Inhibition of 11β-HSD1 Expression by Insulin in Skin: Impact for Diabetic Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Studies
2.2. Isolation of Primary Cells and Cell Culture
2.3. Human Skin
2.4. Stimulation Experiments
2.5. RNA Preparation and Quantitative Real-Time PCR
2.6. Western Blot
2.7. ELISA
2.8. Statistics
3. Results
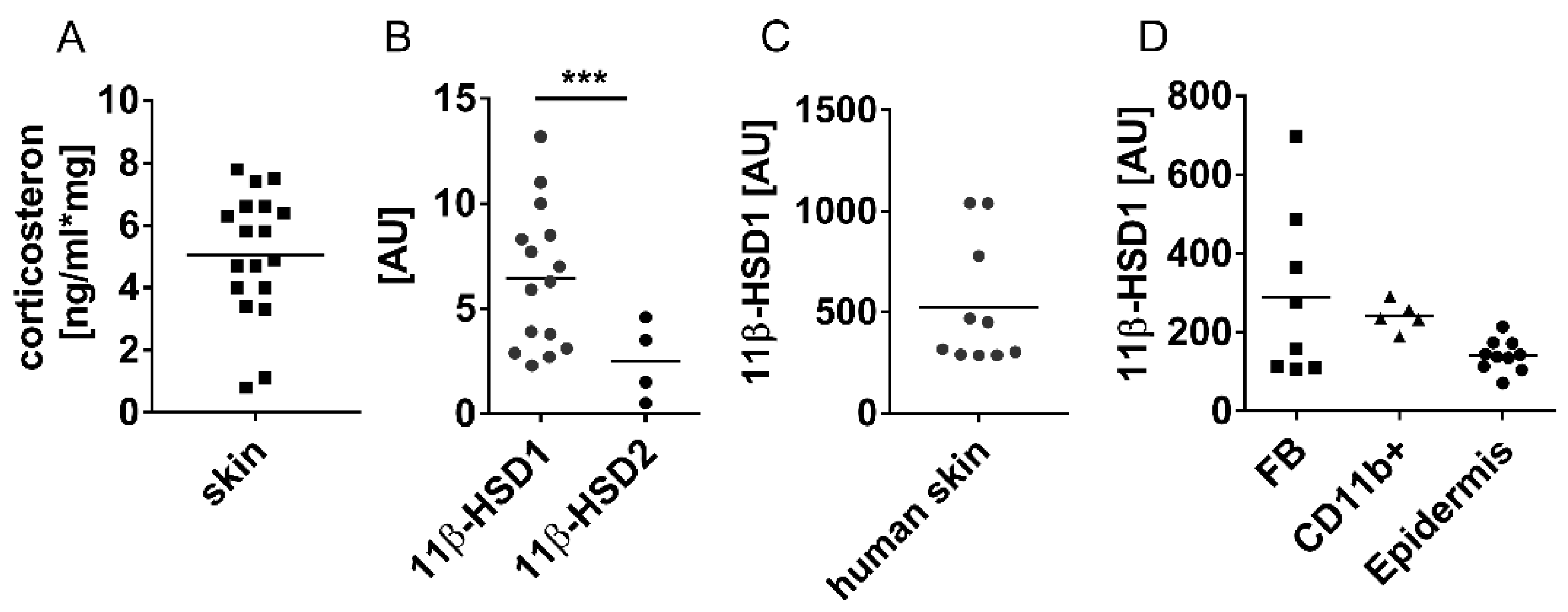
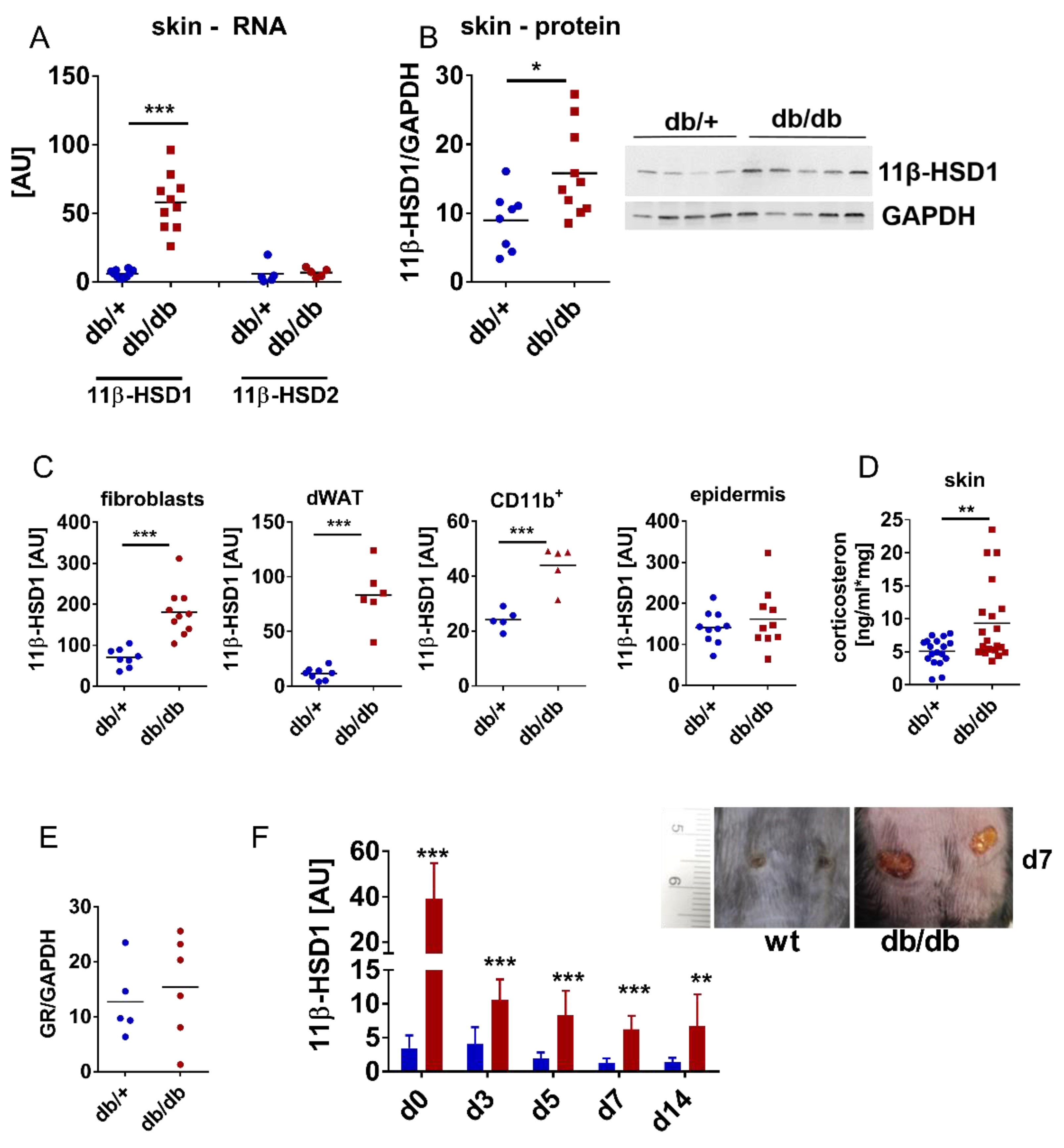
3.1. Cutaneous 11β-HSD1 Expression and Corticosterone Levels are Increased in the Wounds and Skin of Diabetic, Obese Mice
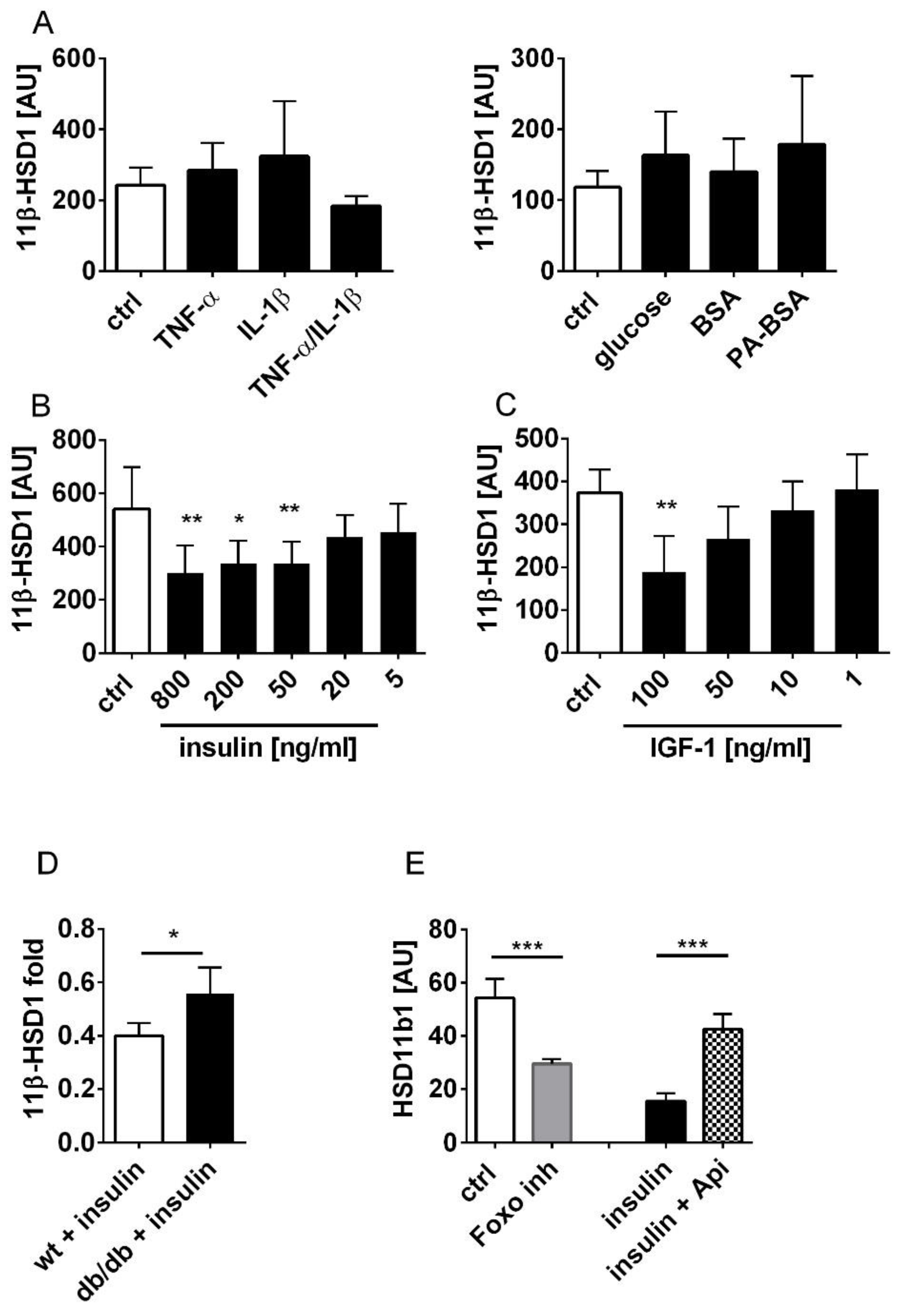
3.2. Insulin and IGF-1 Suppress 11β-HSD1 Expression in Dermal Fibroblasts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Jiang, D.; Hossini, A.M.; Nikolakis, G.; Wlaschek, M.; Scharffetter-Kochanek, K.; Zouboulis, C.C. Diabetes mellitus and the skin. Rev. Endocr. Metab. Disord. 2016, 17, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.L.; Illing, T.; Schliemann, S.; Elsner, P. Cutaneous Manifestations of Diabetes Mellitus: A Review. Am. J. Clin. Dermatol. 2017, 18, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Kimball, A.; Boniakowski, A.; Gallagher, K.A. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr. Diabetes Rep. 2018, 18, 2. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Siegel, K.R.; Gujral, U.P.; Narayan, K.M.V. Type 2 diabetes: A 21st century epidemic. Best Pr. Res. Clin. Endocrinol. Metab. 2016, 30, 331–343. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Otranto, M.; Nascimento, A.P.D.; Monte-Alto-Costa, A. Insulin resistance impairs cutaneous wound healing in mice. Wound Repair Regen. 2013, 21, 464–472. [Google Scholar] [CrossRef]
- Reinke, J.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Seitz, O.; Schürmann, C.; Hermes, N.; Müller, E.; Pfeilschifter, J.; Frank, S.; Goren, I. Wound Healing in Mice with High-Fat Diet- or ob Gene-Induced Diabetes-Obesity Syndromes: A Comparative Study. Exp. Diabetes Res. 2011, 2010, 476969. [Google Scholar] [CrossRef]
- Schoepe, S.; Schacke, H.; May, E.; Asadullah, K. Glucocorticoid therapy-induced skin atrophy. Exp. Dermatol. 2006, 15, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Escoter-Torres, L.; Caratti, G.; Mechtidou, A.; Tuckermann, J.; Uhlenhaut, N.H.; Vettorazzi, S. Fighting the Fire: Mechanisms of Inflammatory Gene Regulation by the Glucocorticoid Receptor. Front. Immunol. 2019, 10, 1859. [Google Scholar] [CrossRef] [PubMed]
- Grose, R.; Werner, S.; Kessler, D.; Tuckermann, J.; Huggel, K.; Durka, S.; Reichardt, H.M.; Werner, S. A role for endogenous glucocorticoids in wound repair. EMBO Rep. 2002, 3, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, A.; Alba, L.; Latorre, V.; Sevilla, L.M.; Pérez, P. Keratinocyte-Targeted Overexpression of the Glucocorticoid Receptor Delays Cutaneous Wound Healing. PLoS ONE 2012, 7, e29701. [Google Scholar] [CrossRef] [PubMed]
- Boix, J.; Sevilla, L.M.; Sáez, Z.; Carceller, E.; Kahlenberg, J.M. Epidermal Mineralocorticoid Receptor Plays Beneficial and Adverse Effects in Skin and Mediates Glucocorticoid Responses. J. Investig. Dermatol. 2016, 136, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Farman, N.; Maubec, E.; Nassar, D.; Desposito, D.; Waeckel, L.; Aractingi, S.; Jaisser, F. Re-Epithelialization of Pathological Cutaneous Wounds Is Improved by Local Mineralocorticoid Receptor Antagonism. J. Investig. Dermatol. 2016, 136, 2080–2089. [Google Scholar] [CrossRef]
- Ahmed, A.; Schmidt, C.; Brunner, T. Extra-Adrenal Glucocorticoid Synthesis in the Intestinal Mucosa: Between Immune Homeostasis and Immune Escape. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Phan, T.S.; Merk, V.M.; Brunner, T. Extra-adrenal glucocorticoid synthesis at epithelial barriers. Genes Immun. 2019, 20, 627–640. [Google Scholar] [CrossRef]
- Tiganescu, A.; Walker, E.A.; Hardy, R.S.; Mayes, A.E.; Stewart, P.M. Localization, Age- and Site-Dependent Expression, and Regulation of 11β-Hydroxysteroid Dehydrogenase Type 1 in Skin. J. Investig. Dermatol. 2011, 131, 30–36. [Google Scholar] [CrossRef]
- Terao, M.; Katayama, I. Local cortisol/corticosterone activation in skin physiology and pathology. J. Dermatol. Sci. 2016, 84, 11–16. [Google Scholar] [CrossRef]
- Morgan, S.A.; McCabe, E.L.; Gathercole, L.L.; Hassan-Smith, Z.K.; Larner, D.P.; Bujalska, I.J.; Stewart, P.M.; Tomlinson, J.W.; Lavery, G.G. 11β-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proc. Natl. Acad. Sci. USA 2014, 111, E2482–E2491. [Google Scholar] [CrossRef] [PubMed]
- Tiganescu, A.; Hupe, M.; Uchida, Y.; Mauro, T.M.; Elias, P.M.; Holleran, W.M. Increased glucocorticoid activation during mouse skin wound healing. J. Endocrinol. 2014, 221, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Tiganescu, A.; Hupe, M.; Uchida, Y.; Mauro, T.; Elias, P.M.; Holleran, W.M. Topical 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibition Corrects Cutaneous Features of Systemic Glucocorticoid Excess in Female Mice. Endocrinology 2018, 159, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Terao, M.; Murota, H.; Kimura, A.; Kato, A.; Ishikawa, A.; Igawa, K.; Miyoshi, E.; Katayama, I. 11β-Hydroxysteroid Dehydrogenase-1 Is a Novel Regulator of Skin Homeostasis and a Candidate Target for Promoting Tissue Repair. PLoS ONE 2011, 6, e25039. [Google Scholar] [CrossRef]
- Schmidt, M.; Gutknecht, D.; Simon, J.C.; Schulz, J.-N.; Eckes, B.; Anderegg, U.; Saalbach, A. Controlling the Balance of Fibroblast Proliferation and Differentiation: Impact of Thy-1. J. Investig. Dermatol. 2015, 135, 1893–1902. [Google Scholar] [CrossRef]
- Schmidt, M.; Gutknecht, D.; Anastassiadis, K.; Eckes, B.; Anderegg, U.; Saalbach, A.; Information, P.E.K.F.C. Thy-1/β3 Integrin Interaction-Induced Apoptosis of Dermal Fibroblasts Is Mediated by Up-Regulation of FasL Expression. J. Investig. Dermatol. 2016, 136, 526–529. [Google Scholar] [CrossRef][Green Version]
- Herbert, D.; Franz, S.; Popkova, Y.; Anderegg, U.; Schiller, J.; Schwede, K.; Lorz, A.; Simon, J.C.; Saalbach, A. High-Fat Diet Exacerbates Early Psoriatic Skin Inflammation Independent of Obesity: Saturated Fatty Acids as Key Players. J. Investig. Dermatol. 2018, 138, 1999–2009. [Google Scholar] [CrossRef]
- Stelzner, K.; Herbert, D.; Popkova, Y.; Lorz, A.; Schiller, J.; Gericke, M.; Klöting, N.; Blüher, M.; Franz, S.; Simon, J.C.; et al. Free fatty acids sensitize dendritic cells to amplify TH1/TH17-immune responses. Eur. J. Immunol. 2016, 46, 2043–2053. [Google Scholar] [CrossRef]
- Hakuno, F.; Takahashi, S.-I. 40 YEARS OF IGF1: IGF1 receptor signaling pathways. J. Mol. Endocrinol. 2018, 61, T69–T86. [Google Scholar] [CrossRef]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef]
- Baida, G.; Bhalla, P.; Kirsanov, K.; Lesovaya, E.; Yakubovskaya, M.; Yuen, K.; Guo, S.; Lavker, R.M.; Readhead, B.; Dudley, J.T.; et al. REDD 1 functions at the crossroads between the therapeutic and adverse effects of topical glucocorticoids. EMBO Mol. Med. 2015, 7, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sivasankar, M.; Kraus, D.H.; Sandulache, V.C.; Amin, M.; Branski, R.C. Glucocorticoids regulate extracellular matrix metabolism in human vocal fold fibroblasts. Laryngoscope 2011, 121, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, J.S.; Coutinho, A.E.; Cailhier, J.-F.; Man, T.Y.; Clay, M.F.; Thomas, G.L.; Harris, H.J.; Mullins, J.J.; Seckl, J.R.; Savill, J.S.; et al. Local Amplification of Glucocorticoids by 11β-Hydroxysteroid Dehydrogenase Type 1 Promotes Macrophage Phagocytosis of Apoptotic Leukocytes. J. Immunol. 2006, 176, 7605–7611. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.K.; Kaplan, N.; Tsoi, L.C.; Xing, X.; Liang, Y.; Swindell, W.R.; Hoover, P.; Aravind, M.; Baida, G.; Clark, M.; et al. Endogenous Glucocorticoid Deficiency in Psoriasis Promotes Inflammation and Abnormal Differentiation. J. Investig. Dermatol. 2017, 137, 1474–1483. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Daynes, R.A. Macrophages from 11β-Hydroxysteroid Dehydrogenase Type 1-Deficient Mice Exhibit an Increased Sensitivity to Lipopolysaccharide Stimulation Due to TGF-β-Mediated Up-Regulation of SHIP1 Expression. J. Immunol. 2007, 179, 6325–6335. [Google Scholar] [CrossRef]
- Chapman, K.E.; Coutinho, A.E.; Zhang, Z.; Kipari, T.; Savill, J.S.; Seckl, J.R. Changing glucocorticoid action: 11β-Hydroxysteroid dehydrogenase type 1 in acute and chronic inflammation. J. Steroid Biochem. Mol. Biol. 2013, 137, 82–92. [Google Scholar] [CrossRef]
- Joost, S.; Annusver, K.; Jacob, T.; Sun, X.; Dalessandri, T.; Sivan, U.; Sequeira, I.; Sandberg, R.; Kasper, M. The Molecular Anatomy of Mouse Skin during Hair Growth and Rest. Cell Stem Cell 2020, 26, 441–457. [Google Scholar] [CrossRef]
- Vukelic, S.; Stojadinovic, O.; Pastar, I.; Rabach, M.; Krzyzanowska, A.; Lebrun, E.; Davis, S.C.; Resnik, S.; Brem, H.; Tomic-Canic, M. Cortisol Synthesis in Epidermis Is Induced by IL-1 and Tissue Injury. J. Biol. Chem. 2011, 286, 10265–10275. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier: NCT03313297, Glucocorticoids and Skin Healing in Diabetes (GC-SHealD). Available online: https://clinicaltrials.gov/ct2/show/NCT03313297?term=tiganescu&draw=2&rank=1 (accessed on 6 April 2020).
- Tiganescu, A.; Tahrani, A.A.; Morgan, S.A.; Otranto, M.; Desmoulière, A.; Abrahams, L.; Amrein, K. ‘11HSD blockade prevents age-induced skin structure and function defects’. J. Clin. Invest. 2013, 123, 3051–3060. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, P.; Guo, C.; Zhu, X.; Sun, K. Expression of 11β-Hydroxysteroid Dehydrogenase Type 1 in Human Fetal Lung and Regulation of Its Expression by Interleukin-1β and Cortisol. J. Clin. Endocrinol. Metab. 2009, 94, 306–313. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.; Zhu, X.; Guo, C.; Sun, K. Stimulation of 11β-HSD1 expression by IL-1β via a C/EBP binding site in human fetal lung fibroblasts. Endocrinology 2009, 36, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, I.D.; Kostadinova, R.M.; Goldring, C.E.; Nawrocki, A.R.; Frey, F.J.; Frey, B.M. Tumor necrosis factor-α upregulates 11β-hydroxysteroid dehydrogenase type 1 expression by CCAAT/enhancer binding protein-β in HepG2 cells. Am. J. Physiol. Metab. 2009, 296, E367–E377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Vatankhah, N.; Jahangiri, Y.; Landry, G.J.; Moneta, G.L.; Azarbal, A.F. Effect of systemic insulin treatment on diabetic wound healing. Wound Repair Regen. 2017, 25, 288–291. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E. Effects of insulin on wound healing: A review of animal and human evidences. Life Sci. 2017, 174, 59–67. [Google Scholar] [CrossRef]
- Han, X.; Tao, Y.; Deng, Y.; Yu, J.; Sun, Y.; Jiang, G.-J. Metformin accelerates wound healing in type 2 diabetic db/db mice. Mol. Med. Rep. 2017, 16, 8691–8698. [Google Scholar] [CrossRef]
- Qing, L.; Fu, J.; Wu, P.; Zhou, Z.; Yu, F.; Tang, J.-Y. Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway. Am. J. Transl. Res. 2019, 11, 655–668. [Google Scholar]
- Hrynyk, M.; Neufeld, R.J. Insulin and wound healing. Burns 2014, 40, 1433–1446. [Google Scholar] [CrossRef]
- Sridharan, K.; Sivaramakrishnan, G. Efficacy of topical insulin in wound healing: A preliminary systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2017, 25, 279–287. [Google Scholar] [CrossRef]
- Stephen, S.; Agnihotri, M.; Kaur, S. A Randomized, Controlled Trial to Assess the Effect of Topical Insulin Versus Normal Saline in Pressure Ulcer Healing. Ostomy/Wound Manag. 2016, 62, 16–23. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brazel, C.B.; Simon, J.C.; Tuckermann, J.P.; Saalbach, A. Inhibition of 11β-HSD1 Expression by Insulin in Skin: Impact for Diabetic Wound Healing. J. Clin. Med. 2020, 9, 3878. https://doi.org/10.3390/jcm9123878
Brazel CB, Simon JC, Tuckermann JP, Saalbach A. Inhibition of 11β-HSD1 Expression by Insulin in Skin: Impact for Diabetic Wound Healing. Journal of Clinical Medicine. 2020; 9(12):3878. https://doi.org/10.3390/jcm9123878
Chicago/Turabian StyleBrazel, Christina B., Jan C. Simon, Jan P. Tuckermann, and Anja Saalbach. 2020. "Inhibition of 11β-HSD1 Expression by Insulin in Skin: Impact for Diabetic Wound Healing" Journal of Clinical Medicine 9, no. 12: 3878. https://doi.org/10.3390/jcm9123878
APA StyleBrazel, C. B., Simon, J. C., Tuckermann, J. P., & Saalbach, A. (2020). Inhibition of 11β-HSD1 Expression by Insulin in Skin: Impact for Diabetic Wound Healing. Journal of Clinical Medicine, 9(12), 3878. https://doi.org/10.3390/jcm9123878




