Neurochemical Changes in the Brain and Neuropsychiatric Symptoms in Clinically Isolated Syndrome
Abstract
1. Introduction
2. Methods
2.1. Participants and Procedures
2.2. Clinical and Neuropsychological Assessments
- Trail Making Test (TMT). Two parts of the TMT from the Halstead-Reitan battery [23] were administered as follows: TMT A with numbers and TMT B with numbers and letters. The test score was the time to complete each task.
- Stroop Color-Word Test (SCWT). Two stimuli were used for the SCWT [24] as follows: a card with the names of colors written in black ink and a card with the names of colors written in an incongruent color. The measures of performance were the time to name the colors written in ink of an incongruent color and the number of errors made.
- Symbol Digit Modalities Test (SDMT) [25] is a simple substitution task. The written version of the SDMT was used. The test score was the number of correctly paired numbers with given geometric figures in 90 s.
- Verbal Fluency Test. In the Verbal Fluency Test [26], participants are asked to generate words beginning with each of the letters F, A, and S, excluding proper nouns (1 min for each letter). The measure of performance was the total number of words generated, excluding repetitions.
- Neurotest. Participants were administered the Single Reaction Time (SRT) test and the Go/no-go task of the Polish version of Neurotest, a computer neuropsychological test battery comprising 11 tools. In the SRT task, the participant is asked to press a computer key as quickly as possible when a green circle appears on the screen (the stimulus appears 25 times). The measure of performance is the average response time. In the Go/no-go task, the participant is told to react when a green square appears on the screen (“go” reaction) and not to react when a blue square appears (“no-go” reaction). The stimuli appear in a random order (75 green squares and 25 blue squares). The test outcomes are the response time for correct “go” reactions and the number of incorrect “no-go” reactions (indicating perseverative errors).
- California Verbal Learning Test (CVLT). The Polish version of the CVLT [27] was used to assess verbal memory. The following outcomes were evaluated: number of words immediately recalled from the first list of 16 words (list A) in the 1st and 5th trials, total number of words immediately recalled from the 1st trial to the 5th trial, short delayed free recall (after learning interference list of words—list B), and long delayed free recall (recalling the words from list A after 20 min).
2.3. MR Examination and Analysis
2.4. Statistical Analysis
3. Results
3.1. Neuropsychological Performance in CIS and HP Groups and Associations with Demographic Factors and BDI/HADS and EDSS Scores
3.2. Classic MRI Findings
3.3. H-MRS Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khalil, M.; Enzinger, C.; Langkammer, C.; Petrovic, K.; Loitfelder, M.; Tscherner, M.; Jehna, M.; Bachmaier, G.; Wallner-Blazek, M.; Ropele, S.; et al. Cognitive impairment in relation to MRI metrics in patients with clinically isolated syndrome. Mult. Scler. J. 2011, 17, 173–180. [Google Scholar] [CrossRef]
- Feuillet, L.; Reuter, F.; Audoin, B.; Malikova, I.; Barrau, K.; Cherif, A.A.; Pelletier, J. Early cognitive impairment in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult. Scler. J. 2007, 13, 124–127. [Google Scholar] [CrossRef]
- Diker, S.; Has, A.C.; Kurne, A.; Göçmen, R.; Oğuz, K.K.; Karabudak, R. The association of cognitive impairment with gray matter atrophy and cortical lesion load in clinically isolated syndrome. Mult. Scler. Relat. Disord. 2016, 10, 14–21. [Google Scholar] [CrossRef]
- Hynčicová, E.; Vyhnálek, M.; Kalina, A.; Martinkovič, L.; Nikolai, T.; Lisy, J.; Hort, J.; Meluzínová, E.; Jan, L. Cognitive impairment and structural brain changes in patients with clinically isolated syndrome at high risk for multiple sclerosis. J. Neurol. 2017, 264, 482–493. [Google Scholar] [CrossRef]
- Roosendaal, S.D.; Bendfeldt, K.; Vrenken, H.; Polman, C.H.; Borgwardt, S.J.; Radue, E.W.; Kappos, L.; Pelletier, D.; Hauser, S.L.; Matthews, P.M.; et al. Grey matter volume in a large cohort of MS patients: Relation to MRI parameters and disability. Mult. Scler. J. 2011, 17, 1098–1106. [Google Scholar] [CrossRef]
- Uher, T.; Blahova-Dusankova, J.; Horakova, D.; Bergsland, N.; Tyblova, M.; Benedict, R.H.B.; Kalincik, T.; Ramasamy, D.P.; Seidl, Z.; Hagemeier, J.; et al. Longitudinal MRI and neuropsychological assessment of patients with clinically isolated syndrome. J. Neurol. 2014, 261, 1735–1744. [Google Scholar] [CrossRef]
- Koçak, M.; Ağan, K.; Mollahasanoglu, A.; Ekinci, G.; Elmaci, N.T.; Günal, D. Changes in the normal appearing white-gray matter and cognitive impairment in clinically isolated syndrome. J. Neurol. Sci. Turk. 2015, 32, 661–669. [Google Scholar]
- Wattjes, M.P.; Harzheim, M.; Lutterbey, G.G.; Bogdanow, M.; Schild, H.H.; Träber, F. High field MR imaging and 1H-MR spectroscopy in clinically isolated syndromes suggestive of multiple sclerosis: Correlation between metabolic alterations and diagnostic MR imaging criteria. J. Neurol. 2008, 255, 56–63. [Google Scholar] [CrossRef]
- Fernando, K.T.M.; McLean, M.A.; Chard, D.T.; MacManus, D.G.; Dalton, C.M.; Miszkiel, K.A.; Gordon, R.M.; Plant, G.T.; Thompson, A.; Miller, D.H. Elevated white matter myo-inositol in clinically isolated syndromes suggestive of multiple sclerosis. Brain 2004, 127, 1361–1369. [Google Scholar] [CrossRef]
- Vingara, L.K.; Yu, H.J.; Wagshul, M.E.; Serafin, D.; Christodoulou, C.; Pelczer, I.; Krupp, L.B.; Maletić-Savatić, M. Metabolomic approach to human brain spectroscopy identifies associations between clinical features and the frontal lobe metabolome in multiple sclerosis. NeuroImage 2013, 82, 586–594. [Google Scholar] [CrossRef]
- Christodoulou, C.; Krupp, L.B.; Liang, Z.; Huang, W.; Melville, P.; Roque, C.; Scherl, W.F.; Morgan, T.; MacAllister, W.S.; Li, L.; et al. Cognitive performance and MR markers of cerebral injury in cognitively impaired MS patients. Neurology 2003, 60, 1793–1798. [Google Scholar] [CrossRef]
- Glanz, B.I.; Holland, C.M.; Gauthier, S.A.; Amunwa, E.L.; Liptak, Z.; Houtchens, M.K.; Sperling, R.A.; Khoury, S.J.; Guttmann, C.R.G.; Weiner, H.L. Cognitive dysfunction in patients with clinically isolated syndromes or newly diagnosed multiple sclerosis. Mult. Scler. J. 2007, 13, 1004–1010. [Google Scholar] [CrossRef]
- Stuke, H.; Hanken, K.; Hirsch, J.; Klein, J.; Wittig, F.; Kastrup, A.; Hildebrandt, H. Cross-Sectional and Longitudinal Relationships between Depressive Symptoms and Brain Atrophy in MS Patients. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Hyncicova, E.; Kalina, A.; Vyhnalek, M.; Nikolai, T.; Martinkovic, L.; Lisy, J.; Hort, J.; Meluzinova, E.; Jan, L. Health-related quality of life, neuropsychiatric symptoms and structural brain changes in clinically isolated syndrome. PLoS ONE 2018, 13, e0200254. [Google Scholar] [CrossRef]
- Feinstein, A.; O’Connor, P.; Akbar, N.; Moradzadeh, L.; Scott, C.J.M.; Lobaugh, N.J. Diffusion tensor imaging abnormalities in depressed multiple sclerosis patients. Mult. Scler. J. 2010, 16, 189–196. [Google Scholar] [CrossRef]
- De Stefano, N.; Filippi, M. MR Spectroscopy in Multiple Sclerosis. J. Neuroimaging 2007, 17, 31S–35S. [Google Scholar] [CrossRef]
- Llufriu, S.; Kornak, J.; Ratiney, H.; Oh, J.; Brenneman, D.; Cree, B.A.C.; Sampat, M.; Hauser, S.L.; Nelson, S.J.; Pelletier, D. Magnetic Resonance Spectroscopy Markers of Disease Progression in Multiple Sclerosis. JAMA Neurol. 2014, 71, 840–847. [Google Scholar] [CrossRef]
- Cianfoni, A.; Law, M.; Re, T.; Dubowitz, D.; Rumboldt, Z.; Imbesi, S. Clinical pitfalls related to short and long echo times in cerebral MR spectroscopy. J. Neuroradiol. 2011, 38, 69–75. [Google Scholar] [CrossRef]
- Miller, D.H.; Chard, D.T.; Ciccarelli, O. Clinically isolated syndromes. Lancet Neurol. 2012, 11, 157–169. [Google Scholar] [CrossRef]
- Filippi, M.; Rocca, M.A.; Ciccarelli, O.; De Stefano, N.; Evangelou, N.; Kappos, L.; Rovira, A.; Sastre-Garriga, J.; Tintorè, M.; Frederiksen, J.L.; et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016, 15, 292–303. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Smith, A. Symbol Digit Modalities Test: Manual; Western Psychological Services: Los Angeles, CA, USA, 1982. [Google Scholar]
- Benton, A. Problems of Test Construction in the Field of Aphasia. Cortex 1967, 3, 32–58. [Google Scholar] [CrossRef]
- Łojek, E.; Stańczak, J. Kalifornijski Test Uczenia się językowego (CVLT); Psychological Test Laboratory of the Polish Psychological Association: Warsaw, Poland, 2010. [Google Scholar]
- Benedict, R.H.; Weinstock-Guttman, B.; Fishman, I.; Sharma, J.; Tjoa, C.W.; Bakshi, R. Prediction of neuropsychological impairment in multiple sclerosis: Comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch. Neurol. 2004, 61, 226–230. [Google Scholar] [CrossRef]
- Wiedermann, D.; Schuff, N.; Matson, G.B.; Soher, B.J.; Du, A.T.; Maudsley, A.A.; Weiner, M.W. Short echo time multislice proton magnetic resonance spectroscopic imaging in human brain: Metabolite distributions and reliability. Magn. Reson. Imaging 2001, 19, 1073–1080. [Google Scholar] [CrossRef]
- Nagae-Poetscher, L.M.; Bonekamp, D.; Barker, P.B.; Brant, L.J.; Kaufmann, W.E.; Horská, A. Asymmetry and gender effect in functionally lateralized cortical regions: A proton MRS imaging study. J. Magn. Reson. Imaging 2003, 19, 27–33. [Google Scholar] [CrossRef]
- Lenhard, W.; Lenhard, A. Calculation of Effect Sizes. Available online: https://www.psychometrica.de/effect_size.html (accessed on 12 December 2019).
- Tintore, M.; Rovira, A.; Martínez, M.J.; Río, J.; Díaz-Villoslada, P.; Brieva, L.; Borràs, C.; Grivé, E.; Capellades, J.; Montalban, X. Isolated demyelinating syndromes: Comparison of different MR imaging criteria to predict conversion to clinically definite multiple sclerosis. Am. J. Neuroradiol. 2000, 21, 702–706. [Google Scholar]
- Potagas, C.; Giogkaraki, E.; Koutsis, G.; Mandellos, D.; Tsirempolou, E.; Sfagos, C.; Vassilopoulos, D. Cognitive impairment in different MS subtypes and clinically isolated syndromes. J. Neurol. Sci. 2008, 267, 100–106. [Google Scholar] [CrossRef]
- Benedict, R.H.; Bruce, J.M.; Dwyer, M.G.; Abdelrahman, N.; Hussein, S.; Weinstock-Guttman, B.; Garg, N.; Munschauer, F.; Zivadinov, R. Neocortical Atrophy, Third Ventricular Width, and Cognitive Dysfunction in Multiple Sclerosis. Arch. Neurol. 2006, 63, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Assouad, R.; Louapre, C.; Tourbah, A.; Papeix, C.; Galanaud, D.; Lubetzki, C.; Stankoff, B. Clinical and MRI characterization of MS patients with a pure and severe cognitive onset. Clin. Neurol. Neurosurg. 2014, 126, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Heesen, C.; Schulz, K.; Fiehler, J.; Von Der Mark, U.; Otte, C.; Jung, R.; Poettgen, J.; Krieger, T.; Gold, S.M. Correlates of cognitive dysfunction in multiple sclerosis. Brain Behav. Immun. 2010, 24, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Staffen, W.; Zauner, H.; Mair, A.; Kutzelnigg, A.; Kapeller, P.; Stangl, H.; Ladurner, G. Magnetic resonance spectroscopy of memory and frontal brain region in early multiple sclerosis. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Gadea, M.; Martínez-Bisbal, M.C.; Martí-Bonmatí, L.; Espert, R.; Casanova, B.; Coret, F.; Celda, B. Spectroscopic axonal damage of the right locus coeruleus relates to selective attention impairment in early stage relapsing-remitting multiple sclerosis. Brain 2004, 127, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K. The prefrontal cortex and cognitive control. Nat. Rev. Neurosci. 2000, 1, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.C. Frontal lobes and human memory: Insights from functional neuroimaging. Brain 2001, 124, 849–881. [Google Scholar] [CrossRef]
- Pokryszko-Dragan, A.; Bladowska, J.; Zimny, A.; Slotwinski, K.; Zagrajek, M.; Gruszka, E.; Bilinska, M.; Sasiadek, M.; Podemski, R. Magnetic resonance spectroscopy findings as related to fatigue and cognitive performance in multiple sclerosis patients with mild disability. J. Neurol. Sci. 2014, 339, 35–40. [Google Scholar] [CrossRef]
- Schuff, N.; Ezekiel, F.; Gamst, A.C.; Amend, D.L.; Capizzano, A.A.; Maudsley, A.A.; Weiner, M. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn. Reson. Med. 2001, 45, 899–907. [Google Scholar] [CrossRef]
- Baker, E.H.; Basso, G.; Barker, P.B.; Smith, M.A.; Bonekamp, D.; Horska, A. Regional apparent metabolite concentrations in young adult brain measured by1H MR spectroscopy at 3 Tesla. J. Magn. Reson. Imaging 2008, 27, 489–499. [Google Scholar] [CrossRef]
- Li, Y.; Jewells, V.; Kim, M.; Chen, Y.; Moon, A.; Armao, D.; Troiani, L.; Markovic-Plese, S.; Lin, W.; Shen, D. Diffusion tensor imaging based network analysis detects alterations of neuroconnectivity in patients with clinically early relapsing-remitting multiple sclerosis. Hum. Brain Mapp. 2013, 34, 3376–3391. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, H.; Yang, Y.; Zhang, K.; Gao, X.; Li, X.; Ni, L.; Lin, P.; Niu, C. Metabolic changes in normal appearing white matter in multiple sclerosis patients using multivoxel magnetic resonance spectroscopy imaging. Medicine 2017, 96, e6534. [Google Scholar] [CrossRef] [PubMed]
- Kalkers, N.F.; Vrenken, H.; Uitdehaag, B.M.J.; Polman, C.H.; Barkhof, F. Brain atrophy in multiple sclerosis: Impact of lesions and of damage of whole brain tissue. Mult. Scler. J. 2002, 8, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, R.; Czarnecki, D.; Shaikh, Z.A.; Priore, R.L.; Janardhan, V.; Kaliszky, Z.; Kinkel, P.R. Brain MRI lesions and atrophy are related to depression in multiple sclerosis. NeuroReport 2000, 11, 1153–1158. [Google Scholar] [CrossRef]
- Mayberg, H.S. Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. Br. Med Bull. 2003, 65, 193–207. [Google Scholar] [CrossRef]
- Lai, C.-H. Fear Network Model in Panic Disorder: The Past and the Future. Psychiatry Investig. 2018, 16, 16–26. [Google Scholar] [CrossRef]
- Davies, S.E.C.; Newcombe, J.; Williams, S.R.; McDonald, W.I.; Clark, J.B. High Resolution Proton NMR Spectroscopy of Multiple Sclerosis Lesions. J. Neurochem. 2002, 64, 742–748. [Google Scholar] [CrossRef]
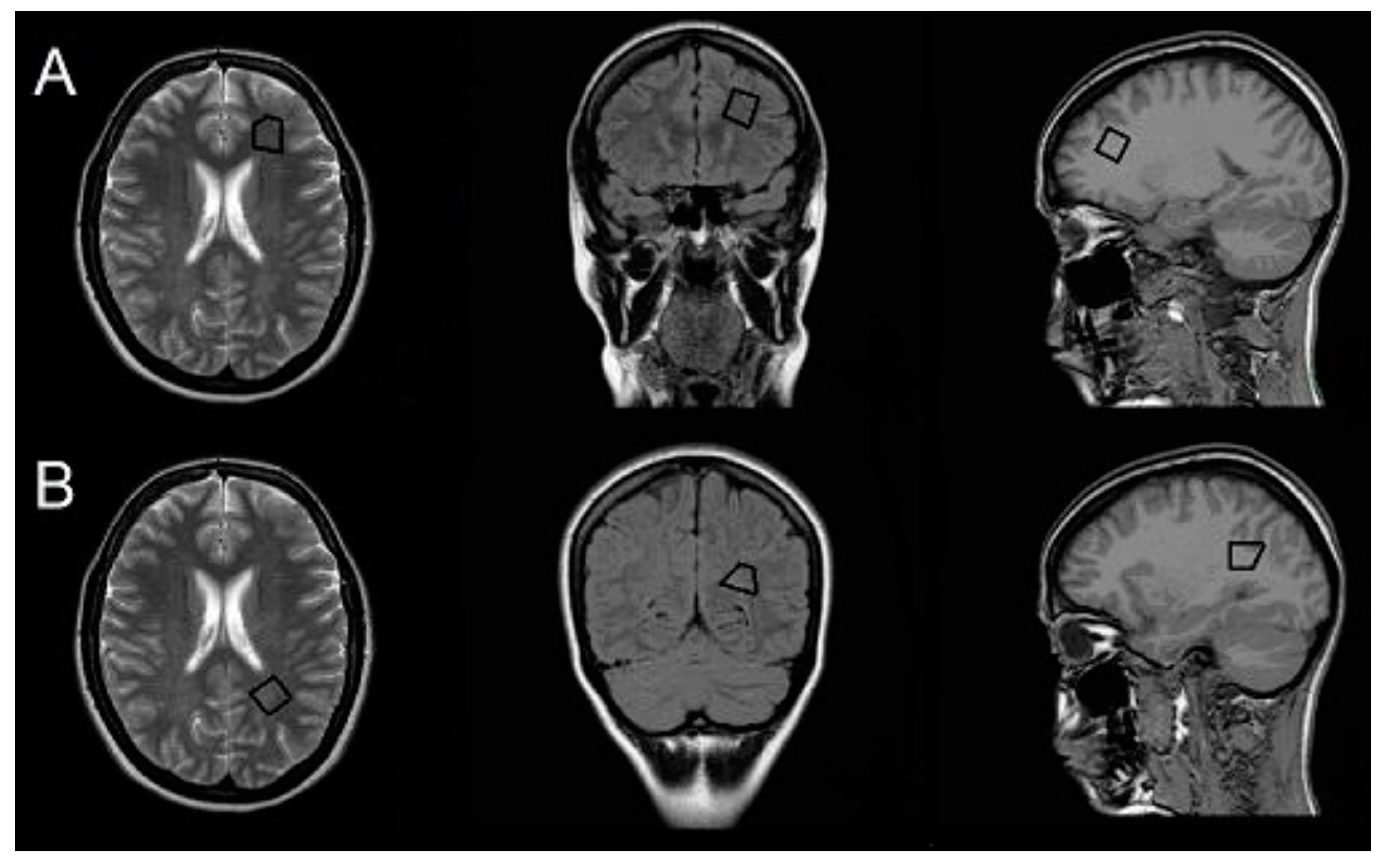
| Characteristic | CIS Group (n = 40) | HP Group (n = 40) | p-Value |
|---|---|---|---|
| Age (years) | 32 (26.5–38.0) | 30 (26.5–39.5) | 0.92 |
| Female-to-male ratio | 29:11 | 28:12 | 0.8 |
| Education (years) | 15 (12–17) | 16 (13–17) | 0.37 |
| Disease duration (months) | 5 (3–8) | ||
| CIS type | |||
| Optic neuritis | 19 (47.5) | ||
| Transverse myelitis | 9 (22.5) | ||
| Brainstem/cerebellar | 9 (22.5) | ||
| Cerebral | 3 (7.5) | ||
| EDSS | 1.25 (1.0–1.5) |
| Test | CIS Group (n = 40) | HP Group (n = 40) | Effect Size | p-Value |
|---|---|---|---|---|
| TMT A (s) | 29.87 ± 10.45 | 26.82 ± 7.06 | 0.43 | 0.13 |
| TMT B (s) | 64.50 (45.85–76.70) | 50.95 (44.05–61.35) | 0.56 | 0.02 |
| SCWT time (s) | 53.10 (47.35–59.30) | 46.90 (39.50–55.10) | 0.51 | 0.03 |
| SCWT errors | 0 (0–1.00) | 0 (0–1.00) | 0.13 | 0.57 |
| SDMT | 47.88 ± 5.1 | 55.50 ± 7.15 | 1.07 | <0.0001 |
| Verbal Fluency Test | 31.0 (26.0–36.0) | 33.5 (28.0–33.5) | 0.18 | 0.43 |
| SRT (s) | 261.5 (236.0–288.5) | 247.0 (220.0–280.5) | 0.22 | 0.32 |
| Go/no-go RT (s) | 317.0 (294.0–366.5) | 299.5 (278.5–341.5) | 0.35 | 0.12 |
| No-go errors | 3.0 (1.5–4.5) | 3.0 (2.0–5.5) | 0.21 | 0.35 |
| CVLT TT | 60.30 ± 7.07 | 62.90 ± 5.42 | 0.41 | 0.07 |
| CVLT T1 | 9.0 (8.0–10.0) | 9.0 (8.0–10.0) | 0.14 | 0.55 |
| CVLT T5 | 14,0 (13.0–15.5) | 15.0 (14.0–16.0) | 0.25 | 0.27 |
| CVLT SDR | 13.0 (11.0–14.0) | 14.0 (13.0–15.0) | 0.50 | 0.04 |
| CVLT LDR | 13.5 (12.5–15.0) | 14.0 (13.0–15.0) | 0.23 | 0.32 |
| BDI | 5.0 (3.0–11.5) | 3 (1–7) | 0.51 | 0.03 |
| HADS-D | 2.0 (1.0–4.5) | 1.0 (0.5–4.0) | 0.36 | 0.12 |
| HADS-A | 5.5 (4.0–8.0) | 3.5 (1.5–6.5) | 0.65 | 0.006 |
| 1H-MRS Ratio | Frontal VOI | Parietal VOI | Effect Size | p-Value |
|---|---|---|---|---|
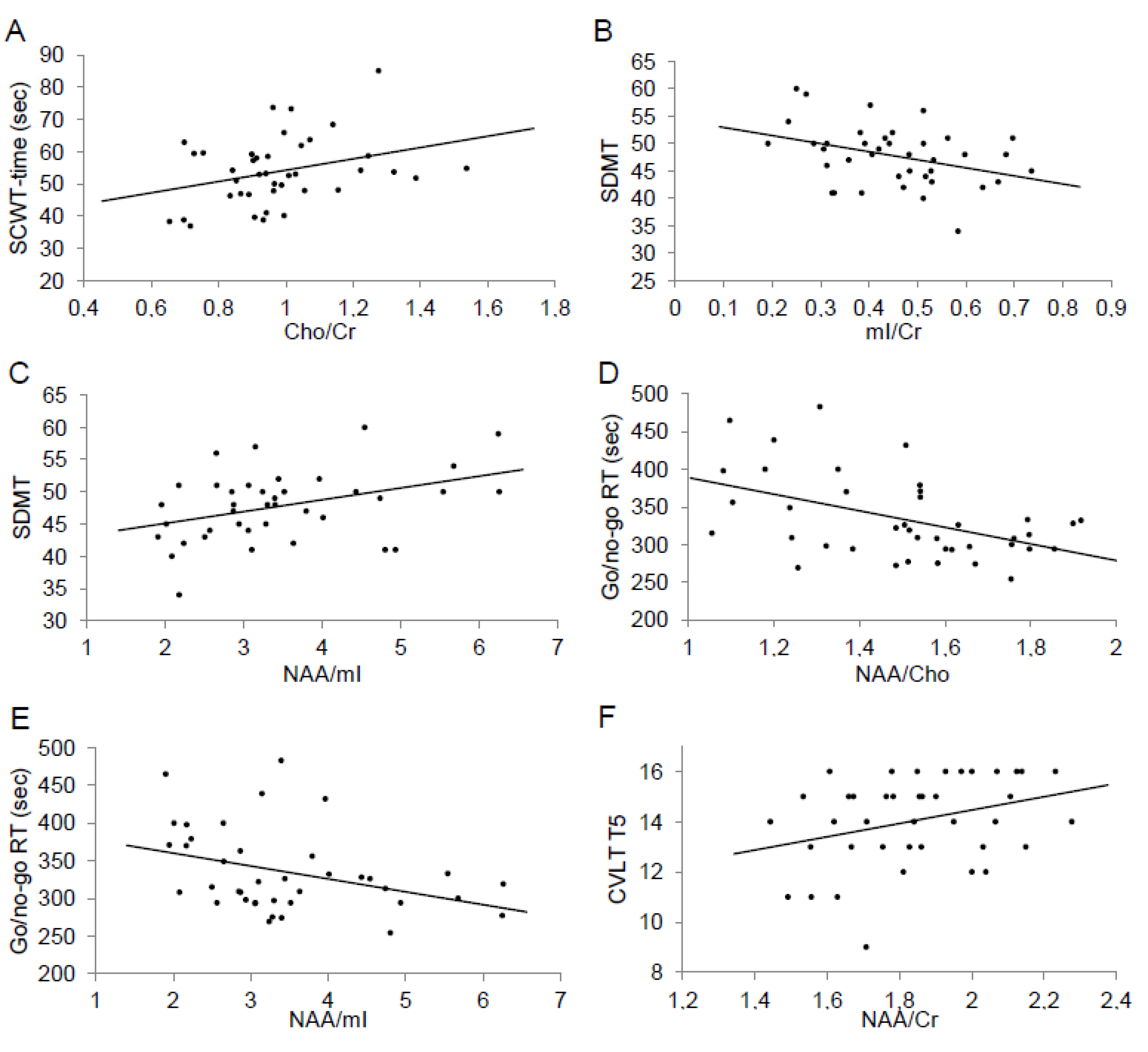
| NAA/Cr | 1.85 (0.21) | 2.05 (0.31) | 0.75 | <0.001 |
| mI/Cr | 0.45 (0.13) | 0.46 (0.13) | 0.13 | 0.45 |
| Cho/Cr | 0.98 (0.19) | 0.92 (0.15) | 0.32 | 0.05 |
| NAA/Cho | 1.50 (0.24) | 1.78 (0.26) | 1.08 | <0.001 |
| NAA/mI | 3.48 (1.17) | 3.80 (1.27) | 0.25 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guenter, W.; Bieliński, M.; Bonek, R.; Borkowska, A. Neurochemical Changes in the Brain and Neuropsychiatric Symptoms in Clinically Isolated Syndrome. J. Clin. Med. 2020, 9, 3909. https://doi.org/10.3390/jcm9123909
Guenter W, Bieliński M, Bonek R, Borkowska A. Neurochemical Changes in the Brain and Neuropsychiatric Symptoms in Clinically Isolated Syndrome. Journal of Clinical Medicine. 2020; 9(12):3909. https://doi.org/10.3390/jcm9123909
Chicago/Turabian StyleGuenter, Wojciech, Maciej Bieliński, Robert Bonek, and Alina Borkowska. 2020. "Neurochemical Changes in the Brain and Neuropsychiatric Symptoms in Clinically Isolated Syndrome" Journal of Clinical Medicine 9, no. 12: 3909. https://doi.org/10.3390/jcm9123909
APA StyleGuenter, W., Bieliński, M., Bonek, R., & Borkowska, A. (2020). Neurochemical Changes in the Brain and Neuropsychiatric Symptoms in Clinically Isolated Syndrome. Journal of Clinical Medicine, 9(12), 3909. https://doi.org/10.3390/jcm9123909





