Eosinophilic Esophagitis in Children in North-Eastern Poland
Abstract
1. Introduction
2. Materials and Methods
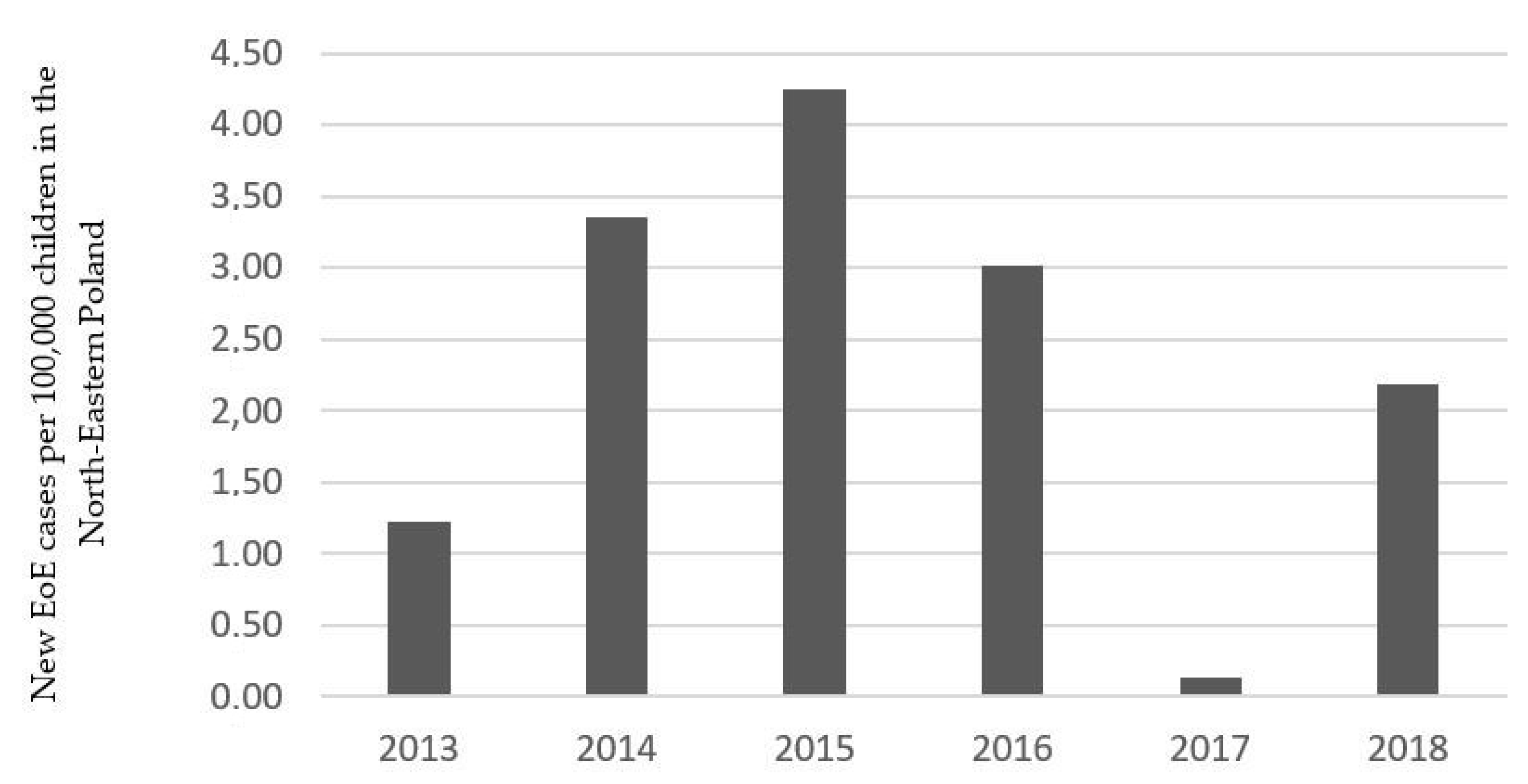
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lucendo, A.J.; Molina-Infante, J.; Arias, Á.; von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Dias, J.A.; Bove, M.; González-Cervera, J.; Larsson, H.; et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur. Gastroenterol. J. 2017, 5, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Liacouras, C.A.; Molina-Infante, J.; Furuta, G.T.; Spergel, J.M.; Zevit, N.; Spechler, S.J.; Attwood, S.E.; Straumann, A.; Aceves, S.S.; et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018, 155, 1022–1033.e10. [Google Scholar] [CrossRef] [PubMed]
- González-Cervera, J.; Lucendo, A.J. Eosinophilic Esophagitis: An Evidence-Based Approach to Therapy. J. Investig. Allergol. Clin. Immunol. 2016, 26, 8–18. [Google Scholar] [PubMed]
- González-Cervera, J.; Arias, Á.; Redondo-González, O.; Cano-Mollinedo, M.M.; Terreehorst, I.; Lucendo, A.J. Association between atopic manifestations and eosinophilic esophagitis: A systematic review and meta-analysis. Ann. Allergy Asthma Immunol. 2017, 118, 582–590.e2. [Google Scholar] [CrossRef]
- Arias, Á.; Pérez-Martínez, I.; Tenías, J.M.; Lucendo, A.J. Systematic review with meta-analysis: The incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment. Pharmacol. Ther. 2016, 43, 3–15. [Google Scholar] [CrossRef]
- Syed, A.A.; Andrews, C.N.; Shaffer, E.; Urbanski, S.J.; Beck, P.; Storr, M. The rising incidence of eosinophilic oesophagitis is associated with increasing biopsy rates: A population-based study. Aliment. Pharmacol. Ther. 2012, 36, 950–958. [Google Scholar] [CrossRef]
- Alexander, E.S.; Martin, L.J.; Collins, M.H.; Kottyan, L.C.; Sucharew, H.; He, H.; Mukkada, V.A.; Succop, P.A.; Abonia, J.P.; Foote, H.; et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J. Allergy Clin. Immunol. 2014, 134, 1084–1092.e1. [Google Scholar] [CrossRef]
- Weerasekera, K.; Sim, D.; Coughlan, F.; Inns, S. Eosinophilic esophagitis incidence in New Zealand: High but not increasing. Clin. Exp. Gastroenterol. 2019, 12, 367–374. [Google Scholar] [CrossRef]
- Robson, J.; O’Gorman, M.; McClain, A.; Mutyala, K.; Davis, C.; Barbagelata, C.; Wheeler, J.; Firszt, R.; Smith, K.; Patel, R.; et al. Incidence and Prevalence of Pediatric Eosinophilic Esophagitis in Utah Based on a 5-Year Population-Based Study. Clin. Gastroenterol. Hepatol. 2019, 17, 107–114.e1. [Google Scholar] [CrossRef]
- Ristic, N.; Jankovic, R.; Dragutinovic, N.; Atanaskovic-Markovic, M.; Radusinovic, M.; Stevic, M.; Ristic, M.; Ristic, M.; Milovanovic, T. Diagnosis of Eosinophilic Esophagitis in Children: A Serbian Single-Center Experience from 2010 to 2017. Med. Princ. Pract. 2019, 28, 449–456. [Google Scholar] [CrossRef]
- Dellon, E.S.; Jensen, E.T.; Martin, C.F.; Shaheen, N.J.; Kappelman, M.D. Prevalence of eosinophilic esophagitis in the United States. Clin. Gastroenterol. Hepatol. 2014, 12, 589–596.e1. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.N.; Srivastava, S.; Teh, M.; Quak, S.H.; Aw, M.M. Eosinophilic oesophagitis in children: An uncommon occurrence in a predominantly Chinese population in Singapore. Singap. Med. J. 2017, 58, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Sherrill, J.D.; Gao, P.S.; Stucke, E.M.; Blanchard, C.; Collins, M.H.; Putnam, P.E.; Franciosi, J.P.; Kushner, J.P.; Abonia, J.P.; Assa’ad, A.H.; et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J. Allergy Clin. Immunol. 2010, 126, 160–165.e3. [Google Scholar] [CrossRef] [PubMed]
- Aceves, S.S.; Newbury, R.O.; Dohil, R.; Schwimmer, J.; Bastian, J.F. Distinguishing eosinophilic esophagitis in pediatric patients: Clinical, endoscopic, and histologic features of an emerging disorder. J. Clin. Gastroenterol. 2007, 41, 252–256. [Google Scholar] [CrossRef]
- Pinheiro, M.I.; de Góes Cavalcanti, L.P.; Honório, R.S.; de Alencar Moreno, L.H.; Fortes, M.C.; da Silva, C.A. Eosinophilic esophagitis in brazilian pediatric patients. Clin. Med. Insights Pediatr. 2013, 7, 41–48. [Google Scholar] [CrossRef]
- Gómez Torrijos, E.; Sánchez Miranda, P.; Donado Palencia, P.; Castro Jimenez, A.; Rodriguez Sánchez, J.; Mendez Díaz, Y.; Moreno Lozano, L.; Garcia Rodriguez, R. Eosinophilic Esophagitis: Demographic, Clinical, Endoscopic, Histologic, and Atopic Characteristics of Children and Teenagers in a Region in Central Spain. J. Investig. Allergol. Clin. Immunol. 2017, 27, 104–110. [Google Scholar] [CrossRef]
- Mehta, P.; Furuta, G.T.; Brennan, T.; Henry, M.L.; Maune, N.C.; Sundaram, S.S.; Menard-Katcher, C.; Atkins, D.; Takurukura, F.; Giffen, S.; et al. Nutritional State and Feeding Behaviors of Children With Eosinophilic Esophagitis and Gastroesophageal Reflux Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 603–608. [Google Scholar] [CrossRef]
- Hoofien, A.; Dias, J.A.; Malamisura, M.; Rea, F.; Chong, S.; Oudshoorn, J.; Nijenhuis-Hendriks, D.; Otte, S.; Papadopoulou, A.; Romano, C.; et al. Pediatric Eosinophilic Esophagitis: Results of the European Retrospective Pediatric Eosinophilic Esophagitis Registry (RetroPEER). J. Pediatr. Gastroenterol. Nutr. 2019, 68, 552–558. [Google Scholar] [CrossRef]
- Fiocchi, A.; Pecora, V.; Petersson, C.J.; Dahdah, L.; Borres, M.P.; Amengual, M.J.; Huss-Marp, J.; Mazzina, O.; Di Girolamo, F. Sensitization pattern to inhalant and food allergens in symptomatic children at first evaluation. Ital. J. Pediatr. 2015, 41, 96. [Google Scholar] [CrossRef]
- Daniluk, U.; Alifier, M.; Kaczmarski, M.; Stasiak-Barmuta, A.; Lebensztejn, D. Longitudinal observation of children with enhanced total serum IgE. Ann. Allergy Asthma Immunol. 2015, 114, 404–410.e4. [Google Scholar] [CrossRef]
- Hiremath, G.; Byramji, D.; Pacheco, A.; Constantine, G.; Davis, C.; Shulman, R.; Olive, A. Eosinophilic Esophagitis in Children and Its Relationship with Parental Allergies: Texas Children’s Hospital Experience. Dig. Dis. Sci. 2016, 61, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Homan, M.; Blagus, R.; Jeverica, A.K.; Orel, R. Pediatric Eosinophilic Esophagitis in Slovenia: Data From a Retrospective 2005-2012 Epidemiological Study. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Samoliński, B.; Sybilski, A.J.; Raciborski, F.; Tomaszewska, A.; Samel-Kowalik, P.; Walkiewicz, A.; Lusawa, A.; Borowicz, J.; Gutowska-Ślesik, J.; Trzpil, L.; et al. Prevalence of rhinitis in Polish population according to the ECAP (Epidemiology of Allergic Disorders in Poland) study. Otolaryngol. Pol. 2009, 63, 324–330. [Google Scholar] [CrossRef]
- Lipiec, A.; Sybilski, A.; Rapiejko, P.; Furmańczyk, K.; Namysłowski, A.; Zieliński, W.; Malkiewicz, M.; Bilińska, D.; Chłopek, K.; Samoliński, B. Prevalence of allergic rhinitis and asthma in Poland in relation to pollen counts. Adv. Dermatol. Allergol. 2019, 37, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Fahey, L.; Robinson, G.; Weinberger, K.; Giambrone, A.E.; Solomon, A.B. Correlation Between Aeroallergen Levels and New Diagnosis of Eosinophilic Esophagitis in New York City. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.J.; Arias, Á.; Tenias, J.M. Systematic review: The association between eosinophilic oesophagitis and coeliac disease. Aliment. Pharmacol. Ther. 2014, 40, 422–434. [Google Scholar] [CrossRef]
- Johnsson, M.; Bove, M.; Bergquist, H.; Olsson, M.; Fornwall, S.; Hassel, K.; Wold, A.E.; Wennerås, C. Distinctive blood eosinophilic phenotypes and cytokine patterns in eosinophilic esophagitis, inflammatory bowel disease and airway allergy. J. Innate Immun. 2011, 3, 594–604. [Google Scholar] [CrossRef]
- von Arnim, U.; Wex, T.; Link, A.; Messerschmidt, M.; Venerito, M.; Miehlke, S.; Malfertheiner, P. Helicobacter pylori infection is associated with a reduced risk of developing eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2016, 43, 825–830. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Gutierrez-Junquera, C.; Savarino, E.; Penagini, R.; Modolell, I.; Bartolo, O.; Prieto-García, A.; Mauro, A.; Alcedo, J.; Perelló, A.; et al. Helicobacter pylori infection does not protect against eosinophilic esophagitis: Results from a large multicenter case-control study. Am. J. Gastroenterol. 2018, 113, 972–979. [Google Scholar] [CrossRef]
- Kia, L.; Hirano, I. Distinguishing GERD from eosinophilic oesophagitis: Concepts and controversies. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 379–386. [Google Scholar] [CrossRef]
- Kim, H.P.; Vance, R.B.; Shaheen, N.J.; Dellon, E.S. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 988–996.e5. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, N. Distinct features in the clinical presentations of eosinophilic esophagitis in children and adults: Is this the same disease? Dig. Dis. 2014, 32, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Liacouras, C.A.; Spergel, J.M.; Ruchelli, E.; Verma, R.; Mascarenhas, M.; Semeao, E.; Flick, J.; Kelly, J.; Brown–Whitehorn, T.; Mamula, P.; et al. Eosinophilic esophagitis: A 10-year experience in 381 children. Clin. Gastroenterol. Hepatol. 2005, 3, 1198–1206. [Google Scholar] [CrossRef]
- Dellon, E.S.; Gibbs, W.B.; Fritchie, K.J.; Rubinas, T.C.; Wilson, L.A.; Woosley, J.T.; Shaheen, N.J. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1305–1313. [Google Scholar] [CrossRef]
- Botan, V.; Dos Santos Borges, T.K.; Rocha Alves, É.; Claudino Pereira Couto, S.; Bender Kohnert Seidler, H.; Muniz-Junqueira, M.I. Enhanced activation of eosinophils in peripheral blood and implications for eosinophilic esophagitis diagnosis. J. Gastroenterol. Hepatol. 2017, 32, 1318–1327. [Google Scholar] [CrossRef]
- Schlag, C.; Miehlke, S.; Heiseke, A.; Brockow, K.; Krug, A.; von Arnim, U.; Straumann, A.; Vieth, M.; Bussmann, C.; Mueller, R.; et al. Peripheral blood eosinophils and other non-invasive biomarkers can monitor treatment response in eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2015, 42, 1122–1130. [Google Scholar] [CrossRef]
- Hines, B.T.; Rank, M.A.; Wright, B.L.; Marks, L.A.; Hagan, J.B.; Straumann, A.; Greenhawt, M.; Dellon, E.S. Minimally invasive biomarker studies in eosinophilic esophagitis: A systematic review. Ann. Allergy Asthma Immunol. 2018, 121, 218–228. [Google Scholar] [CrossRef]
- Zdanowicz, K.; Kucharska, M.; Reszec, J.; Lebensztejn, D.M.; Daniluk, U. Immunohistochemical markers for eosinophilic esophagitis. Scand. J. Gastroenterol. 2020, 55, 1–7. [Google Scholar] [CrossRef]
| EoE Group | Non-EoE Group | p Value | |
|---|---|---|---|
| Number of patients | 36 (8.31%) | 397 (91.7%) | - |
| Age (year) | 10 (2–17) | 13 (1–18) | <0.001 |
| Male (n) | 28 (77.78%) | 197 (49.62%) | 0.002 |
| BMI | |||
| <10 percentile | 10 (27.78%) | 73 (18.39%) | NS |
| >90 percentile | 3 (8.33%) | 46 (11.59%) | NS |
| Co-existing conditions | |||
| Food allergy | 4 (11.11%) | 10 (2.52%) | 0.02 |
| Seasonal allergy | 9 (23.08%) | 35 (8.82%) | 0.005 |
| Diabetes mellitus type 1 | 0 (0%) | 1 (0.25%) | NA |
| Celiac disease | 4 (11.11%) | 28 (6.30%) | NS |
| IBD | 4 (11.11%) | 23 (5.79%) | NS |
| Symptoms | |||
| Abdominal pain (n) | 25 (69.4%) | 297 (74.81%) | NS |
| Failure of thrive (n) | 11 (30.6%) | 76 (19.14%) | NS |
| Dysphagia (n) | 8 (22.2%) | 29 (7.30%) | 0.006 |
| Halitosis (n) | 7 (19.4%) | 51 (12.85%) | NS |
| Vomiting (n) | 6 (16.7%) | 89 (22.42%) | NS |
| Lack of appetite (n) | 6 (16.7%) | 54 (13.60%) | NS |
| Heartburn (n) | 1 (2.78%) | 42 (10.58%) | NS |
| Nausea (n) | 3 (8.33%) | 65 (16.37%) | NS |
| Weight loss (n) | 2 (5.56%) | 45 (11.34%) | NS |
| Eructation (n) | 3 (8.33%) | 55 (13.85%) | NS |
| Regurgitation (n) | 2 (5.56%) | 9 (2.28%) | NS |
| Chest pain (n) | 0 (0%) | 10 (2.52%) | NA |
| EoE | Non-EoE | p | |
|---|---|---|---|
| Endoscopy and histology findings | |||
| Longitudinal furrowing (n) | 25 (69.4%) | 19 (4.76%) | <0.001 |
| Decrease vascular pattern (n) | 11 (30.6%) | 18 (4.53%) | <0.001 |
| Whitish exudates (n) | 7 (19.4%) | 9 (2.37%) | <0.001 |
| Esophageal erosion (n) | 5 (13.89%) | 40 (10.08%) | NS |
| Mucosal esophageal erythema (n) | 4 (11.11%) | 41 (10.33%) | NS |
| Papules/plaques (n) | 4 (11.11%) | 46 (11.59%) | NS |
| Trachealization/rings (n) | 3 (8.33%) | 5 (1.26%) | 0.02 |
| Esophageal polyp (n) | 1 (2.78%) | 6 (1.51%) | NS |
| Esophageal hernia (n) | 3 (8.33%) | 36 (9.07%) | NS |
| Helicobacter pylori infection (n) | 16 (44.44%) | 181 (45.59%) | NS |
| Median eosinophil counts (range) | 22 (15–45) | 0 (0–12) | - |
| Number of endoscopic changes: | |||
| 0 | 4 (11.11%) | 249 (65.70%) | <0.001 |
| 1 | 10 (27.78%) | 91 (24.01%) | NS |
| 2 | 14 (38.89%) | 43 (11.35%) | <0.001 |
| 3 | 7 (19.44%) | 10 (2.52%) | <0.001 |
| Laboratory results | |||
| Eosinophilia (n) (>400 eos/μL) | 20 (55.56%) | 26 (6.55%) | <0.001 |
| Hb (median, min-max) (g/dL) | 13.05 (10.7–17.0) | 13.30 (8.1–17.2) | NS |
| CRP (median, min-max) (mg/dL) | 0.2 (0.0–4.8) | 0.2(0.0–52.1) | NS |
| PLT (median, min-max) (×103/μL) | 253 (152–439) | 266 (86–643) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdanowicz, K.; Kucharska, M.; Sobaniec-Lotowska, M.E.; Lebensztejn, D.M.; Daniluk, U. Eosinophilic Esophagitis in Children in North-Eastern Poland. J. Clin. Med. 2020, 9, 3869. https://doi.org/10.3390/jcm9123869
Zdanowicz K, Kucharska M, Sobaniec-Lotowska ME, Lebensztejn DM, Daniluk U. Eosinophilic Esophagitis in Children in North-Eastern Poland. Journal of Clinical Medicine. 2020; 9(12):3869. https://doi.org/10.3390/jcm9123869
Chicago/Turabian StyleZdanowicz, Katarzyna, Magdalena Kucharska, Maria Elzbieta Sobaniec-Lotowska, Dariusz Marek Lebensztejn, and Urszula Daniluk. 2020. "Eosinophilic Esophagitis in Children in North-Eastern Poland" Journal of Clinical Medicine 9, no. 12: 3869. https://doi.org/10.3390/jcm9123869
APA StyleZdanowicz, K., Kucharska, M., Sobaniec-Lotowska, M. E., Lebensztejn, D. M., & Daniluk, U. (2020). Eosinophilic Esophagitis in Children in North-Eastern Poland. Journal of Clinical Medicine, 9(12), 3869. https://doi.org/10.3390/jcm9123869





