The Specific Bile Acid Profile of Shock: A Hypothesis Generating Appraisal of the Literature
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shin, D.-J.; Wang, L. Bile Acid-Activated Receptors: A Review on FXR and Other Nuclear Receptors. In Bile Acids and Their Receptors; Fiorucci, S., Distrutti, E., Eds.; Springer International Publishing: Cham, Swizerland, 2019; pp. 51–72. [Google Scholar]
- Guo, C.; Chen, W.-D.; Wang, Y.-D. TGR5, Not Only a Metabolic Regulator. Front. Physiol. 2016, 7, 646. [Google Scholar] [CrossRef] [PubMed]
- Fryer, R.M.; Ng, K.J.; Mazurek, S.G.N.; Patnaude, L.; Skow, D.J.; Muthukumarana, A.; Gilpin, K.E.; Dinallo, R.M.; Kuzmich, D.; Lord, J.; et al. G Protein–Coupled Bile Acid Receptor 1 Stimulation Mediates Arterial Vasodilation through a KCa1.1 (BKCa)–Dependent Mechanism. J. Pharmacol. Exp. Ther. 2014, 348, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Thounaojam, M.C.; Bartoli, M.; Khurana, S. Deoxycholylglycine, a conjugated secondary bile acid, reduces vascular tone by attenuating Ca2+ sensitivity via rho kinase pathway. Toxicol. Appl. Pharmacol. 2018, 348, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Trauner, M.; Boyer, J.L. Bile salt transporters: Molecular characterization, function, and regulation. Physiol. Rev. 2003, 83, 633–671. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Yoon, S.; Tindberg, N.; Jarvelainen, H.A.; Lindros, K.O.; Ingelman-Sundberg, M. Hepatic expression of multiple acute phase proteins and down-regulation of nuclear receptors after acute endotoxin exposure. Biochem. Pharmacol. 2004, 67, 1389–1397. [Google Scholar] [CrossRef]
- Cherrington, N.J.; Slitt, A.L.; Li, N.; Klaassen, C.D. Lipopolysaccharide-mediated regulation of hepatic transporter mRNA levels in rats. Drug Metab. Dispos. 2004, 32, 734–741. [Google Scholar] [CrossRef]
- Vincent, J.L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and mortality of septic shock in Europe and North America: A systematic review and meta-analysis. Crit. Care 2019, 23, 196. [Google Scholar] [CrossRef]
- Kolte, D.; Khera, S.; Aronow, W.S.; Mujib, M.; Palaniswamy, C.; Sule, S.; Jain, D.; Gotsis, W.; Ahmed, A.; Frishman, W.H.; et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J. Am. Heart Assoc. 2014, 3, e000590. [Google Scholar] [CrossRef]
- Standl, T.; Annecke, T.; Cascorbi, I.; Heller, A.R.; Sabashnikov, A.; Teske, W. The Nomenclature, Definition and Distinction of Types of Shock. Dtsch. Arztebl. Int. 2018, 115, 757–768. [Google Scholar] [CrossRef]
- Janssens, U.; Graf, J. Shock—What are the basics? Internist 2004, 45, 258–266. [Google Scholar] [CrossRef]
- Luo, L.; Aubrecht, J.; Li, D.; Warner, R.L.; Johnson, K.J.; Kenny, J.; Colangelo, J.L. Assessment of serum bile acid profiles as biomarkers of liver injury and liver disease in humans. PLoS ONE 2018, 13, e0193824. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Amano, K.; Nakano, M.; Masubuchi, N.; Sugihara, M.; Matsuura, T. Analysis of the Serum Bile Acid Composition for Differential Diagnosis in Patients with Liver Disease. Gastroenterol. Res. Pract. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Drolz, A.; Roedl, K.; Rutter, K.; Ferlitsch, A.; Fauler, G.; Trauner, M.; Fuhrmann, V. Serum bile acids as marker for acute decompensation and acute-on-chronic liver failure in patients with non-cholestatic cirrhosis. Liver Int. 2017, 37, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Trottier, J.; Bialek, A.; Caron, P.; Straka, R.J.; Milkiewicz, P.; Barbier, O. Profiling circulating and urinary bile acids in patients with biliary obstruction before and after biliary stenting. PLoS ONE 2011, 6, e22094. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Dorko, K.; Antoine, D.J.; Clarke, J.I.; Gholami, P.; Li, F.; Kumer, S.C.; Schmitt, T.M.; Forster, J.; Fan, F.; et al. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol. Appl. Pharmacol. 2015, 283, 168–177. [Google Scholar] [CrossRef]
- Chen, J.; Deng, W.; Wang, J.; Shao, Y.; Ou, M.; Ding, M. Primary bile acids as potential biomarkers for the clinical grading of intrahepatic cholestasis of pregnancy. Int. J. Gynecol. Obstet. 2013, 122, 5–8. [Google Scholar] [CrossRef]
- Horvatits, T.; Drolz, A.; Rutter, K.; Roedl, K.; Fauler, G.; Muller, C.; Kluge, S.; Trauner, M.; Schenk, P.; Fuhrmann, V. Serum bile acids in patients with hepatopulmonary syndrome. Z. Gastroenterol. 2017, 55, 361–367. [Google Scholar] [CrossRef]
- Vanwijngaerden, Y.-M.; Wauters, J.; Langouche, L.; Vander Perre, S.; Liddle, C.; Coulter, S.; Vanderborght, S.; Roskams, T.; Wilmer, A.; Van den Berghe, G.; et al. Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology 2011, 54, 1741–1752. [Google Scholar] [CrossRef]
- Horvatits, T.; Drolz, A.; Rutter, K.; Roedl, K.; Langouche, L.; Van den Berghe, G.; Fauler, G.; Meyer, B.; Hülsmann, M.; Heinz, G.; et al. Circulating bile acids predict outcome in critically ill patients. Ann. Intensive Care 2017, 7, 48. [Google Scholar] [CrossRef]
- LaRue, J.; Stratagoules, E.; Martinez, J. Deoxycholic acid-induced apoptosis is switched to necrosis by bcl-2 and calphostin C. Cancer Lett. 2000, 152, 107–113. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, B.; Qi, M.; Yao, Y.; Xu, L.; Ji, R.; Chen, W.; Wang, J.; Huang, S.; Ma, L.; et al. TUDCA Ameliorates Liver Injury Via Activation of SIRT1-FXR Signaling in a Rat Hemorrhagic Shock Model. Shock 2020, 53, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Haak, B.W.; Prescott, H.C.; Wiersinga, W.J. Therapeutic Potential of the Gut Microbiota in the Prevention and Treatment of Sepsis. Front. Immunol. 2018, 9, 2042. [Google Scholar] [CrossRef] [PubMed]
- Rius, M.; Nies, A.T.; Hummel-Eisenbeiss, J.; Jedlitschky, G.; Keppler, D. Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology 2003, 38, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Soroka, C.J.; Lee, J.M.; Azzaroli, F.; Boyer, J.L. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology 2001, 33, 783–791. [Google Scholar] [CrossRef]
- Zollner, G.; Trauner, M. Mechanisms of cholestasis. Clin. Liver Dis. 2008, 12, 1–26. [Google Scholar] [CrossRef]
- Donner, M.G.; Warskulat, U.; Saha, N.; Haussinger, D. Enhanced expression of basolateral multidrug resistance protein isoforms Mrp3 and Mrp5 in rat liver by LPS. Biol. Chem. 2004, 385, 331–339. [Google Scholar] [CrossRef]
- Trauner, M.; Arrese, M.; Lee, H.; Boyer, J.L.; Karpen, S.J. Endotoxin downregulates rat hepatic ntcp gene expression via decreased activity of critical transcription factors. J. Clin. Investig. 1998, 101, 2092–2100. [Google Scholar] [CrossRef]
- Oswald, M.; Kullak-Ublick, G.A.; Paumgartner, G.; Beuers, U. Expression of hepatic transporters OATP-C and MRP2 in primary sclerosing cholangitis. Liver 2001, 21, 247–253. [Google Scholar] [CrossRef]
- Anwer, M.S. Cellular regulation of hepatic bile acid transport in health and cholestasis. Hepatology 2004, 39, 581–590. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, J.; Wang, J.; Yang, Y.; Huang, J.; Gong, H.; Cui, H.; Chen, D. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit. Care 2016, 20, 332. [Google Scholar] [CrossRef]
- Luo, L.; Han, W.; Du, J.; Yang, X.; Duan, M.; Xu, C.; Zeng, Z.; Chen, W.; Chen, J. Chenodeoxycholic Acid from Bile Inhibits Influenza A Virus Replication via Blocking Nuclear Export of Viral Ribonucleoprotein Complexes. Molecules 2018, 23, 3315. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Li, N.; Li, Q.; Zhang, C.E.; Feng, W.W.; Li, G.Q.; Li, R.Y.; Tu, C.; Han, X.; Bai, Z.F.; et al. Screening for biomarkers of liver injury induced by Polygonum multiflorum: A targeted metabolomic study. Front. Pharmacol. 2015, 6, 217. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.-D.; Zhu, R.-X.; Wu, Z.-Q.; Lyu, S.-Y.; Zhao, L.-X.; Du, Z.-J.; Pan, X.-T. Gut Microbiota Disruption in Septic Shock Patients: A Pilot Study. Med. Sci. Monit. 2018, 24, 8639–8646. [Google Scholar] [CrossRef] [PubMed]
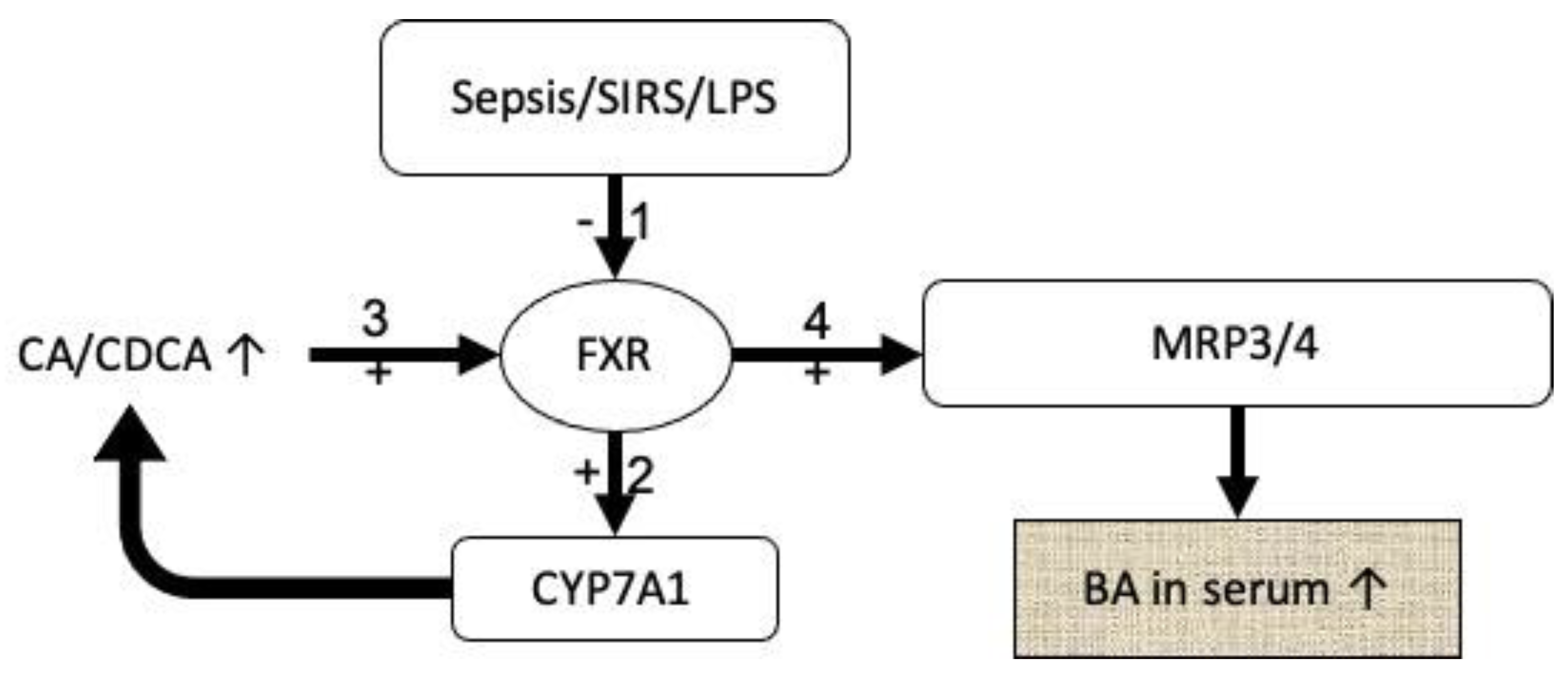
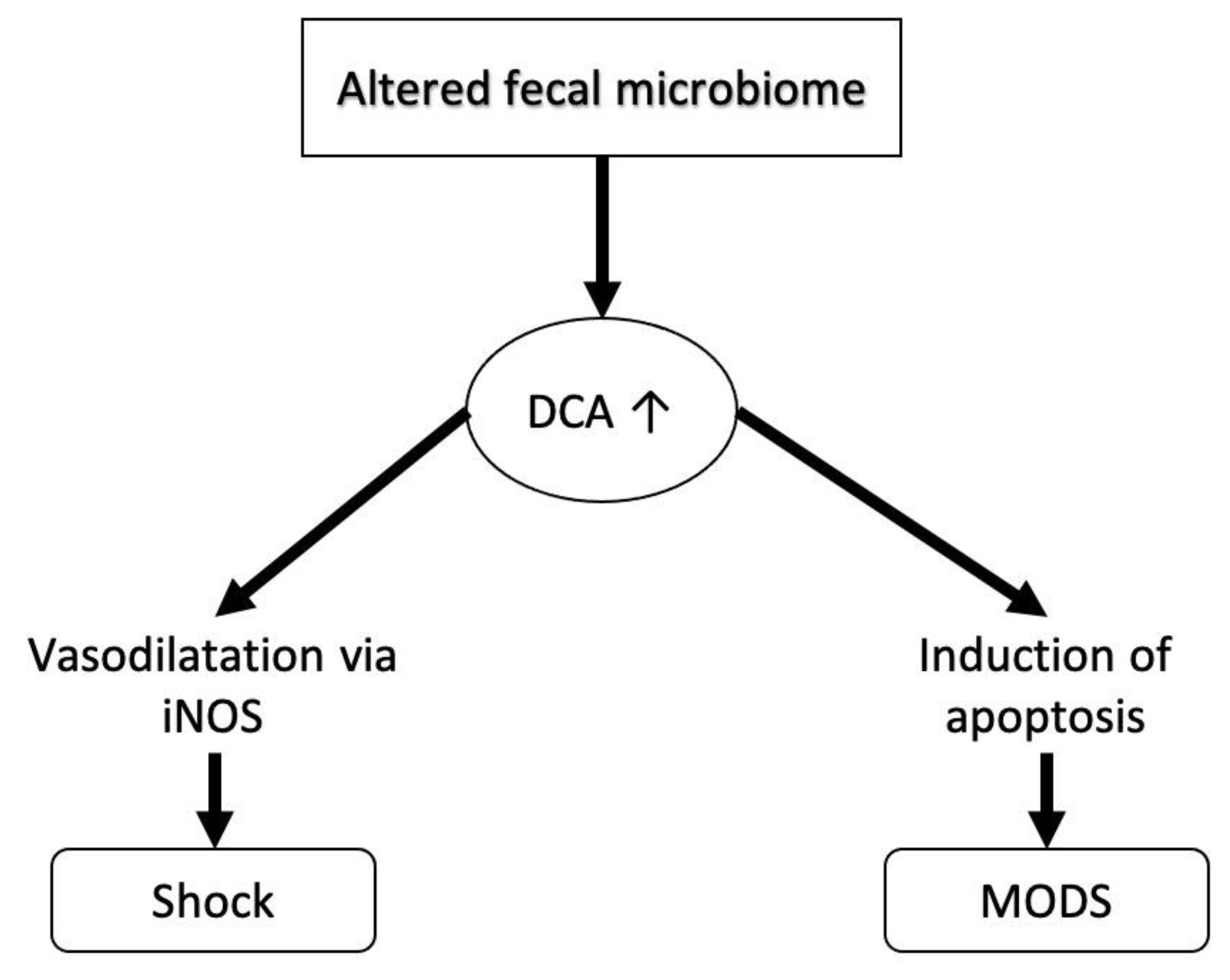
| Bile Acid | Abbreviation |
|---|---|
| Cholinic acid | CA |
| Taurocholic acid | TCA |
| Glycocholic acid | GCA |
| Chenodeoxycholic acid | CDCA |
| Taurochenodeoxycholic acid | TCDCA |
| Glycochenodeoxycholic acid | GCDCA |
| Ursodeoxycholic acid | UDC |
| Tauroursodeoxycholic acid | TUDCA |
| Glycoursodeoxycholic acid | GUDCA |
| Litocholic acid | LCA |
| Taurolitocholic acid | TLCA |
| Glycolitocholic acid | GLCA |
| Disease Category | Disease State Investigated | CA | TCA # | GCA | CDCA | TCDCA # | GCDCA # | DCA # | TDCA # | GDCA | UDCA | TUDCA # | GUDCA # | LCA # | TLCA # | GLCA # | TBA # |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver | General liver disease [12] | +210 * | +9622 * | +2769 * | +141 * | +5225 * | +1279 * | −57 * | +593 * | +215 * | n/a | n/a | n/a | n/a | n/a | n/a | +969 * |
| Drug induced liver injury [12] | +1343 * | +7856 * | +5159 * | +423 * | +5959 * | +2776 * | −46 * | +1519 * | +1192 * | n/a | n/a | n/a | n/a | n/a | n/a | +2192 * | |
| Viral hepatitis [13] | +33 | +1331 | +559 | −26 | +659 | +522 | −52 | +250 | +48 | +20 | +1100 | +326 | +135 | +150 | +229 | +420 | |
| Alcoholic liver disease [13] | +103 | +1119 | +653 | +60 | +599 | +589 | −70 | +77 | −7 | +482 * | +2589 | +1482 * | +114 | +82 | +103 | +460 | |
| Non-alcoholic fatty liver disease [13] | +192 | +619 | +426 | +70 | +265 | +382 | −25 | +186 | +61 | +65 | +511 | +245 | +236 | +67 | +300 | +366 | |
| Other liver diseases [13] | +28 | +3 | +1060 | +54 | +957 | +513 | −72 | +191 | +14 | +14 | +1584 | +416 | +73 | +133 | +227 | +457 | |
| Acutely decompensated chronic cirrhosis [14] | +25 | n/a | +228 * | +102 * | +356 * | +136 * | −63 | −100 | −82 | +33 | 0 | +413 * | −14 | −100 | +67 | +195 | |
| Biliary Tract | Biliary obstruction [15] | +5 | +30,221 * | +24,030 * | −67 * | +13,692 * | +3599 * | −95 * | +375 * | +70 | −85 * | +1398 * | n/a | +88 * | +50 | +36 | +5778 * |
| Obstructive cholestasis [16] | 0 | +3 * | +2 * | −55 | +2 * | +689 * | −90 * | +300 * | +375 * | 0 | n/a | n/a | 0 | n/a | n/a | n/a | |
| Severe cholestasis in pregnancy [17] | +134 * | +5411 * | +1053 * | −60 | +210 * | +342 * | −26 | +1082 * | +125 * | −37 | +73 * | n/a | 0 | −25 | 0 | +767 * | |
| Biliary tract disease [13] | +9 | +4295 * | +1491 | +70 | +1723 | +798 | −88 * | +309 | +14 | −55 * | +1323 | +276 | +72 | +193 | +114 | +709 | |
| Other | Hepatopulmonary syndrome [18] | +63 * | +645 * | +216 * | +856 * | +464 * | +210 * | −53 | −100 | −100 | +25 | −100 | +75 | −38 | −33 | −100 | +210 * |
| General critical illness [19] | +25 | +2050 * | +8200 * | −10 | +3800 * | +3300 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | n/a | +1010 * | |
| Shock States | Cardiogenic shock [20] | 0 | +650 * | +1067 * | −10 | +2600 * | +580 * | +100 * | +533 * | +2800 * | +300 * | +800 * | +200 * | +25 * | +600 * | n/a | +90 * |
| Septic shock [20] | 0 | +1150 * | +1933 * | +120 | +4900 * | +1820 * | +80 * | +333 * | +5500 * | +600 * | +1000 * | +350 * | 0 | +500 * | n/a | +590 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harnisch, L.-O.; Moerer, O. The Specific Bile Acid Profile of Shock: A Hypothesis Generating Appraisal of the Literature. J. Clin. Med. 2020, 9, 3844. https://doi.org/10.3390/jcm9123844
Harnisch L-O, Moerer O. The Specific Bile Acid Profile of Shock: A Hypothesis Generating Appraisal of the Literature. Journal of Clinical Medicine. 2020; 9(12):3844. https://doi.org/10.3390/jcm9123844
Chicago/Turabian StyleHarnisch, Lars-Olav, and Onnen Moerer. 2020. "The Specific Bile Acid Profile of Shock: A Hypothesis Generating Appraisal of the Literature" Journal of Clinical Medicine 9, no. 12: 3844. https://doi.org/10.3390/jcm9123844
APA StyleHarnisch, L.-O., & Moerer, O. (2020). The Specific Bile Acid Profile of Shock: A Hypothesis Generating Appraisal of the Literature. Journal of Clinical Medicine, 9(12), 3844. https://doi.org/10.3390/jcm9123844





