. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Tu, M.; Sugano, A.; Yamamori, I.; Iba, A.; Yusa, K.; Kaneko, M.; Ota, S.; et al. Effect of timing of collection of salivary metabolomic biomarkers on oral cancer detection. Amino Acids 2017, 49, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Gleber-Netto, F.O.; Yakob, M.; Li, F.; Feng, Z.; Dai, J.; Kao, H.-K.; Chang, Y.-L.; Chang, K.-P.; Wong, D.T. Salivary Biomarkers for Detection of Oral Squamous Cell Carcinoma in a Taiwanese Population. Clin. Cancer Res. 2016, 22, 3340–3347. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Sugano, A.; Nakamura, M.; Kaneko, M.; Ota, S.; Hiwatari, K.; Enomoto, A.; Soga, T.; et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci. Rep. 2016, 6, 31520. [Google Scholar] [CrossRef]
- Kojima, S.; Zhou, B.; Teramukai, S.; Hara, A.; Kosaka, N.; Matsuo, Y.; Suzuki, H.; Torigoe, S.; Suzuki, T.; Uno, K.; et al. Cancer screening of healthy volunteers using whole-body 18F-FDG-PET scans: The Nishidai clinic study. Eur. J. Cancer 2007, 43, 1842–1848. [Google Scholar] [CrossRef]
- Nishizawa, S.; Kojima, S.; Teramukai, S.; Inubushi, M.; Kodama, H.; Maeda, Y.; Okada, H.; Zhou, B.; Nagai, Y.; Fukushima, M. Prospective Evaluation of Whole-Body Cancer Screening with Multiple Modalities Including [18F]Fluorodeoxyglucose Positron Emission Tomography in a Healthy Population: A Preliminary Report. J. Clin. Oncol. 2009, 27, 1767–1773. [Google Scholar] [CrossRef]
- Ono, K.; Ochiai, R.; Yoshida, T.; Kitagawa, M.; Omagari, J.; Kobayashi, H.; Yamashita, Y. The detection rates and tumor clinical/pathological stages of whole-body FDG-PET cancer screening. Ann. Nucl. Med. 2007, 21, 65–72. [Google Scholar] [CrossRef]
- Terauchi, T.; Murano, T.; Daisaki, H.; Kanou, D.; Shoda, H.; Kakinuma, R.; Hamashima, C.; Moriyama, N.; Kakizoe, T. Evaluation of whole-body cancer screening using 18F-2-deoxy-2-fluoro-d-glucose positron emission tomography: A preliminary report. Ann. Nucl. Med. 2008, 22, 379–385. [Google Scholar] [CrossRef]
- Synakiewicz, A.; Sawicka-Zukowska, M.; Adrianowska, N.; Galezowska, G.; Ratajczyk, J.; Owczarzak, A.; Konieczna, L.; Stachowicz-Stencel, T. Amino acid profiles as potential biomarkers for pediatric cancers: A preliminary communication. Biomark. Med. 2017, 11, 619–627. [Google Scholar] [CrossRef]
- Nakamoto, Y.; Higashi, T.; Sakahara, H.; Tamaki, N.; Kogire, M.; Doi, R.; Hosotani, R.; Imamura, M.; Konishi, J. Delayed18F-fluoro-2-deoxy-D-glucose positron emission tomography scan for differentiation between malignant and benign lesions in the pancreas. Cancer 2000, 89, 2547–2554. [Google Scholar] [CrossRef]
- Lan, X.-L.; Zhang, Y.; Wu, Z.-J.; Jia, Q.; Wei, H.; Gao, Z.-R. The value of dual time point 18F-FDG PET imaging for the differentiation between malignant and benign lesions. Clin. Radiol. 2008, 63, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Su, M.; Tian, Y.; Li, F.; Li, L.; Kuang, A.; Zeng, J. Dual-time point PET/CT with F-18 FDG for the differentiation of malignant and benign bone lesions. Skelet. Radiol. 2009, 38, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.K.; Yoo, I.; Kim, S.H.; O, J.H.; Choi, W.H.; Na, S.J.; Park, S.Y. The clinical value of dual-time point 18F-FDG PET/CT for differentiating extrahepatic cholangiocarcinoma from benign disease. Clin. Nucl. Med. 2013, 38, e106-11. [Google Scholar] [CrossRef] [PubMed]
- Keyes, J.W. SUV: Standard uptake or silly useless value? J. Nucl. Med. 1995, 36, 1836–1839. [Google Scholar] [PubMed]
- Sugawara, Y.; Zasadny, K.R.; Neuhoff, A.W.; Wahl, R.L. Reevaluation of the Standardized Uptake Value for FDG: Variations with Body Weight and Methods for Correction. Radiology 1999, 213, 521–525. [Google Scholar] [CrossRef]
- Langen, K.J.; Braun, U.; Kops, E.R.; Herzog, H.; Kuwert, T.; Nebeling, B.; E Feinendegen, L. The influence of plasma glucose levels on fluorine-18-fluorodeoxyglucose uptake in bronchial carcinomas. J. Nucl. Med. 1993, 34, 355–359. [Google Scholar]
- Hamberg, L.M.; Hunter, G.J.; Alpert, N.M.; Choi, N.C.; Babich, J.W.; Fischman, A.J. The dose uptake ratio as an index of glucose metabolism: Useful parameter or oversimplification? J. Nucl. Med. 1994, 35, 1308–1312. [Google Scholar]
- Iwano, S.; Ito, S.; Tsuchiya, K.; Kato, K.; Naganawa, S. What causes false-negative PET findings for solid-type lung cancer? Lung Cancer 2013, 79, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, A.; Khan, S.R.; Bharwani, N.; Stewart, V.; Rockall, A.G.; Khan, S.; Barwick, T.D. FDG PET/CT Pitfalls in Gynecologic and Genitourinary Oncologic Imaging. RadioGraphics 2017, 37, 577–594. [Google Scholar] [CrossRef]
- Adams, H.L.; Jaunoo, S.S. Clinical significance of incidental findings on staging positron emission tomography for oesophagogastric malignancies. Ann. R. Coll. Surg. Engl. 2014, 96, 207–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sugimoto, M. Salivary metabolomics for cancer detection. Expert Rev. Proteom. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Oral Cancer | Control | |
|---|---|---|---|
| n | 26 | 44 | |
| Age | Average (SD) | 73 (29–94) | 68 (21–90) |
| Sex | male | 12 (46.2) | 16 (36.4) |
| female | 14 (53.8) | 28 (63.6) | |
| Smoking habit | yes | 14 (53.8) | 9 (20.5) ** |
| Periodontitis | yes | 17 (65.4) | 29 (65.9) |
| Stage | I | 5 (19.2) | |
| II | 6 (23.1) | ||
| III | 7 (26.9) | ||
| IV | 8 (30.8) | ||
| Standard Uptake Value | early phase (n = 26) | 10.2 (2.8–19.4) | |
| delayed phase (n = 23) | 11.3 (1.8–22.4) | ||
| Histological type | Squamous cell carcinoma | 22 (84.6) | |
| Malignant melanoma | 2 (7.7) | ||
| Adenoid cystic carcinoma | 1 (3.8) | ||
| Unknown | 1 (3.8) | ||
| Salivary Metabolites | SUVmax in Delayed Phase | |
|---|---|---|
| Spearman’s Rank Correlation Coefficient (R) | p-Value | |
| N-acetylneuraminate | 0.492 | 0.017 |
| Pyruvate | 0.467 | 0.025 |
| Hexanoate | 0.484 | 0.019 |
| Homovanillate | 0.455 | 0.029 |
| 3-methylhistidine | 0.473 | 0.023 |
| 3-phenylpropionate | 0.462 | 0.026 |
| Pipecolate | 0.521 | 0.011 |
| p-hydroxyphenylacetate | 0.453 | 0.030 |
| Isethionate | 0.482 | 0.020 |
| Crotonate | 0.417 | 0.048 |
| o-phosphoserine | 0.468 | 0.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, S.; Hiraka, T.; Kirii, K.; Sugimoto, M.; Shimamoto, H.; Sugano, A.; Kitabatake, K.; Toyoguchi, Y.; Kanoto, M.; Nemoto, K.; et al. Relationship between Standard Uptake Values of Positron Emission Tomography/Computed Tomography and Salivary Metabolites in Oral Cancer: A Pilot Study. J. Clin. Med. 2020, 9, 3958. https://doi.org/10.3390/jcm9123958
Ishikawa S, Hiraka T, Kirii K, Sugimoto M, Shimamoto H, Sugano A, Kitabatake K, Toyoguchi Y, Kanoto M, Nemoto K, et al. Relationship between Standard Uptake Values of Positron Emission Tomography/Computed Tomography and Salivary Metabolites in Oral Cancer: A Pilot Study. Journal of Clinical Medicine. 2020; 9(12):3958. https://doi.org/10.3390/jcm9123958
Chicago/Turabian StyleIshikawa, Shigeo, Toshitada Hiraka, Kazukuni Kirii, Masahiro Sugimoto, Hiroaki Shimamoto, Ayako Sugano, Kenichiro Kitabatake, Yuuki Toyoguchi, Masafumi Kanoto, Kenji Nemoto, and et al. 2020. "Relationship between Standard Uptake Values of Positron Emission Tomography/Computed Tomography and Salivary Metabolites in Oral Cancer: A Pilot Study" Journal of Clinical Medicine 9, no. 12: 3958. https://doi.org/10.3390/jcm9123958
APA StyleIshikawa, S., Hiraka, T., Kirii, K., Sugimoto, M., Shimamoto, H., Sugano, A., Kitabatake, K., Toyoguchi, Y., Kanoto, M., Nemoto, K., Soga, T., Tomita, M., & Iino, M. (2020). Relationship between Standard Uptake Values of Positron Emission Tomography/Computed Tomography and Salivary Metabolites in Oral Cancer: A Pilot Study. Journal of Clinical Medicine, 9(12), 3958. https://doi.org/10.3390/jcm9123958