Differentiation of Traumatic Osteoporotic and Non-Osteoporotic Vertebral AO A3 Fractures by Analyzing the Posterior Edge Morphology—A Retrospective Feasibility Study
Abstract
1. Introduction
2. Material and Methods
2.1. CT-Datasets
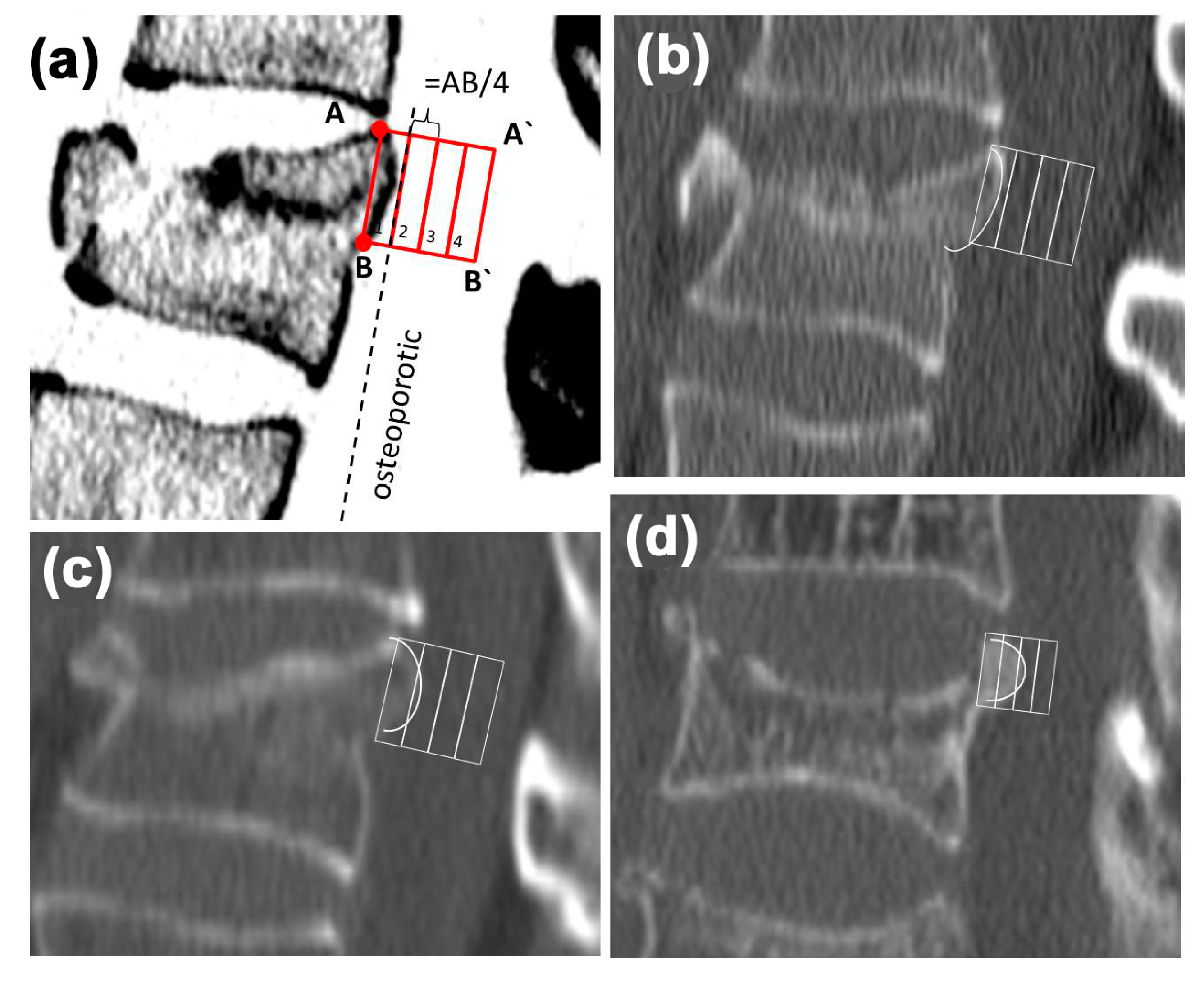
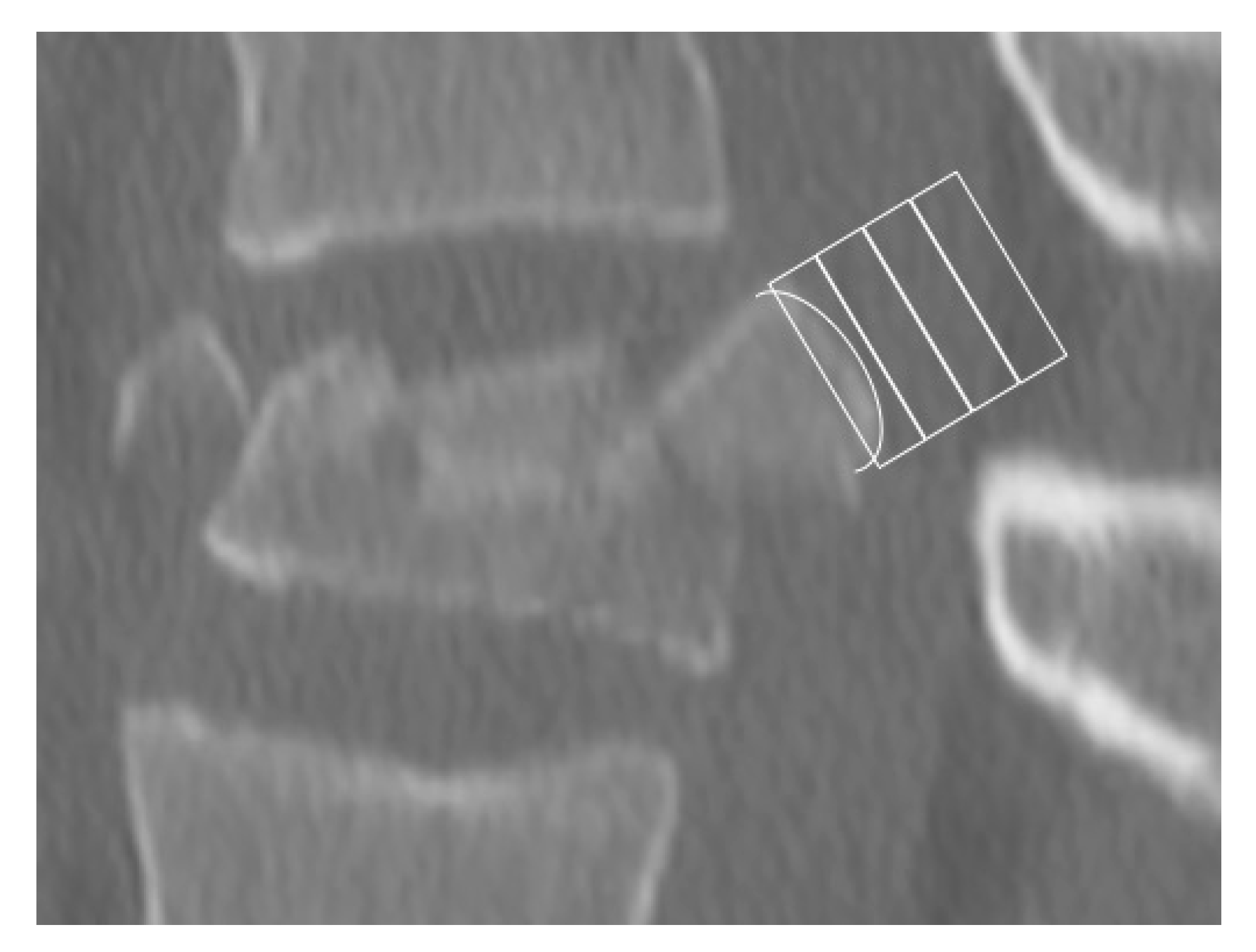
2.2. Morphological Analysis—Bulging
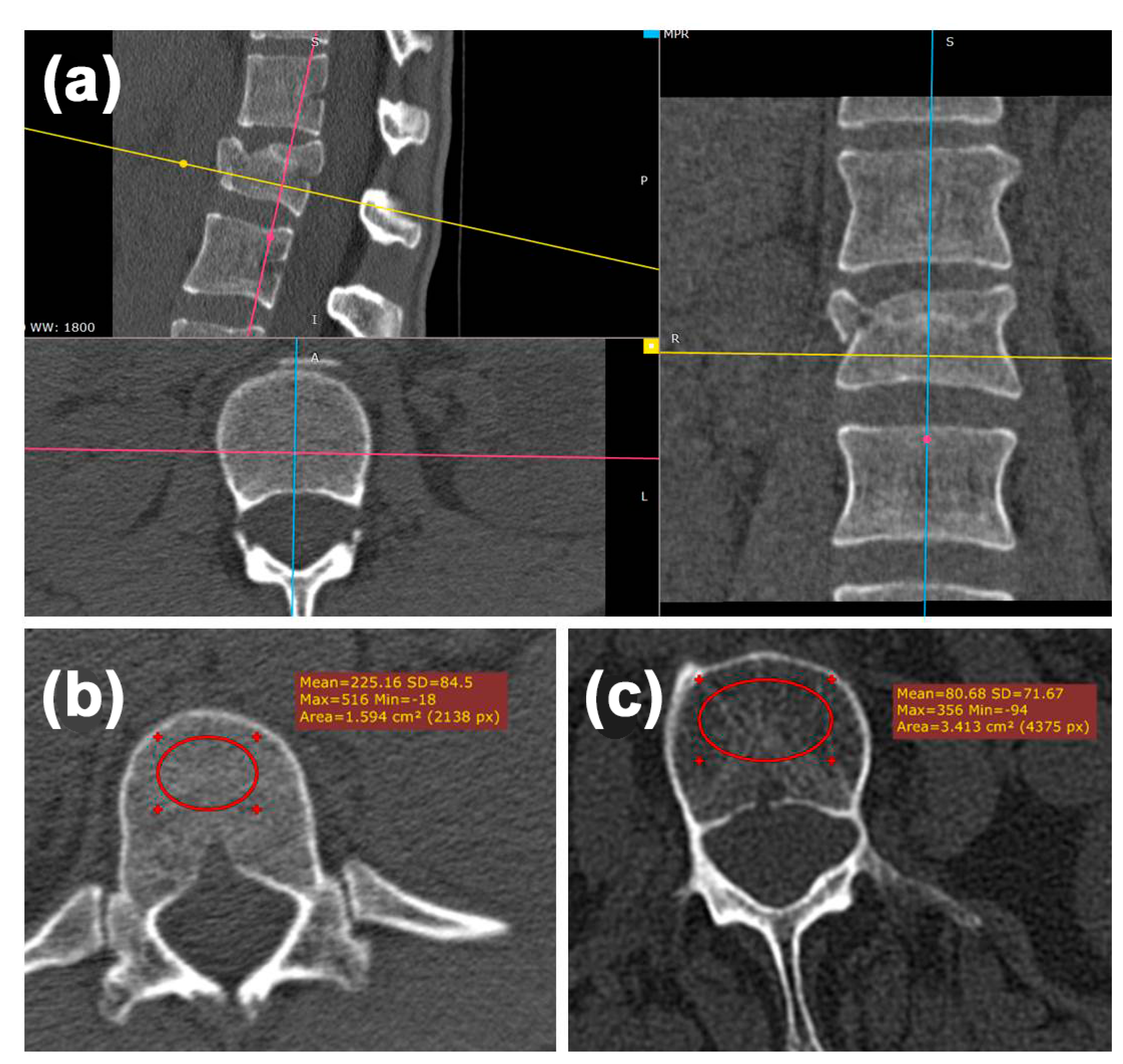
2.3. Radiologic Analysis—Hounsfield Units (HU)
2.4. Statistical Methods
3. Results
3.1. Patient’s Data
3.2. Morphologic Analysis—Bulging
3.3. Radiologic Analysis—Hounsfield Units
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Heyde, C.-E.; Ertel, W.; Kayser, R. Management of spine injuries in polytraumatized patients. Der Orthopäde 2005, 34, 889–905. [Google Scholar] [CrossRef]
- Becker, S.; Chavanne, A.; Spitaler, R.; Kropik, K.; Aigner, N.; Ogon, M.; Redl, H. Assessment of different screw augmentation techniques and screw designs in osteoporotic spines. Eur. Spine J. 2008, 17, 1462–1469. [Google Scholar] [CrossRef]
- Krappinger, D.; Kastenberger, T.; Schmid, R. Augmented posterior instrumentation for the treatment of osteoporotic vertebral body fractures. Oper. Orthop. Traumatol. 2012, 24, 4–12. [Google Scholar] [CrossRef]
- Zou, J.; Mei, X.; Gan, M.; Wang, G.; Lu, J.; Yang, H. Is kyphoplasty reliable for osteoporotic vertebral compression fracture with vertebral wall deficiency? Injury 2010, 41, 360–364. [Google Scholar] [CrossRef]
- Burval, D.J.; McLain, R.F.; Milks, R.; Inceoglu, S. Primary Pedicle Screw Augmentation in Osteoporotic Lumbar Vertebrae: Biomechanical analysis of pedicle fixation strength. Spine 2007, 32, 1077–1083. [Google Scholar] [CrossRef]
- Bostelmann, R.; Keiler, A.; Steiger, H.J.; Scholz, A.; Cornelius, J.F.; Schmoelz, W. Effect of augmentation techniques on the failure of pedicle screws under cranio-caudal cyclic loading. Eur. Spine J. 2015, 26, 181–188. [Google Scholar] [CrossRef]
- Sawakami, K.; Yamazaki, A.; Ishikawa, S.; Ito, T.; Watanabe, K.; Endo, N. Polymethylmethacrylate Augmentation of Pedicle Screws Increases the Initial Fixation in Osteoporotic Spine Patients. J. Spinal Disord. Tech. 2012, 25, E28–E35. [Google Scholar] [CrossRef]
- Schnake, K.J.; Blattert, T.R.; Liljenqvist, U. Cement augmentation of pedicle screws: Pros and cons. Der Orthopäde 2016, 45, 755–759. [Google Scholar] [CrossRef]
- Lee, S.; Chung, C.K.; Oh, S.H.; Park, S.B. Correlation between Bone Mineral Density Measured by Dual-Energy X-Ray Absorptiometry and Hounsfield Units Measured by Diagnostic CT in Lumbar Spine. J. Korean Neurosurg. Soc. 2013, 54, 384–389. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; Del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic Screening for Osteoporosis Using Abdominal Computed Tomography Scans Obtained for Other Indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [CrossRef]
- Heyde, C.-E.; Rohlmann, A.; Weber, U.; Kayser, R. Stabilisierung der osteoporotischen Wirbelsäule unter biomechanischen Gesichtspunkten. Der Orthopäde 2010, 39, 407–416. [Google Scholar] [CrossRef]
- Klöckner, P.D.C. Instrumental fixation in spinal surgery. Particular characteristics in patients with manifest osteoporosis. Der Orthopäde 2010, 39, 432–436. [Google Scholar] [CrossRef]
- Janssen, I.; Ryang, Y.-M.; Gempt, J.; Bette, S.; Gerhardt, J.; Kirschke, J.S.; Meyer, B. Risk of cement leakage and pulmonary embolism by bone cement-augmented pedicle screw fixation of the thoracolumbar spine. Spine J. 2017, 17, 837–844. [Google Scholar] [CrossRef]
- Mueller, J.U.; Baldauf, J.; Marx, S.; Kirsch, M.; Schroeder, H.W.S.; Pillich, D.T. Cement leakage in pedicle screw augmentation: A prospective analysis of 98 patients and 474 augmented pedicle screws. J. Neurosurg. Spine 2016, 25, 103–109. [Google Scholar] [CrossRef]
- Schreiber, J.J.; Anderson, P.A.; Rosas, H.G.; Buchholz, A.L.; Au, A.G. Hounsfield Units for Assessing Bone Mineral Density and Strength: A Tool for Osteoporosis Management. J. Bone Jt. Surg.-Am. Vol. 2011, 93, 1057–1063. [Google Scholar] [CrossRef]
- Choi, M.K.; Kim, S.M.; Lim, J.K. Diagnostic efficacy of Hounsfield units in spine CT for the assessment of real bone mineral density of degenerative spine: Correlation study between T-scores determined by DEXA scan and Hounsfield units from CT. Acta Neurochir. 2016, 158, 1421–1427. [Google Scholar] [CrossRef]
- Scheyerer, M.J.; Ullrich, B.; Osterhoff, G.; Spiegl, U.A.; Schnake, K.J. Arbeitsgruppe Osteoporotische Frakturen der Sektion Wirbelsaule der Deutschen Gesellschaft fur Orthopadie und U Hounsfield units as a measure of bone density-applications in spine surgery. Der Unfallchirurg 2019, 122, 654–661. [Google Scholar] [CrossRef]
- Hoppe, S.; Uhlmann, M.; Schwyn, R.; Suhm, N.; Benneker, L.M. Intraoperative Mechanical Measurement of Bone Quality With the DensiProbe. J. Clin. Densitom. 2015, 18, 109–116. [Google Scholar] [CrossRef]
- Klos, K.; Mückley, T.; Wahnert, D.; Zwipp, H.; Gueorguiev, B.G.; Schwieger, K.; Hofmann, G.O.; Windolf, M. The use of DensiProbe in hindfoot arthrodesis. Can fusion failure be predicted by mechanical bone strength determination? Z. Orthop. Unfallchirurgie 2010, 149, 206–211. [Google Scholar] [CrossRef]
- Klos, K.; Windolf, M.; Schwieger, K.; Kuhn, P.; Hänni, M.; Gueorguiev, B.; Hofmann, G.O.; Mückley, T. Intraoperative Mechanical Bone Strength Determination in Tibiotalocalcaneal Fusion: A Biomechanical Investigation. Foot Ankle Int. 2009, 30, 1183–1189. [Google Scholar] [CrossRef]
- Röderer, G.; Brianza, S.; Schiuma, D.; Schwyn, R.; Scola, A.; Gueorguiev, B.; Gebhard, F.; Tami, A. Mechanical Assessment of Local Bone Quality to Predict Failure of Locked Plating in a Proximal Humerus Fracture Model. Orthopedics 2013, 36, e1134–e1140. [Google Scholar] [CrossRef]
- Röderer, G.; Scola, A.; Schmölz, W.; Gebhard, F.; Windolf, M.; Hofmann-Fliri, L. Biomechanical in vitro assessment of screw augmentation in locked plating of proximal humerus fractures. Injury 2013, 44, 1327–1332. [Google Scholar] [CrossRef]
- Suhm, N.; Hengg, C.; Schwyn, R.; Windolf, M.; Quarz, V.; Hänni, M. Mechanical torque measurement predicts load to implant cut-out: A biomechanical study investigating DHS® anchorage in femoral heads. Arch. Orthop. Trauma Surg. 2006, 127, 469–474. [Google Scholar] [CrossRef]
- Popp, A.W.; Schwyn, R.; Schiuma, D.; Keel, M.J.; Lippuner, K.; Benneker, L.M. DensiProbe Spine: An intraoperative measurement of bone quality in spinal instrumentation. A clinical feasibility study. Spine J. 2013, 13, 1223–1229. [Google Scholar] [CrossRef]
- Suhm, N.; Haenni, M.; Schwyn, R.; Hirschmann, M.; Müller, A.M. Quantification of bone strength by intraoperative torque measurement: A technical note. Arch. Orthop. Trauma Surg. 2008, 128, 613–620. [Google Scholar] [CrossRef][Green Version]
| Box | Osteoporotic (n = 34) | Non-Osteoporotic (n = 51) |
|---|---|---|
| 1 | 0 | 49 (96.1%) |
| 2 | 30 (88.2%) | 2 (3.9%) |
| 3 | 4 (11.8%) | 0 |
| 4 | 0 | 0 |
| Cut-Off Box 2 | Osteoporosis | Total | |
|---|---|---|---|
| + | − | ||
| Test + | 34 (100%) true positive | 2 (4%) false positive | 36 |
| Test − | 0 (0%) false negative | 50 (96%) true negative | 50 |
| Cut-Off HU < 110 | |||
| Test + | 32 (94%) true positive | 3 (6%) false positive | 35 |
| Test − | 2 (6%) false negative | 49 (94%) true negative | 51 |
| HU Fractured Vertebra | HU Vertebra Above | HU Vertebra Below | |
|---|---|---|---|
| All | 184.6 (70) | 147.6 (74) | 137.5 (72) |
| Non-osteoporotic | 220.9 (59) | 193.9 (56) | 182.9 (54) |
| Osteoporotic | 129.1 (45) | 76.8 (28) | 68.2 (24) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vordemvenne, T.; Wähnert, D.; Klingebiel, S.; Lohmaier, J.; Hartensuer, R.; Raschke, M.J.; Roßlenbroich, S. Differentiation of Traumatic Osteoporotic and Non-Osteoporotic Vertebral AO A3 Fractures by Analyzing the Posterior Edge Morphology—A Retrospective Feasibility Study. J. Clin. Med. 2020, 9, 3910. https://doi.org/10.3390/jcm9123910
Vordemvenne T, Wähnert D, Klingebiel S, Lohmaier J, Hartensuer R, Raschke MJ, Roßlenbroich S. Differentiation of Traumatic Osteoporotic and Non-Osteoporotic Vertebral AO A3 Fractures by Analyzing the Posterior Edge Morphology—A Retrospective Feasibility Study. Journal of Clinical Medicine. 2020; 9(12):3910. https://doi.org/10.3390/jcm9123910
Chicago/Turabian StyleVordemvenne, Thomas, Dirk Wähnert, Sebastian Klingebiel, Jens Lohmaier, René Hartensuer, Michael J. Raschke, and Steffen Roßlenbroich. 2020. "Differentiation of Traumatic Osteoporotic and Non-Osteoporotic Vertebral AO A3 Fractures by Analyzing the Posterior Edge Morphology—A Retrospective Feasibility Study" Journal of Clinical Medicine 9, no. 12: 3910. https://doi.org/10.3390/jcm9123910
APA StyleVordemvenne, T., Wähnert, D., Klingebiel, S., Lohmaier, J., Hartensuer, R., Raschke, M. J., & Roßlenbroich, S. (2020). Differentiation of Traumatic Osteoporotic and Non-Osteoporotic Vertebral AO A3 Fractures by Analyzing the Posterior Edge Morphology—A Retrospective Feasibility Study. Journal of Clinical Medicine, 9(12), 3910. https://doi.org/10.3390/jcm9123910







