The Pharmacological Approach to Oncologic Patients with Acute Coronary Syndrome
Abstract
1. Introduction
2. Methods
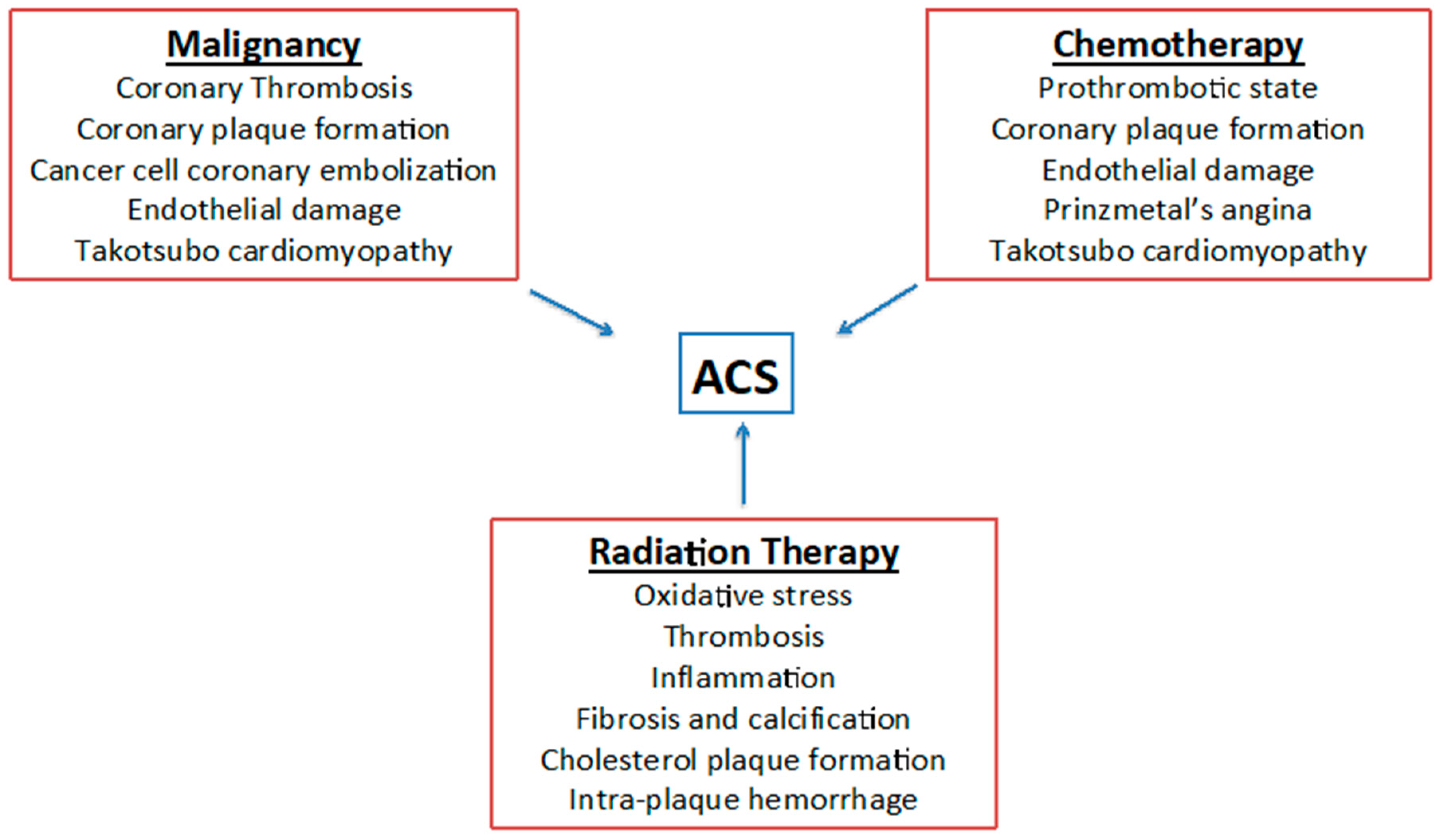
3. Cardiovascular Toxicities Induced by Chemotherapy and Radiotherapy
4. Clinical Presentation of ACS in Cancer Patients
5. Management of Acute Coronary Syndrome
5.1. Interventional Approach
- Balloon angioplasty should be advised for patients with platelet count <30,000/µL, who are not candidates for DAPT, or when a noncardiac procedure or surgery is necessary as soon as possible;
- BMS should be considered for patients with platelet count >30,000/µL, who need a noncardiac procedure, or surgery or chemotherapy, that can be delayed for >4 weeks;
- New-generation DES should be recommended for patients with platelet count >30,000/µL, who do not have an immediate need for a noncardiac procedure or surgery or chemotherapy.
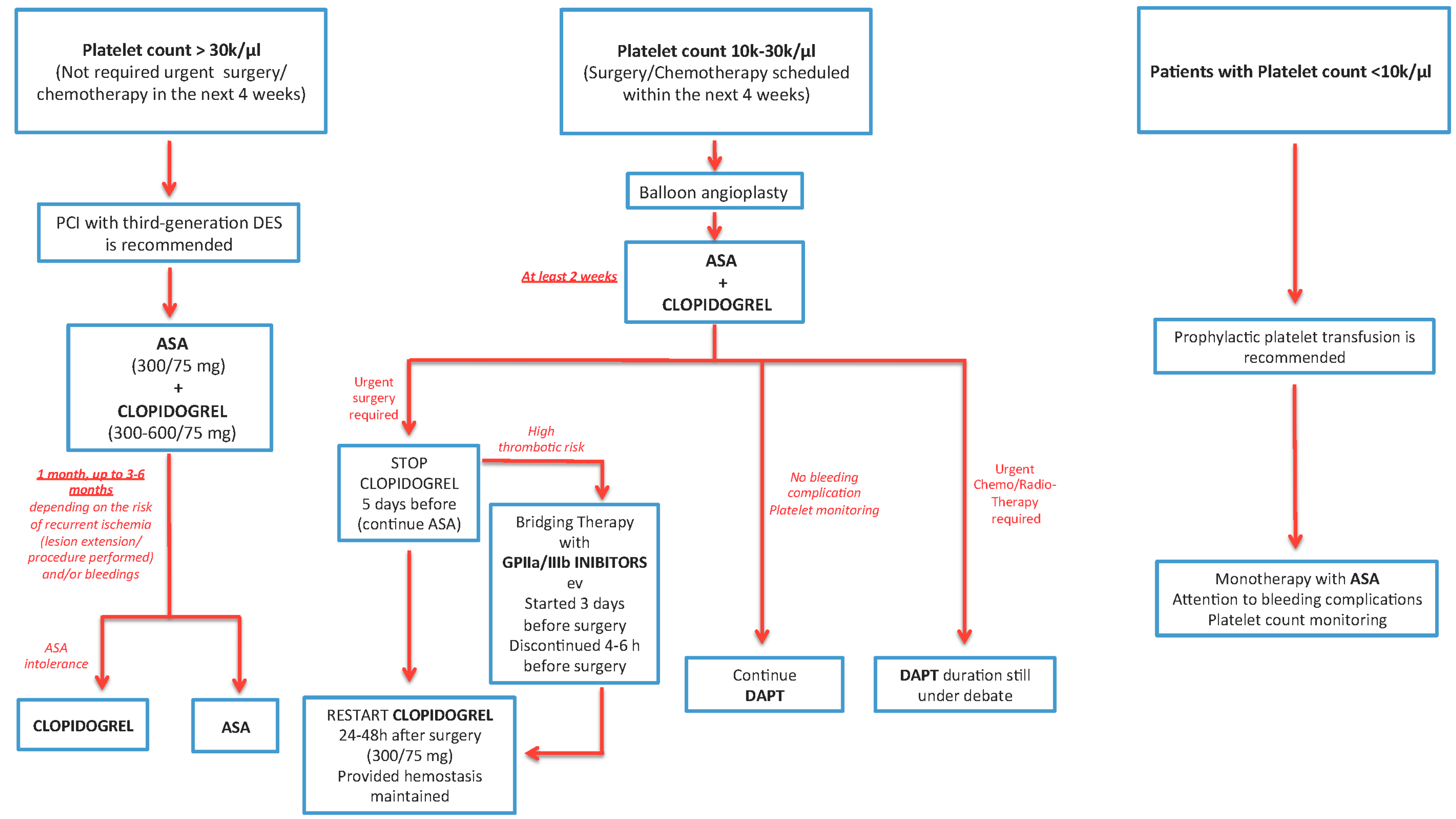
5.2. Thrombocytopenia in Cancer Patients
- PCIs are safe in patients with a platelet count between 40 k and 50 k/µL and no thrombotic disorders [40].
- The starting dose of unfractioned heparin (UFH) if the platelet count is 50,000/μL or lower should be 30 to 50 U/kg. If the platelet count exceeds 50,000/µL, UFH at a dose of 50 to 70 U/kg or bivalirudin IV should be used. If the activated clotting time (ACT) is less than 250 s during the infusion of UFH, the heparin dose should be increased. ACT monitoring is crucial for patient safety in this clinical setting [25].
- For a platelet count <30,000/µL, revascularization and DAPT should be decided after a multidisciplinary evaluation and a risk/benefit analysis.
- If the platelet count is higher than 10,000/µL, ASA can be continued; DAPT with Clopidogrel is allowed if the platelet count exceeds 30,000/µl. Prasugrel, Ticagrelor, and IIB-IIIA inhibitors should not be used if the platelet count is <50,000/µL [41].
- If the platelet count is <50,000/µL, the duration of DAPT may be reduced to 2 weeks after PTCA alone, 4 weeks after BMS, and 6 months after second- or third-generation DES.
- Prophylactic platelet transfusion can be performed if the platelet count is below 20,000/µL and the patient has a high fever, leukocytosis, a sudden decrease in the platelet count, or other coagulation disorders, or if the patient is on chemotherapy due to bladder, ovarian, colon cancer, or melanoma. Therapeutic platelet transfusion is recommended in thrombocytopenic patients who develop bleeding during or after catheterization; after, platelet transfusion is recommended to repeat platelet count [42].
- If the platelet count is <10,000/µL, platelet transfusion should be performed, see Figure 2.
6. Medical Therapy
6.1. Antiplatelet Therapy
6.1.1. Patients with Platelet Count >30 k/μL and Who Do not Require Urgent Surgery/Chemotherapy (in the Next 4 Weeks)
6.1.2. Patients with a Platelet Count of 10–30 k/μL or Who have Surgery/Chemotherapy is Scheduled within the Next 4 Weeks
6.1.3. Patients with a Platelet Count of <10 k/μL
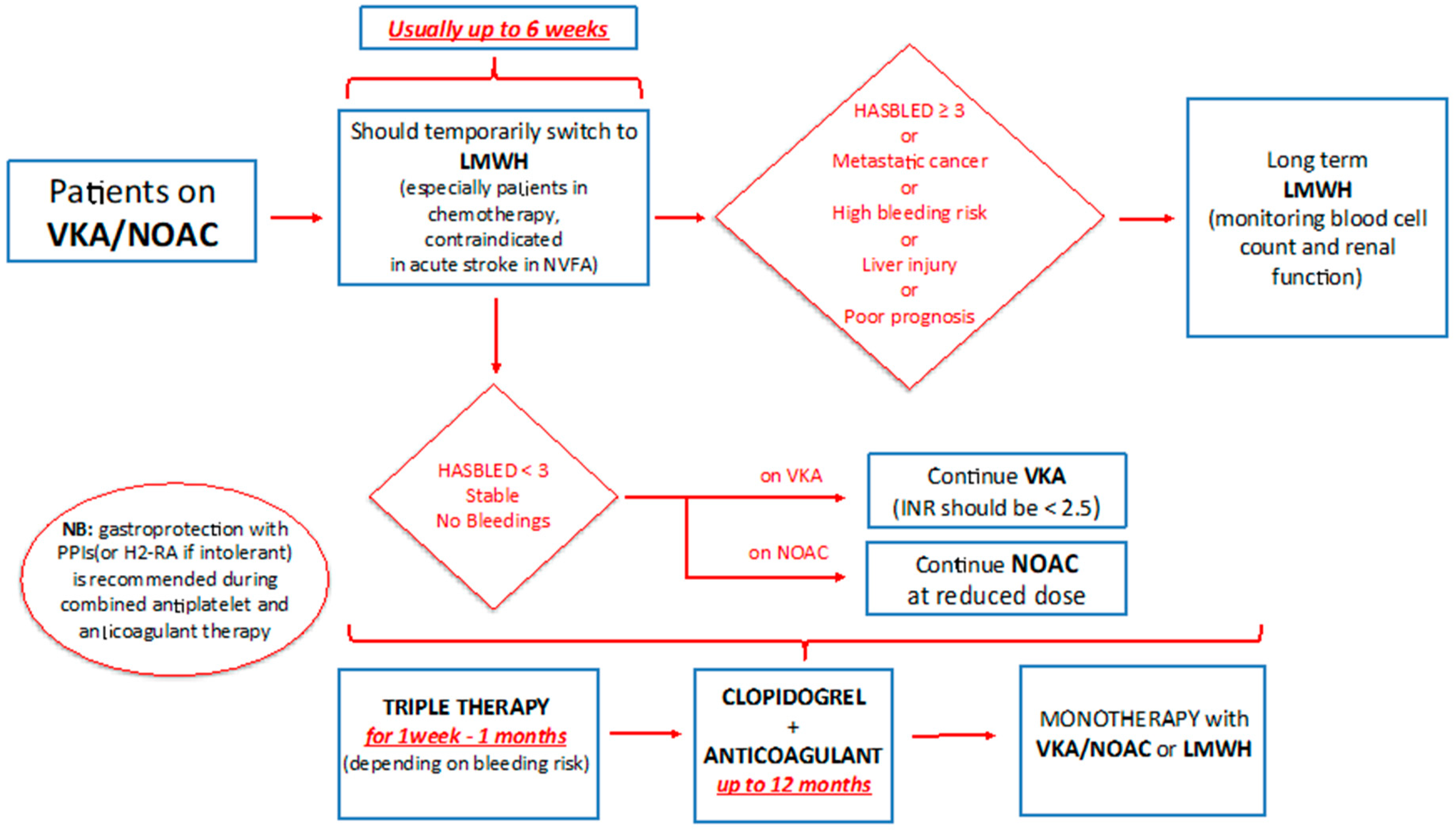
6.2. Triple Therapy
Atrial Fibrillation and ACS in Cancer Patients
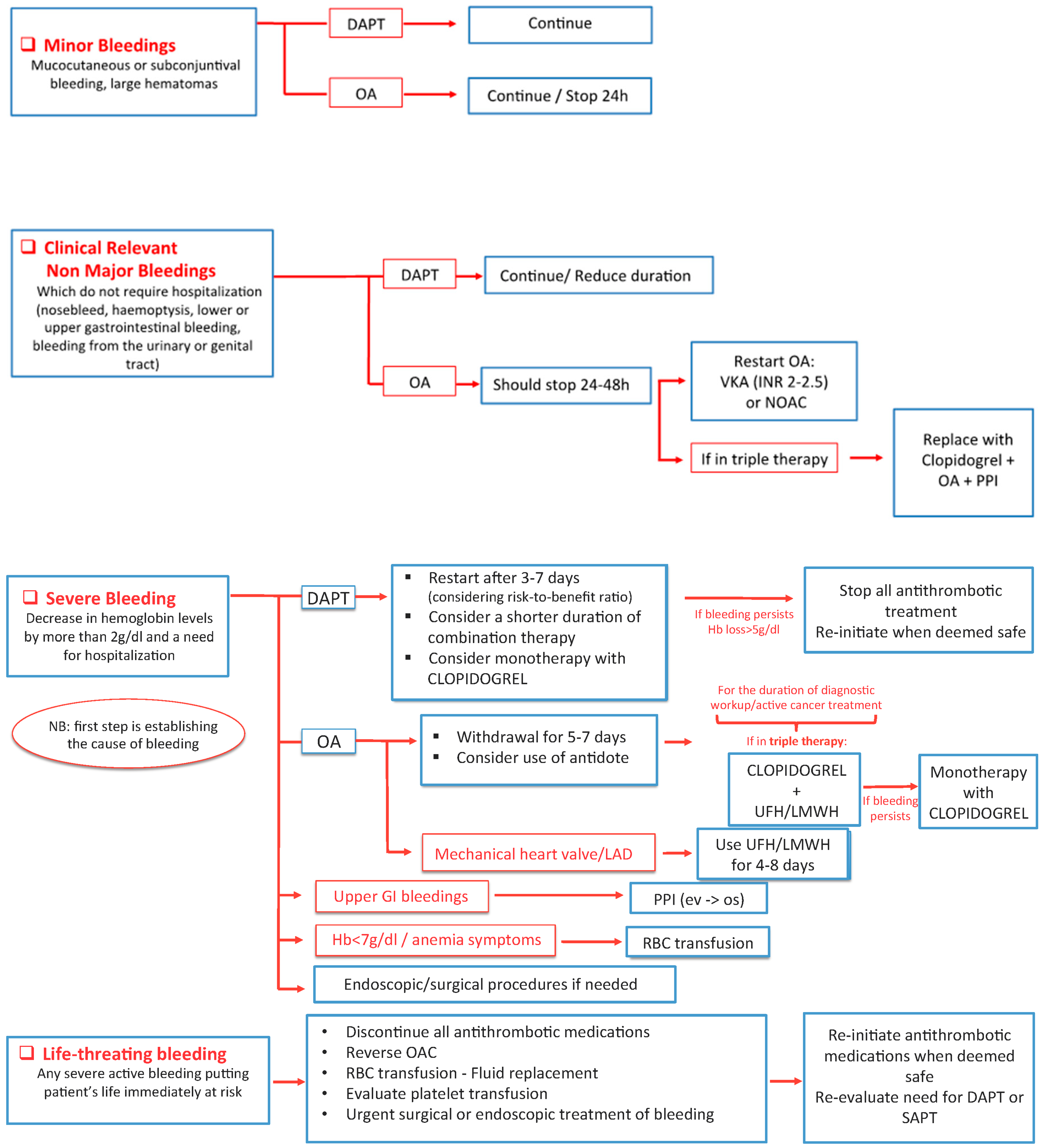
7. Complications Management: Bleedings
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yusuf, S.W.; Cipolla, C.; Durand, J.-B.; Lenihan, D.J. Cancer and cardiovascular disease. Cardiol. Res. Pract. 2011, 2011, 1. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Chen, H.-Y.; Saczynski, J.S.; McManus, D.D.; Lessard, D.; Yarzebski, J.; Lapane, K.L.; Gore, J.M. The impact of cardiac and noncardiac comorbidities on the short-term outcomes of patients hospitalized with acute myocardial infarction: A population-based perspective. Clin. Epidemiol. 2013, 5, 439-48. [Google Scholar] [CrossRef]
- Yeh, E.T.; Chang, H.-M. Cancer and Clot. J. Am. Coll. Cardiol. 2017, 70, 939–941. [Google Scholar] [CrossRef]
- Hasin, T.; Iakobishvili, Z.; Weisz, G. Associated risk of malignancy in patients with cardiovascular disease: Evidence and possible mechanism. Am. J. Med. 2017, 130, 780–785. [Google Scholar] [CrossRef]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Elkind, M.S.; Panageas, K.S.; DeAngelis, L.M. Risk of arterial thromboembolism in patients with cancer. J. Am. Coll. Cardiol. 2017, 70, 926–938. [Google Scholar] [CrossRef]
- Yusuf, S.W.; Daraban, N.; Abbasi, N.; Lei, X.; Durand, J.-B.; Daher, I.N. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin. Cardiol. 2012, 35, 443–450. [Google Scholar] [CrossRef]
- Polk, A.; Vaage-Nilsen, M.; Vistisen, K.; Nielsen, D.L. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat. Rev. 2013, 39, 974–984. [Google Scholar] [CrossRef]
- Karabay, K.O.; Yildiz, O.; Aytekin, V. Multiple coronary thrombi with cisplatin. J. Invasive Cardiol. 2014, 26, E18–E20. [Google Scholar]
- Jafri, M.; Protheroe, A. Cisplatin-associated thrombosis. Anti-Cancer Drugs 2008, 19, 927–929. [Google Scholar] [CrossRef]
- Katayama, M.; Imai, Y.; Hashimoto, H.; Kurata, M.; Nagai, K.; Tamita, K.; Morioka, S.; Furukawa, Y. Fulminant fatal cardiotoxicity following cyclophosphamide therapy. J. Cardiol. 2009, 54, 330–334. [Google Scholar] [CrossRef]
- Gupta, S.; Ghosh, J.; Bajpai, J.; Maheshwari, A.; Shah, K. Acute non-ST elevation myocardial infarction following paclitaxel administration for ovarian carcinoma: A case report and review of literature. J. Cancer Res. Ther. 2012, 8, 442-4. [Google Scholar] [CrossRef]
- Chen, X.-L.; Lei, Y.-H.; Liu, C.-F.; Yang, Q.-F.; Zuo, P.-Y.; Liu, C.-Y.; Chen, C.-Z.; Liu, Y.-W. Angiogenesis inhibitor bevacizumab increases the risk of ischemic heart disease associated with chemotherapy: A meta-analysis. PLoS ONE 2013, 8, e66721. [Google Scholar] [CrossRef] [PubMed]
- Kappers, M.H.W.; Smedts, F.M.; Horn, T.; Van Esch, J.H.; Sleijfer, S.; Leijten, F.; Wesseling, S.; Strevens, H.; Danser, A.H.J.; Meiracker, A.H.V.D. The Vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension 2011, 58, 295–302. [Google Scholar] [CrossRef]
- Naib, T.; Steingart, R.M.; Chen, C.L. Sorafenib-associated multivessel coronary artery vasospasm. Herz 2011, 36, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Quintás-Cardama, A.; Kantarjian, H.; Cortes, J. Nilotinib-associated vascular events. Clin. Lymphoma Myeloma Leuk. 2012, 12, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Gogia, A.; Khurana, S.; Paramanik, R. Acute myocardial infarction after first dose of rituximab infusion. Turk. J. Hematol. 2014, 31, 95–96. [Google Scholar] [CrossRef]
- Ziehr, D.R.; Chen, M.-H.; Zhang, D.; Braccioforte, M.H.; Moran, B.J.; Mahal, B.A.; Hyatt, A.S.; Basaria, S.; Beard, C.J.; Beckman, J.A.; et al. Association of androgen-deprivation therapy with excess cardiac-specific mortality in men with prostate cancer. BJU Int. 2014, 116, 358–365. [Google Scholar] [CrossRef]
- Nanda, A.; Chen, M.-H.; Braccioforte, M.H.; Moran, B.J.; D’Amico, A.V. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease–induced congestive heart failure or myocardial infarction. JAMA 2009, 302, 866–873. [Google Scholar] [CrossRef]
- Amir, E.; Seruga, B.; Niraula, S.; Carlsson, L.; Ocaña, A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2011, 103, 1299–1309. [Google Scholar] [CrossRef]
- Stewart, F.A.; Heeneman, S.; Poele, J.T.; Kruse, J.; Russell, N.S.; Gijbels, M.; Daemen, M. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE—Mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am. J. Pathol. 2006, 168, 649–658. [Google Scholar] [CrossRef]
- Lee, M.S.; Finch, W.; Mahmud, E. Cardiovascular complications of radiotherapy. Am. J. Cardiol. 2013, 112, 1688–1696. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, 37, 267–315. [Google Scholar] [CrossRef]
- Munoz, E.; Iliescu, G.; Vejpongsa, P.; Charitakis, K.; Karimzad, K.; Lopez-Mattei, J.; Yusuf, S.W.; Marmagkiolis, K.; Iliescu, C. Takotsubo stress cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 1143–1144. [Google Scholar] [CrossRef]
- Bisceglia, I.; Canale, M.L.; Lestuzzi, C.; Parrini, I.; Russo, G.; Colivicchi, F.; Gabrielli, D.; Gulizia, M.M.; Iliescu, C.A. Acute coronary syndromes in cancer patients. J. Cardiovasc. Med. 2020. [Google Scholar] [CrossRef]
- Iliescu, C.A.; Grines, C.L.; Herrmann, J.; Yang, E.H.; Cilingiroglu, M.; Charitakis, K.; Hakeem, A.; Toutouzas, K.P.; Leesar, M.A.; Marmagkiolis, K. SCAI Expert consensus statement: Evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of india, and sociedad Latino Americana de Cardiologıa interve. Catheter. Cardiovasc. Interv. 2016, 87, E202–E223. [Google Scholar] [CrossRef]
- Patel, M.R.; Dehmer, G.J.; Hirshfeld, J.W.; Smith, P.K.; Spertus, J.A.; Masoudi, F. AACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update: A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J. Thorac. Cardiovasc. Surg. 2012, 143, 780–803. [Google Scholar]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; A Goudevenos, J.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2017, 39, 119–177. [Google Scholar] [CrossRef]
- Guddati, A.K.; Joy, P.S.; Kumar, G. Analysis of outcomes of percutaneous coronary intervention in metastatic cancer patients with acute coronary syndrome over a 10-year period. J. Cancer Res. Clin. Oncol. 2016, 142, 471–479. [Google Scholar] [CrossRef]
- Roule, V.; Verdier, L.; Blanchart, K.; Ardouin, P.; Lemaitre, A.; Bignon, M.; Sabatier, R.; Alexandre, J.; Beygui, F. Systematic review and meta-analysis of the prognostic impact of cancer among patients with acute coronary syndrome and/or percutaneous coronary intervention. BMC Cardiovasc. Disord. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Styczkiewicz, K.; Styczkiewicz, M.; Myćka, M.; Mędrek, S.; Kondraciuk, T.; Czerkies-Bieleń, A.; Wiśniewski, A.; Szmit, S.; Jankowski, P. Clinical presentation and treatment of acute coronary syndrome as well as 1-year survival of patients hospitalized due to cancer. Medicine 2020, 99, e18972. [Google Scholar] [CrossRef]
- Banasiak, W.; Zymliński, R.; Undas, A. Optimal management of cancer patients with acute coronary syndrome. Pol. Arch. Intern. Med. 2018, 128. [Google Scholar] [CrossRef]
- Iannaccone, M.; D’Ascenzo, F.; Vadalà, P.; Wilton, M.M.S.B.; Noussan, P.; Colombo, F.; Raposeiras-Roubín, S.; Abu-Assi, E.; González-Juanatey, J.R.; Henriques, J.P.S.; et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: A BleeMACS substudy. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 631–638. [Google Scholar] [CrossRef]
- Aronson, D.; Brenner, B. Arterial thrombosis and cancer. Thromb. Res. 2018, 164, S23–S28. [Google Scholar] [CrossRef]
- Iliescu, C.; Cilingiroglu, M.; Giza, D.E.; Rosales, O.; Lebeau, J.; Guerrero-Mantilla, I.; Lopez-Mattei, J.; Song, J.; Silva, G.; Loyalka, P.; et al. “Bringing on the light” in a complex clinical scenario: Optical coherence tomography–guided discontinuation of antiplatelet therapy in cancer patients with coronary artery disease (PROTECT-OCT registry). Am. Heart J. 2017, 194, 83–91. [Google Scholar] [CrossRef]
- Iannaccone, M.; D’Ascenzo, F.; De Filippo, O.; Gagliardi, M.; A Southern, D.; Raposeiras-Roubín, S.; Abu-Assi, E.; Henriques, J.P.S.; Saucedo, J.; González-Juanatey, J.R.; et al. Optimal medical therapy in patients with malignancy undergoing percutaneous coronary intervention for acute coronary syndrome: A BleeMACS sub-study. Am. J. Cardiovasc. Drugs 2016, 17, 61–71. [Google Scholar] [CrossRef]
- Chew, D.; Mahaffey, K.W.; White, H.D.; Huang, Z.; Hoekstra, J.W.; Ferguson, J.J.; Califf, R.M.; Aylward, P.E. Coronary artery bypass surgery in patients with acute coronary syndromes is difficult to predict. Am. Heart J. 2008, 155, 841–847. [Google Scholar] [CrossRef]
- Nathan, S.; Rao, S.V. Radial versus femoral access for percutaneous coronary intervention: Implications for vascular complications and bleeding. Curr. Cardiol. Rep. 2012, 14, 502–509. [Google Scholar] [CrossRef]
- Hakim, D.A.; Dangas, G.D.; Caixeta, A.; Nikolsky, E.; Lansky, A.; Moses, J.W.; Claessen, B.E.; Sanidas, E.; White, H.D.; Ohman, E.M.; et al. Impact of baseline thrombocytopenia on the early and late outcomes after ST-elevation myocardial infarction treated with primary angioplasty: Analysis from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial. Am. Heart J. 2011, 161, 391–396. [Google Scholar] [CrossRef]
- Sarkiss, M.G.; Yusuf, S.W.; Warneke, C.L.; Botz, G.; Lakkis, N.; Hirch-Ginsburg, C.; Champion, J.C.; Swafford, J.; Shaw, A.D.S.; Lenihan, D.J.; et al. Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes. Cancer 2006, 109, 621–627. [Google Scholar] [CrossRef]
- Schiffer, C.A.; Bohlke, K.; Delaney, M.; Hume, H.; Magdalinski, A.J.; McCullough, J.J.; Omel, J.L.; Rainey, J.M.; Rebulla, P.; Rowley, S.D.; et al. Platelet transfusion for patients with cancer: American Society of Clinical Oncology Clinical Practice guideline update. J. Clin. Oncol. 2018, 36, 283–299. [Google Scholar] [CrossRef]
- McCarthy, C.P.; Steg, G.; Bhatt, D.L. The management of antiplatelet therapy in acute coronary syndrome patients with thrombocytopenia: A clinical conundrum. Eur. Heart J. 2017, 38, 3488–3492. [Google Scholar] [CrossRef]
- Iliescu, C.; Durand, J.-B.; Kroll, M. Cardiovascular interventions in thrombocytopenic cancer patients. Tex. Heart Inst. J. 2011, 38, 259–260. [Google Scholar]
- Feher, A.; Kampaktsis, P.N.; Parameswaran, R.; Stein, E.M.; Steingart, R.; Gupta, D. Aspirin is associated with improved survival in severely thrombocytopenic cancer patients with acute myocardial infarction. Oncology 2017, 22, 213–221. [Google Scholar] [CrossRef]
- Rohrmann, S.; Witassek, F.; Erne, P.; Rickli, H.; Radovanovic, D. Treatment of patients with myocardial infarction depends on history of cancer. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 639–645. [Google Scholar] [CrossRef]
- Syed, W.Y.; Cezar, I.; Jaya, D.B.; Iyad, N.D. Antiplatelet therapy and percutaneous coronary intervention in patients with acute coronary syndrome and thrombocytopenia. Tex. Heart Inst. J. 2010, 37, 336–340. [Google Scholar]
- Lagerqvist, B.; James, S.K.; Stenestrand, U.; Lindbäck, J.; Nilsson, T.; Wallentin, L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N. Engl. J. Med. 2007, 356, 1009–1019. [Google Scholar] [CrossRef]
- Rossini, R.; Tarantini, G.; Visconti, L.O.; Bramucci, E.; Castiglioni, B.; De Servi, S.; Lettieri, C.; Lettino, M.; Piccaluga, E.; Savonitto, S.; et al. Perioperative management of antiplatelet therapy in patients with coronary stents undergoing cardiac and non-cardiac surgery: A consensus document from Italian cardiological, surgical and anaesthesiological societies. EuroIntervention 2014, 10, 38–46. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Ording, A.G.; Horváth-Puhó, E.; Adelborg, K.; Pedersen, L.; Prandoni, P.; Sørensen, H.T. Thromboembolic and bleeding complications during oral anticoagulation therapy in cancer patients with atrial fibrillation: A Danish nationwide population-based cohort study. Cancer Med. 2017, 6, 1165–1172. [Google Scholar] [CrossRef]
- Shah, S.; Norby, F.L.; Datta, Y.H.; Lutsey, P.L.; MacLehose, R.F.; Chen, L.Y.; Alonso, A. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2018, 2, 200–209. [Google Scholar] [CrossRef]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into onco-cardiology: Atrial fibrillation in cancer. JACC 2014, 63, 945–953. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Antman, E.M. Management of bleeding with non–vitamin K antagonist oral anticoagulants in the era of specific reversal agents. Circulation 2016, 134, 248–261. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; A Byrne, R.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef]
- Eisenberg, M.J.; Richard, P.R.; Libersan, D.; Filion, K.B. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation 2009, 119, 1634–1642. [Google Scholar] [CrossRef]
- Desai, J.; Granger, C.B.; Weitz, J.I.; Aisenberg, J. Novel oral anticoagulants in gastroenterology practice. Gastrointest. Endosc. 2013, 78, 227–239. [Google Scholar] [CrossRef]
- Desai, J.; Kolb, J.M.; I Weitz, J.; Aisenberg, J. Gastrointestinal bleeding with the new oral anticoagulants–defining the issues and the management strategies. Thromb. Haemost. 2013, 110, 205–212. [Google Scholar] [CrossRef]
- Qureshi, W.; Mittal, C.; Patsias, I.; Garikapati, K.; Kuchipudi, A.; Cheema, G.; Elbatta, M.; Alirhayim, Z.; Khalid, F. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am. J. Cardiol. 2014, 113, 662–668. [Google Scholar] [CrossRef]
- Halvorsen, S.; Storey, R.F.; Rocca, B.; Sibbing, D.; Berg, J.T.; Grove, E.L.; Weiss, T.W.; Collet, J.-P.; Andreotti, F.; Gulba, D.C.; et al. Management of antithrombotic therapy after bleeding in patients with coronary artery disease and/or atrial fibrillation: Expert consensus paper of the European Society of Cardiology Working Group on Thrombosis. Eur. Heart J. 2016, 38, 1455–1462. [Google Scholar] [CrossRef]

| Incidence | Presentations | Cancer Therapy | |
|---|---|---|---|
| Antimetabolites | |||
| 5-Fluorouracil [7] | 0.1–18% | Angina, Vasospasm, ACS | Colorectal, pancreas, gastric breast |
| Alkylating agents | |||
| Cisplatin [8] | 0.2–12% | Angina, coronary thrombosis, ACS, progression of CAD | Ovarian, Testicular, Bladder, squamous cell of head and neck, mesothelioma |
| Antitumor antibiotics | |||
| Bleomycine [9] | <2% | Angina, vasospasm, ACS | Testicular, cervix, Hodgkin’s and non-Hodgkins lymphoma |
| Anti-microtubule agents | |||
| Vinblastine [9] | <5% | Angina, ACS | Testicular, lymphoma, breast cancer |
| Monoclonal antibodies | |||
| Rituximab [16] | <1% | Vasospasm, ACS, Takotsubo cardiomyopathy | Non-Hodgkin’s lymphoma, ChronicLymphocytic Leukemia |
| Tyrosine kinase inhibitors | |||
| Sorafenib [14] | 1% | Angina, ACS | Liver, renal cell, thyroid cancer |
| Sunitinib [12,13] | 5–8% | ACS, Takotsubo cardiomyopathy, progression of CAD | Pancreas, renal cell, gastrointestinal stromal tumor |
| Nilotinib [15] | 8–12% | ACS, progression of CAD, peripheral artery disease | Chronic myeloid Leukemia |
| Hormone therapy | |||
| Aromatase inhibitors (anastrozole) [19] | 2% | Angina, ACS | Breast cancer |
| Anti-androgens (bicalbutide) [17] | 1–33% | ACS, progression of CAD | Prostate cancer |
| Gonadotropin-releasing hormone agonist (goserelin) [18] | 1–2% | Angina, ACS | Prostate cancer |
| Gonadothropin-releasing hormone antagonist (degarelix) [18] | <1% | ACS | Prostate cancer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radmilovic, J.; Di Vilio, A.; D’Andrea, A.; Pastore, F.; Forni, A.; Desiderio, A.; Ragni, M.; Quaranta, G.; Cimmino, G.; Russo, V.; et al. The Pharmacological Approach to Oncologic Patients with Acute Coronary Syndrome. J. Clin. Med. 2020, 9, 3926. https://doi.org/10.3390/jcm9123926
Radmilovic J, Di Vilio A, D’Andrea A, Pastore F, Forni A, Desiderio A, Ragni M, Quaranta G, Cimmino G, Russo V, et al. The Pharmacological Approach to Oncologic Patients with Acute Coronary Syndrome. Journal of Clinical Medicine. 2020; 9(12):3926. https://doi.org/10.3390/jcm9123926
Chicago/Turabian StyleRadmilovic, Juri, Alessandro Di Vilio, Antonello D’Andrea, Fabio Pastore, Alberto Forni, Alfonso Desiderio, Massimo Ragni, Gaetano Quaranta, Giovanni Cimmino, Vincenzo Russo, and et al. 2020. "The Pharmacological Approach to Oncologic Patients with Acute Coronary Syndrome" Journal of Clinical Medicine 9, no. 12: 3926. https://doi.org/10.3390/jcm9123926
APA StyleRadmilovic, J., Di Vilio, A., D’Andrea, A., Pastore, F., Forni, A., Desiderio, A., Ragni, M., Quaranta, G., Cimmino, G., Russo, V., Scherillo, M., & Golino, P. (2020). The Pharmacological Approach to Oncologic Patients with Acute Coronary Syndrome. Journal of Clinical Medicine, 9(12), 3926. https://doi.org/10.3390/jcm9123926







