Adolescent Sport Participation and Age at Menarche in Relation to Midlife Body Composition, Bone Mineral Density, Fitness, and Physical Activity
Abstract
1. Introduction
2. Materials and Methods
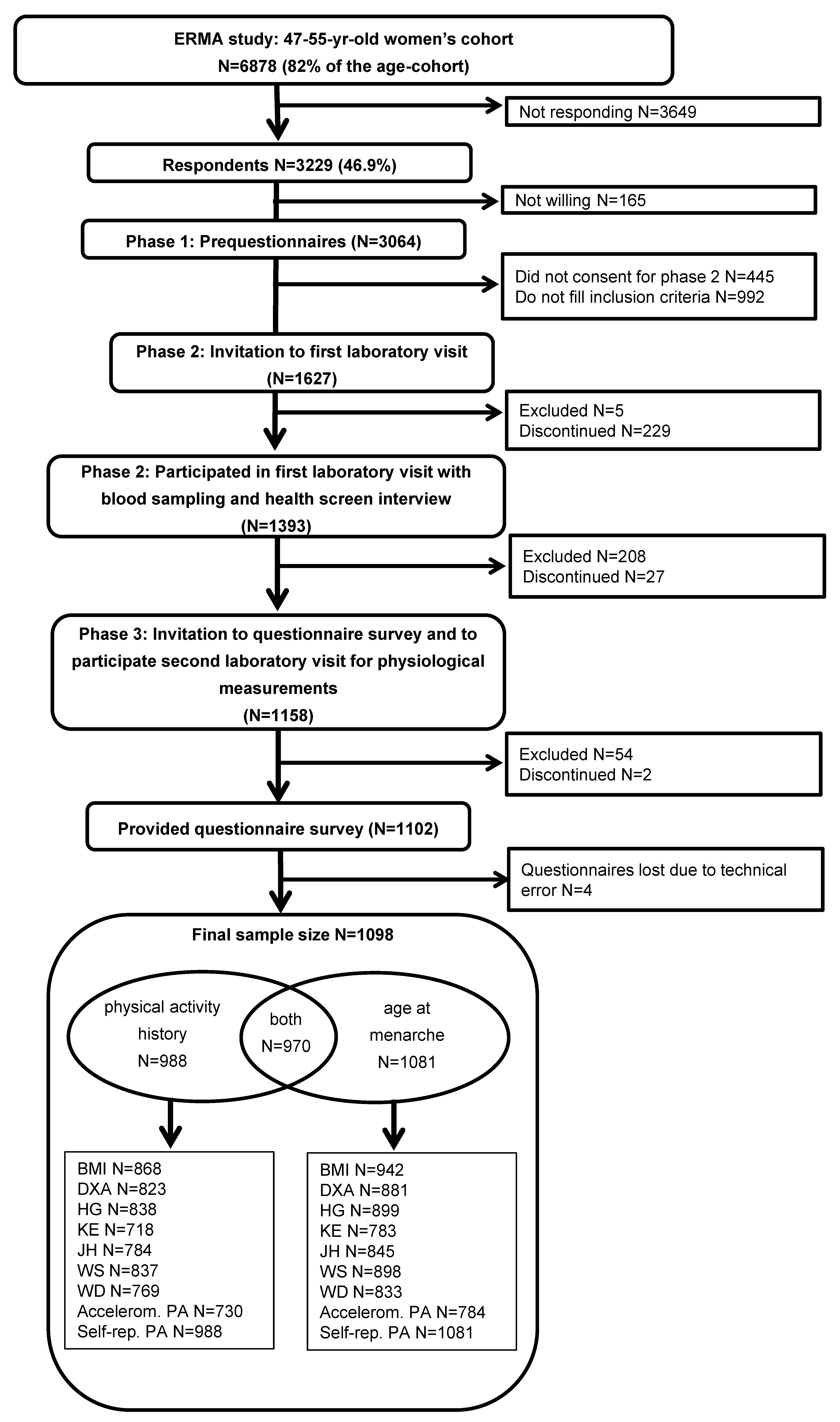
2.1. Participants
2.2. Assessment Methods
2.2.1. Adolescent Predictors
PA Level
AAM
2.2.2. Middle Age Characteristics
Anthropometrics and Body Composition
Physical Performance Tests
Accelerometer-Measured PA
Self-Reported PA
Background Variables
2.3. Statistical Analysis
3. Results
3.1. PA Participation from Childhood to Midlife
3.2. Midlife Characteristics According to Adolescence PA
3.3. AAM and Midlife Characteristics
4. Discussion
4.1. PA Participation from Childhood to Midlife
4.2. Association between PA in Adolescence and AAM
4.3. Competitive Sport in Adolescence and Midlife Characteristics
4.4. AAM and Midlife Characteristics
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haskell, W.L.; Lee, I.-M.; Pate, R.R.; Powell, K.; Blair, S.N.; Franklin, B.; Macera, C.; Heath, G.W.; Thompson, P.D.; Bauman, A.; et al. Physical Activity and Public Health: Updated Recommendation for Adults From the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Theintz, G.; Buchs, B.; Rizzoli, R.; Slosman, D.; Clavien, H.; Sizonenko, P.C.; Bonjour, J.-P. Longitudinal monitoring of bone mass accumulation in healthy adolescents: Evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J. Clin. Endocrinol. Metab. 1992, 75, 1060–1065. [Google Scholar] [CrossRef]
- Kohrt, W.M.; Bloomfield, S.A.; Little, K.D.; Nelson, M.E.; Yingling, V.R. Physical Activity and Bone Health. Med. Sci. Sports Exerc. 2004, 36, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Zanker, C.L.; Osborne, C.; Oldroyd, B.; Truscott, J.G.; Cooke, C.B. Bone density, body composition and menstrual history of sedentary female former gymnasts, aged 20–32 years. Osteoporos. Int. 2004, 15, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Bass, S.; Pearce, G.; Bradney, M.; Hendrich, E.; Delmas, P.D.; Harding, A.; Seeman, E. Exercise Before Puberty May Confer Residual Benefits in Bone Density in Adulthood: Studies in Active Prepubertal and Retired Female Gymnasts. J. Bone Miner. Res. 1998, 13, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Erlandson, M.C.; Kontulainen, S.; Chilibeck, P.D.; Arnold, C.M.; Faulkner, R.; Baxter-Jones, A.D.G. Higher premenarcheal bone mass in elite gymnasts is maintained into young adulthood after long-term retirement from sport: A 14-year follow-up. J. Bone Miner. Res. 2011, 27, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, A.; Celi, M.; Volpe, S.L.; Sorge, R.; Tarantino, U. Long-term effect of exercise on bone mineral density and body composition in post-menopausal ex-elite athletes: A retrospective study. Eur. J. Clin. Nutr. 2011, 66, 69–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, K.M.; Bennell, K.; Hopper, J.L.; Flicker, L.; Nowson, C.; Sherwin, A.J.; Crichton, K.J.; Harcourt, P.R.; Wark, J.D. Self-reported ballet classes undertaken at age 10–12 years and hip bone mineral density in later life. Osteoporos. Int. 1998, 8, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.K.; Laing, E.M.; Modlesky, C.M.; O’Connor, P.J.; Lewis, R.D. Former college artistic gymnasts maintain higher BMD: A nine-year follow-up. Osteoporos. Int. 2006, 17, 1691–1697. [Google Scholar] [CrossRef]
- Telama, R. Tracking of Physical Activity from Childhood to Adulthood: A Review. Obes. Facts 2009, 2, 187–195. [Google Scholar] [CrossRef]
- Craigie, A.M.; Lake, A.A.; Kelly, S.A.; Adamson, A.J.; Mathers, J.C. Tracking of obesity-related behaviours from childhood to adulthood: A systematic review. Maturitas 2011, 70, 266–284. [Google Scholar] [CrossRef]
- Telama, R.; Yang, X.; Leskinen, E.; Kankaanpää, A.; Hirvensalo, M.; Tammelin, T.; Viikari, J.S.A.; Raitakari, O.T. Tracking of Physical Activity from Early Childhood through Youth into Adulthood. Med. Sci. Sports Exerc. 2014, 46, 955–962. [Google Scholar] [CrossRef]
- Palomäki, S.; Hirvensalo, M.; Smith, K.; Raitakari, O.; Männistö, S.; Hutri-Kähönen, N.; Tammelin, T.H. Does organized sport participation during youth predict healthy habits in adulthood? A 28-year longitudinal study. Scand. J. Med. Sci. Sports 2018, 28, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Nattiv, A.; Joy, E.; Misra, M.; Williams, N.; Mallinson, R.J.; Gibbs, J.C.; Olmsted, M.; Goolsby, M.; Matheson, G.; et al. 2014 Female Athlete Triad Coalition Consensus Statement on Treatment and Return to Play of the Female Athlete Triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. Br. J. Sports Med. 2014, 48, 289. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.K.; Meyer, N.L.; et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Kujala, U.M.; Orava, S.; Parkkari, J.; Kaprio, J.; Sarna, S. Sports Career-Related Musculoskeletal Injuries. Sports Med. 2003, 33, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Friery, K.B. Incidence of injury and disease among former athletes: A review. J. Exerc. Physiol. Online 2008, 11, 26–45. [Google Scholar]
- Schwenk, T.L.; Gorenflo, D.W.; Dopp, R.R.; Hipple, E. Depression and Pain in Retired Professional Football Players. Med. Sci. Sports Exerc. 2007, 39, 599–605. [Google Scholar] [CrossRef]
- Tveit, M.; Rosengren, B.E.; Nilsson, J.-Å.; Karlsson, M.K. Former Male Elite Athletes Have a Higher Prevalence of Osteoarthritis and Arthroplasty in the Hip and Knee Than Expected. Am. J. Sports Med. 2011, 40, 527–533. [Google Scholar] [CrossRef]
- Meczekalski, B.; Katulski, K.; Czyzyk, A.; Podfigurna-Stopa, A. Health in older women athletes. Maturitas 2014, 79, 357–361. [Google Scholar] [CrossRef]
- Chevalley, T.; Bonjour, J.-P.; Ferrari, S.; Rizzoli, R. Deleterious Effect of Late Menarche on Distal Tibia Microstructure in Healthy 20-Year-Old and Premenopausal Middle-Aged Women. J. Bone Miner. Res. 2009, 24, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Iranagh, J.A.; Motalebi, S.A.; Hamid, T.A. Reproductive factors influencing bone mineral density in postmenopausal women. Women Health 2018, 59, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Gerdhem, P.; Obrant, K.J. Bone mineral density in old age: The influence of age at menarche and menopause. J. Bone Miner. Metab. 2004, 22, 372–375. [Google Scholar] [CrossRef]
- Varenna, M.; Binelli, L.; Zucchi, F.; Ghiringhelli, D.; Gallazzi, M.; Sinigaglia, L. Prevalence of Osteoporosis by Educational Level in a Cohort of Postmenopausal Women. Osteoporos. Int. 1999, 9, 236–241. [Google Scholar] [CrossRef]
- Paganini-Hill, A.; Atchison, K.A.; Gornbein, J.A.; Nattiv, A.; Service, S.K.; White, S.C. Menstrual and Reproductive Factors and Fracture Risk: The Leisure World Cohort Study. J. Womens Health 2005, 14, 808–819. [Google Scholar] [CrossRef]
- Cooper, G.S.; Sandler, D.P. Long-term effects of reproductive-age menstrual cycle patterns on peri- and postmenopausal fracture risk. Am. J. Epidemiol. 1997, 145, 804–809. [Google Scholar] [CrossRef]
- Torstveit, M.K.; Sundgot-Borgen, J. Participation in leanness sports but not training volume is associated with menstrual dysfunction: A national survey of 1276 elite athletes and controls. Br. J. Sports Med. 2005, 39, 141–147. [Google Scholar] [CrossRef]
- Calthorpe, L.; Brage, S.; Ong, K.K. Systematic review and meta-analysis of the association between childhood physical activity and age at menarche. Acta Paediatr. 2019, 108, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Hoch, A.Z.; Pajewski, N.M.; Moraski, L.; Carrera, G.F.; Wilson, C.R.; Hoffmann, R.G.; Schimke, J.E.; Gutterman, D.D. Prevalence of the Female Athlete Triad in High School Athletes and Sedentary Students. Clin. J. Sport Med. 2009, 19, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Bubach, S.; Menezes, A.M.B.; Barros, F.C.; Wehrmeister, F.C.; Gonçalves, H.; Assunção, M.C.F.; Horta, B.L. Impact of the age at menarche on body composition in adulthood: Results from two birth cohort studies. BMC Public Health 2016, 16, 1007. [Google Scholar] [CrossRef] [PubMed]
- Trikudanathan, S.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Seely, E.W.; Murabito, J.M.; Fox, C.S. Association of Female Reproductive Factors with Body Composition: The Framingham Heart Study. J. Clin. Endocrinol. Metab. 2013, 98, 236–244. [Google Scholar] [CrossRef]
- Prentice, P.; Viner, R.M. Pubertal timing and adult obesity and cardiometabolic risk in women and men: A systematic review and meta-analysis. Int. J. Obes. 2013, 37, 1036–1043. [Google Scholar] [CrossRef]
- Kovanen, V.; Aukee, P.; Kokko, K.; Finni, T.; Tarkka, I.M.; Tammelin, T.; Kujala, U.M.; Sipilä, S.; Laakkonen, E.K. Design and protocol of Estrogenic Regulation of Muscle Apoptosis (ERMA) study with 47 to 55-year-old women’s cohort: Novel results show menopause-related differences in blood count. Menopause 2018, 25, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Hirvensalo, M.; Lintunen, T.; Rantanen, T. The continuity of physical activity–a retrospective and prospective study among older people. Scand. J. Med. Sci. Sports 2000, 10, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Van Der Ploeg, G.E.; Withers, R.T.; Laforgia, J. Percent body fat via DEXA: Comparison with a four-compartment model. J. Appl. Physiol. 2003, 94, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Bondarev, D.; Laakkonen, E.K.; Finni, T.; Kokko, K.; Kujala, U.M.; Aukee, P.; Kovanen, V.; Sipilä, S. Physical performance in relation to menopause status and physical activity. Menopause 2018, 25, 1432–1441. [Google Scholar] [CrossRef]
- Bosco, C.; Luhtanen, P.; Komi, P.V. A simple method for measurement of mechanical power in jumping. Eur. J. Appl. Physiol. 1983, 50, 273–282. [Google Scholar] [CrossRef]
- Ronkainen, P.H.A.; Kovanen, V.; Alén, M.; Pöllänen, E.; Palonen, E.-M.; Ankarberg-Lindgren, C.; Hämäläinen, E.; Turpeinen, U.; Kujala, U.M.; Puolakka, J.; et al. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: A study with monozygotic twin pairs. J. Appl. Physiol. 2009, 107, 25–33. [Google Scholar] [CrossRef]
- ATS. Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS Statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Laakkonen, E.K.; Kulmala, J.; Aukee, P.; Hakonen, H.; Kujala, U.M.; Lowe, D.A.; Kovanen, V.; Tammelin, T.; Sipilä, S. Female reproductive factors are associated with objectively measured physical activity in middle-aged women. PLoS ONE 2017, 12, e0172054. [Google Scholar] [CrossRef]
- Sasaki, J.; John, D.; Freedson, P.S. Validation and comparison of ActiGraph activity monitors. J. Sci. Med. Sport 2011, 14, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Kujala, U.M.; Kaprio, J.; Sarna, S.; Koskenvuo, M. Relationship of Leisure-Time Physical Activity and Mortality: The Finnish twin cohort. JAMA 1998, 279, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, M.; Sipilä, S.; Kulmala, J.; Hakonen, H.; Tammelin, T.H.; Kujala, U.M.; Kovanen, V.; Laakkonen, E.K. Validity and Reliability of a Single Question for Leisure-Time Physical Activity Assessment in Middle-Aged Women. J. Aging Phys. Act. 2020, 28, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A Second Update of Codes and MET Values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Waller, K.; Kaprio, J.; Kujala, U.M. Associations between long-term physical activity, waist circumference and weight gain: A 30-year longitudinal twin study. Int. J. Obes. 2007, 32, 353–361. [Google Scholar] [CrossRef]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.M.; Rebar, R.W.; Sherman, S.S.; Sluss, P.M.; de Villiers, T.J.; STRAW + 10 Collaborative Group. Executive Summary of the Stages of Reproductive Aging Workshop + 10: Addressing the Unfinished Agenda of Staging Reproductive Aging. J. Clin. Endocrinol. Metab. 2012, 97, 1159–1168. [Google Scholar] [CrossRef]
- He, C.; Kraft, P.; Chen, C.; Buring, J.E.; Paré, G.; Hankinson, S.E.; Chanock, S.J.; Ridker, P.M.; Hunter, D.J.; Chasman, D.I. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat. Genet. 2009, 41, 724–728. [Google Scholar] [CrossRef]
- Day, F.R.; Thompson, D.J.; Helgason, H.; Chasman, D.I.; Finucane, H.; Sulem, P.; Ruth, K.S.; Whalen, S.; Sarkar, A.K.; Albrecht, E.; et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet. 2017, 49, 834–841. [Google Scholar] [CrossRef]
- Zhu, J.; Kusa, T.O.; Chan, Y.-M. Genetics of pubertal timing. Curr. Opin. Pediatr. 2018, 30, 532–540. [Google Scholar] [CrossRef]
- Yermachenko, A.; Dvornyk, V. Nongenetic Determinants of Age at Menarche: A Systematic Review. BioMed Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Valdimarsson, Ö.; Alborg, H.G.; Düppe, H.; Nyquist, F.; Karlsson, M. Reduced Training Is Associated With Increased Loss of BMD. J. Bone Miner. Res. 2005, 20, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Sundh, D.; Mellström, D.; Lorentzon, M. Current Physical Activity Is Independently Associated With Cortical Bone Size and Bone Strength in Elderly Swedish Women. J. Bone Miner. Res. 2016, 32, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Sipila, S.; Törmäkangas, T.; Sillanpää, E.; Aukee, P.; Kujala, U.M.; Kovanen, V.; Laakkonen, E.K. Muscle and bone mass in middle-aged women: Role of menopausal status and physical activity. J. Cachex-Sarcopenia Muscle 2020, 11, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Spector, T.D.; Harris, P.A.; Hart, D.; Cicuttini, F.M.; Nandra, D.; Etherington, J.; Wolman, R.L.; Doyle, D.V. Risk of osteoarthritis associated with long-term weight-bearing sports: A radiologic survey of the hips and knees in female ex-athletes and population controls. Arthritis Rheum. 1996, 39, 988–995. [Google Scholar] [CrossRef]
- Haljaste, K.; Unt, E. Relationships between physical activity and musculoskeletal disorders in former athletes. Coll. Antropol. 2010, 34, 1335–1340. [Google Scholar] [PubMed]
- Tveit, M.; Rosengren, B.E.; Nyquist, F.; Nilsson, J.-Å.; Karlsson, M.K. Former Male Elite Athletes Have Lower Incidence of Fragility Fractures Than Expected. Med. Sci. Sports Exerc. 2013, 45, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Bergström, U.; Jönsson, H.; Gustafson, Y.; Pettersson, U.; Stenlund, H.; Svensson, O. The hip fracture incidence curve is shifting to the right. Acta Orthop. 2009, 80, 520–524. [Google Scholar] [CrossRef]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef]
- Bratland-Sanda, S.; Sundgot-Borgen, J. Eating disorders in athletes: Overview of prevalence, risk factors and recommendations for prevention and treatment. Eur. J. Sport Sci. 2013, 13, 499–508. [Google Scholar] [CrossRef]
- Martinsen, M.; Sundgot-Borgen, J. Higher Prevalence of Eating Disorders among Adolescent Elite Athletes than Controls. Med. Sci. Sports Exerc. 2013, 45, 1188–1197. [Google Scholar] [CrossRef]
- Micali, N.; Martini, M.G.; Thomas, J.J.; Eddy, K.T.; Kothari, R.; Russell, E.; Bulik, C.M.; Treasure, J. Lifetime and 12-month prevalence of eating disorders amongst women in mid-life: A population-based study of diagnoses and risk factors. BMC Med. 2017, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Keski-Rahkonen, A.; Hoek, H.W.; Susser, E.S.; Linna, M.S.; Sihvola, E.; Raevuori, A.; Bulik, C.M.; Kaprio, J.; Rissanen, A. Epidemiology and Course of Anorexia Nervosa in the Community. Am. J. Psychiatry 2007, 164, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.I.; Hiripi, E.; Pope, H.G.; Kessler, R.C. The Prevalence and Correlates of Eating Disorders in the National Comorbidity Survey Replication. Biol. Psychiatry 2007, 61, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, C.; Hayward, C.; De Zwaan, M.; Kraemer, H.C.; Agras, W.S. Coming to Terms With Risk Factors for Eating Disorders: Application of Risk Terminology and Suggestions for a General Taxonomy. Psychol. Bull. 2004, 130, 19–65. [Google Scholar] [CrossRef] [PubMed]
- Keski-Rahkonen, A.; Mustelin, L. Epidemiology of eating disorders in Europe: Prevalence, incidence, comorbidity, course, consequences, and risk factors. Curr. Opin. Psychiatry 2016, 29, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Kirchengast, S.; Gruber, D.; Sator, M.; Huber, J. Impact of the age at menarche on adult body composition in healthy pre- and postmenopausal women. Am. J. Phys. Anthr. 1998, 105, 9–20. [Google Scholar] [CrossRef]
- Pierce, M.B.; Leon, D. Age at menarche and adult BMI in the Aberdeen Children of the 1950s Cohort Study. Am. J. Clin. Nutr. 2005, 82, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, R.; Forouhi, N.G.; Sharp, S.J.; Luben, R.; Bingham, S.A.; Khaw, K.-T.; Wareham, N.J.; Ong, K.K. Early Age at Menarche Associated with Cardiovascular Disease and Mortality. J. Clin. Endocrinol. Metab. 2009, 94, 4953–4960. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.K.; Dickman, P.W.; Adami, H.-O.; Wolk, A. Early anthropometric measures and reproductive factors as predictors of body mass index and obesity among older women. Int. J. Obes. 2005, 29, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, L.-J.; Liu, Y.-Z.; Recker, R.R.; Deng, H. Genetic and environmental correlations between obesity phenotypes and age at menarche. Int. J. Obes. 2006, 30, 1595–1600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hardy, R.; Maddock, J.; Ghosh, A.K.; Hughes, A.D.; Kuh, D. The relationship between pubertal timing and markers of vascular and cardiac structure and function in men and women aged 60–64 years. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Lawlor, D.A.; Iliodromiti, S.; Padmanabhan, S.; Nelson, S.M.; Fraser, A. Age at Menarche and Cardiometabolic Health: A Sibling Analysis in the Scottish Family Health Study. J. Am. Hear. Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, M.; Tehrani, F.R.; Khalili, D.; Cheraghi, L.; Azizi, F. Is there any association between age at menarche and anthropometric indices? A 15-year follow-up population-based cohort study. Eur. J. Pediatr. 2020, 179, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.; Brewer, C.F.; Fabiola Del Greco, M.; Sivakumaran, P.; Bowden, J.; Sheehan, N.A.; Minelli, C. Age at menarche and adult body mass index: A Mendelian randomization study. Int. J. Obes. 2018, 42, 1574–1581. [Google Scholar] [CrossRef]
- Bell, J.A.; Carslake, D.; Wade, K.H.; Richmond, R.C.; Langdon, R.J.; Vincent, E.E.; Holmes, M.V.; Timpson, N.J.; Smith, G.D. Influence of puberty timing on adiposity and cardiometabolic traits: A Mendelian randomisation study. PLoS Med. 2018, 15, e1002641. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, L.-J.; Shen, H.; Deng, H. Genetic and Environmental Correlations between Age at Menarche and Bone Mineral Density at Different Skeletal Sites. Calcif. Tissue Int. 2005, 77, 356–360. [Google Scholar] [CrossRef]
- Parker, S.E.; Troisi, R.; Wise, L.A.; Palmer, J.R.; Titus-Ernstoff, L.; Strohsnitter, W.C.; Hatch, E.E. Menarche, menopause, years of menstruation, and the incidence of osteoporosis: The influence of prenatal exposure to diethylstilbestrol. J. Clin. Endocrinol. Metab. 2013, 99, 594–601. [Google Scholar] [CrossRef]
- Hagemans, M.L.; Van Der Schouw, Y.T.; De Kleijn, M.J.; Van Staveren, W.A.; Pop, V.J.; Leusink, G.L.; Grobbee, D.E. Indicators for the total duration of premenopausal endogenous estrogen exposure in relation to BMD. Hum. Reprod. 2004, 19, 2163–2169. [Google Scholar] [CrossRef]
- Blum, M.; Harris, S.S.; Must, A.; Phillips, S.M.; Rand, W.M.; Dawson-Hughes, B. Weight and Body Mass Index at Menarche are Associated with Premenopausal Bone Mass. Osteoporos. Int. 2001, 12, 588–594. [Google Scholar] [CrossRef]
- Nakaoka, D.; Sugimoto, T.; Kaji, H.; Kanzawa, M.; Yano, S.; Yamauchi, M.; Sugishita, T.; Chihara, K. Determinants of Bone Mineral Density and Spinal Fracture Risk in Postmenopausal Japanese Women. Osteoporos. Int. 2001, 12, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Sioka, C.; Fotopoulos, A.; Georgiou, A.; Xourgia, X.; Papadopoulos, A.; Kalef-Ezra, J.A. Age at menarche, age at menopause and duration of fertility as risk factors for osteoporosis. Climacteric 2010, 13, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hassa, H.; Tanir, H.; Senses, T.; Öge, T.; Sahin-Mutlu, F. Related factors in bone mineral density of lumbal and femur in natural postmenopausal women. Arch. Gynecol. Obstet. 2005, 273, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Y.; Liu, P.-Y.; Deng, H. The impact of reproductive and menstrual history on bone mineral density in Chinese women. J. Clin. Densitom. 2003, 6, 289–296. [Google Scholar] [CrossRef]
- Ozdemir, F.; Demirbag, D.; Rodoplu, M. Reproductive Factors Affecting the Bone Mineral Density in Postmenopausal Women. Tohoku J. Exp. Med. 2005, 205, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-X.; Lei, S.-F.; Deng, F.; Zhang, F.; Liu, Y.-J.; Recker, R.R.; Papasian, C.J.; Deng, H. Bivariate genome-wide linkage analysis for traits BMD and AAM: Effect of menopause on linkage signals. Maturitas 2009, 62, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Chevalley, T.; Bonjour, J.P.; Ferrari, S.; Rizzoli, R. The Influence of Pubertal Timing on Bone Mass Acquisition: A Predetermined Trajectory Detectable Five Years before Menarche. J. Clin. Endocrinol. Metab. 2009, 94, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Liel, Y.; Edwards, J.; Shary, J.; Spicer, K.; Gordon, L.; Bell, N.H. The Effects of Race and Body Habitus on Bone Mineral Density of the Radius, Hip, and Spine in Premenopausal Women*. J. Clin. Endocrinol. Metab. 1988, 66, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, S.L.; Barrett-Connor, E. Relation between Body Size and Bone Mineral Density in Elderly Men and Women. Am. J. Epidemiol. 1993, 138, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Kasagi, F.; Yamada, M.; Kodama, K. Risk Factors for Hip Fracture in a Japanese Cohort. J. Bone Miner. Res. 1997, 12, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; O’Neill, T.; Finn, J.; Lunt, M.; Silman, A.; Felsenberg, D.; Armbrecht, G.; Banzer, D.; Benevolenskaya, L.; Bhalla, A.; et al. Determinants of incident vertebral fracture in men and women: Results from the European Prospective Osteoporosis Study (EPOS). Osteoporos. Int. 2003, 14, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Silman, A.J. Risk factors for Colles’ fracture in men and women: Results from the European Prospective Osteoporosis Study. Osteoporos. Int. 2003, 14, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Gullberg, B.; Kanis, J.A.; Allander, E.; Elffors, L.; Dequeker, J.; Dilsen, G.; Gennari, C.; Lopes Vaz, L.A.; Lyritis, G.; et al. Risk factors for hip fracture in european women: The MEDOS study. J. Bone Miner. Res. 2009, 10, 1802–1815. [Google Scholar] [CrossRef] [PubMed]
- Kvalheim, S.; Sandvik, L.; Winsvold, B.S.; Hagen, K.; Zwart, J.-A. Early menarche and chronic widespread musculoskeletal complaints- Results from the HUNT study. Eur. J. Pain 2015, 20, 458–464. [Google Scholar] [CrossRef]
- Roze, C.; Doyen, C.; Le Heuzey, M.-F.; Armoogum, P.; Mouren, M.-C.; Léger, J. Predictors of late menarche and adult height in children with anorexia nervosa. Clin. Endocrinol. 2007, 67, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Must, A.; Phillips, S.; Naumova, E.; Blum, M.; Harris, S.; Dawson-Hughes, B.; Rand, W.M. Recall of Early Menstrual History and Menarcheal Body Size: After 30 Years, How Well Do Women Remember? Am. J. Epidemiol. 2002, 155, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Casey, V.; Dwyer, J.T.; Coleman, K.A.; Krall, E.A.; Gardner, J.; Valadian, I. Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Ann. Hum. Biol. 1991, 18, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Żarów, R.; Cichocka, B.A. A comparative analysis of estimation of age at menarche by various methods in women participating in the Krakow Longitudinal Growth Study, Poland. Am. J. Hum. Biol. 2008, 20, 146–148. [Google Scholar] [CrossRef]
- Cooper, R.; Blell, M.; Hardy, R.; Black, S.; Pollard, T.M.; Wadsworth, M.E.J.; Pearce, M.S.; Kuh, D. Validity of age at menarche self-reported in adulthood. J. Epidemiol. Commun. Health 2006, 60, 993–997. [Google Scholar] [CrossRef]
- Mostafavifar, A.M.; Best, T.M.; Myer, G.D. Early sport specialisation, does it lead to long-term problems? Br. J. Sports Med. 2013, 47, 1060–1061. [Google Scholar] [CrossRef]
- Mathisen, F.K.S.; Kokko, S.; Tynjälä, J.; Torsheim, T.; Wold, B. Leisure-time physical activity and participation in organized sports: Changes from 1985 to 2014 in Finland and Norway. Scand. J. Med. Sci. Sports 2019, 29, 1232–1242. [Google Scholar] [CrossRef]
- Myer, G.D.; Jayanthi, N.; DiFiori, J.P.; Faigenbaum, A.D.; Kiefer, A.W.; Logerstedt, D.; Micheli, L.J. Sport Specialization, Part I: Does Early Sports Specialization Increase Negative Outcomes and Reduce the Opportunity for Success in Young Athletes? Sports Health 2015, 7, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Sundgot-Borgen, J. Risk and trigger factors for the development of eating disorders in female elite athletes. Med. Sci. Sports Exerc. 1994, 26, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Rauh, M.J.; Tenforde, A.S.; Barrack, M.T.; Rosenthal, M.D.; Nichols, J.F. Associations Between Sport Specialization, Running-Related Injury, and Menstrual Dysfunction Among High School Distance Runners. Athl. Train. Sports Health Care 2018, 10, 260–269. [Google Scholar] [CrossRef]
| Competitive Sport (CS) | Regular PA (RPA) | No Exercise (NE) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| at Age 13–16 (n = 136) | at Age 13–16 (n = 689) | at Age 13–16 (n = 163) | |||||||
| Age | CS | RPA | NE | CS | RPA | NE | CS | RPA | NE |
| 7–12 | 50.7% (69) | 45.6% (62) | 3.7% (5) | 5.8% (40) | 85.5% (589) | 8.7% (60) | 3.7% (6) | 36.8% (60) | 59.5% (97) |
| 17–19 | 48.5% (66) | 47.1% (64) | 4.4% (6) | 2.0% (14) | 88.0% (606) | 10.0% (69) | 1.8% (3) | 29.4% (48) | 68.7% (112) |
| 20–29 | 22.8% (31) | 70.6% (96) | 6.6% (9) | 2.0% (14) | 87.2% (601) | 10.7% (74) | 2.5% (4) | 65.6% (107) | 31.9% (52) |
| 30–39 | 8.8% (12) | 85.3% (116) | 5.9% (8) | 1.6% (11) | 89.3% (615) | 9.1% (63) | 0.6% (1) | 76.7% (125) | 22.7% (37) |
| 40–50 | 1.5% (2) | 91.2% (124) | 7.4% (10) | 1.2% (8) | 94.0% (647) | 4.9% (34) | 0.6% (1) | 95.1% (155) | 4.3% (7) |
| Variable | n | Competitive Sport | n | Regular PA | n | No Exercise | p-Value | Model 1: p-Value a,b | Model 2: p-Value c,d |
|---|---|---|---|---|---|---|---|---|---|
| Background variables | |||||||||
| Age (y) | 136 | 50.9 (50.5–51.2) R,N | 689 | 51.4 (51.3–51.6) | 163 | 51.6 (51.3–51.9) | 0.005e | ||
| Age at menarche (y) | 134 | 677 | 162 | 0.010 | |||||
| ≤12 | 35.1% (47) N | 35.2% (238) N | 38.9% (63) | ||||||
| 13 | 30.6% (41) | 34.3% (232) | 43.2% (70) | ||||||
| ≥14 | 34.3% (46) | 30.6% (207) | 17.9% (29) | ||||||
| Bachelor or higher education | 136 | 48.5% (66) N | 689 | 41.5% (286) | 163 | 34.4% (56) | 0.045 | ||
| Number of parities | 136 | 2.0 (1.8–2.1) | 687 | 2.1 (2.0–2.2) N | 163 | 1.9 (1.7–2.1) | 0.017f | ||
| Used OCP at some point of life | 136 | 6.6% (9) | 689 | 9.9% (68) | 163 | 11.0% (18) | 0.399 | ||
| Used HC during the preceding 10 years | 136 | 62.5% (85) R,N | 689 | 50.8% (350) | 163 | 49.1% (80) | 0.031 | ||
| Menopausal status | 136 | 689 | 161 | 0.893 | |||||
| PRE | 30.1% (41) | 27.4% (189) | 24.5% (40) | ||||||
| EPM | 19.1% (26) | 18.6% (128 | 19.0% (31) | ||||||
| LPM | LPM 21.3% (29) | 19.3% (133) | 20.9% (34) | ||||||
| POST | POST 29.4% (40) | 34.7% (239) | 35.6% (58) | ||||||
| Body composition | |||||||||
| Height (cm) | 113 | 166.4 (165.4–167.4) | 609 | 165.6 (165.1–166.0) | 146 | 164.7 (163.8–165.7) | 0.074 e | 0.188 a | 0.257 c |
| Weight (m) | 113 | 70.3 (68.3–72.3) | 609 | 70.3 (69.4–71.2) | 146 | 68.5 (66.8–70.1) | 0.182 e | 0.046a | 0.038c |
| BMI (kg/m2) | 113 | 25.4 (24.7–26.1) | 609 | 25.6 (25.3–25.9) | 146 | 25.2 (24.7–25.8) | 0.431 e | 0.151 a | 0.124 c |
| Total fat mass (kg) | 101 | 24.0 (22.4–25.5) | 584 | 25.5 (24.8–26.2) | 138 | 24.4 (23.1–25.7) | 0.141 e | 0.041a | 0.037c |
| Fat percentage (%) | 101 | 33.3 (31.8–34.7) R | 584 | 35.4 (34.8–36.0) | 138 | 35.0 (33.8–36.2) | 0.028e | 0.022a | 0.026c |
| Fat mass index (kg/m2) | 101 | 8.7 (8.1–9.3) | 584 | 9.3 (9.0–9.5) | 138 | 9.0 (8.5–9.7) | 0.143 e | 0.065 a | 0.062 c |
| Total lean mass (kg) | 101 | 43.7 (42.8–44.6) R,N | 584 | 42.1 (41.7–42.4) | 138 | 41.4 (40.7–42.1) | <0.001e | 0.001a | 0.001c |
| Lean mass index (kg/m2) | 101 | 15.8 (15.5–16.0) R,N | 584 | 15.3 (15.2–15.4) | 138 | 15.2 (15.0–15.5) | 0.002e | 0.002a | 0.001c |
| ALMI (kg/m2) | 101 | 6.9 (6.7–7.0) R,N | 584 | 6.6 (6.6–6.7) | 138 | 6.6 (6.5–6.7) | <0.001e | 0.001a | 0.001c |
| BMD | |||||||||
| FN BMD (g/cm2) | 101 | 1.00 (0.98–1.03) R,N | 584 | 0.96 (0.95–0.97) | 138 | 0.95 (0.93–0.97) | <0.001e | 0.001a | 0.002c |
| FN T score < −1 | 101 | 14.9% (15) R,N | 584 | 25.7% (150) | 138 | 28.3% (39) | 0.039 | ||
| FN T score ≤ −2.5 | 101 | 0.0% (0) | 584 | 0.9% (5) | 138 | 0.7% (1) | 1.000 | ||
| Physical performance | |||||||||
| Hand grip force (N) | 106 | 333.5 (322.6–344.4) R,N | 590 | 313.5 (308.7–318.4) | 142 | 305.5 (296.4–314.5) | 0.001e | 0.002a | 0.002c |
| Knee extension force (N) | 92 | 509.1 (488.4–529.8) R,N | 508 | 460.6 (452.7–468.4) | 118 | 442.3 (425.6–458.9) | <0.001e | <0.001a | <0.001c |
| Jumping height (cm) | 101 | 21.3 (20.5–22.1) R,N | 547 | 19.0 (18.6–19.3) | 136 | 18.7 (18.0–19.4) | <0.001e | <0.001a | <0.001c |
| Walking speed (m/s) | 106 | 2.9 (2.8–3.0) R,N | 591 | 2.6 (2.6–2.7) | 140 | 2.6 (2.5–2.6) | <0.001e | <0.001a | <0.001c |
| Walking distance in 6 min (m) | 95 | 696.7 (684.8–708.5) R,N | 543 | 667.5 (662.3–672.7) | 131 | 660.7 (651.1–670.2) | <0.001e | <0.001a | 0.001c |
| Self-reported PA | |||||||||
| Leisure-time PA (MET-h/d) | 136 | 4.9 (4.2–5.6) R,N | 689 | 4.2 (3.9–4.4) | 163 | 3.6 (3.2–4.0) | <0.001f | 0.008b | 0.007d |
| Accelerometer-measured PA | |||||||||
| Leisure-time MVPA (min/d) | 88 | 47.6 (41.6–53.6) | 516 | 43.1 (41.1–45.2) | 126 | 40.7 (36.6–44.7) | 0.166 f | 0.178 b | 0.271 d |
| Leisure-time step count (steps/d) | 88 | 7277 (6676–7878) | 516 | 6843 (6601–7084) | 126 | 6763 (6334–7192) | 0.339 e | 0.302 b | 0.384 d |
| Total MVPA (min/d) | 88 | 54.3 (47.7–60.7) | 516 | 50.3 (48.0–52.5) | 126 | 46.7 (42.6–50.8) | 0.188 f | 0.220 b | 0.312 d |
| Total step count (steps/d) | 88 | 8903 (8291–9515) | 516 | 8698 (8450–8947) | 126 | 8510 (8087–8933) | 0.646 f | 0.743 b | 0.761 d |
| Variable | n | AAM ≤ 12 | n | AAM = 13 | n | AAM ≥ 14 | p-Value | Model 1: p-Value a,b | Model 2: p-Value c,d |
|---|---|---|---|---|---|---|---|---|---|
| Background variables | |||||||||
| Age (y) | 391 | 51.3 (51.1–51.5) | 377 | 51.4 (51.2–51.6) | 313 | 51.4 (51.2–51.6) | 0.465 e | ||
| Bachelor or higher education (%) | 391 | 41.2% (161) | 377 | 41.9% (158) | 313 | 40.9% (128) | 0.961 | ||
| Number of parities | 391 | 2.0 (1.9–2.2) | 377 | 2.0 (1.9–2.1) | 313 | 2.0 (1.9–2.2) | 0.937 f | ||
| Used OCP at some point of life | 391 | 7.9% (31) H | 377 | 7.7% (29) H | 313 | 13.1% (41) | 0.025 | ||
| Used HC during the preceding 10 years | 391 | 54.2% (212) | 377 | 48.0% (181) | 313 | 53.7% (168) | 0.172 | ||
| Menopausal status | 390 | 377 | 313 | 0.373 | |||||
| PRE | 24.8% (97) | 29.7% (112) | 29.4% (92) | ||||||
| EPM | 20.2% (79) | 17.2% (65) | 16.3% (51) | ||||||
| LPM | 21.7% (85) | 17.2% (65) | 18.2% (57) | ||||||
| POST | 33.2% (130) | 35.8% (135) | 36.1% (113) | ||||||
| PA at age 13–16 | 346 | 343 | 281 | 0.014 | |||||
| CS | 13.5% (47) H | 12.0% (41) H | 16.4% (46) | ||||||
| RPA | 68.6% (238) | 67.9% (233) | 73.3% (206) | ||||||
| NE | 17.9% (62) | 20.1% (69) | 10.3% (29) | ||||||
| Body composition | |||||||||
| Height (cm) | 335 | 165.3 (164.7–165.9) | 341 | 165.3 (164.7–166.0) | 266 | 166.3 (165.6–166.9) | 0.073 e | 0.122 a | 0.135 c |
| Weight (m) | 335 | 71.7 (70.5–72.8) M,H | 341 | 69.6 (68.4–70.7) | 266 | 68.0 (66.8–69.3) | <0.001 e | <0.001 a | <0.001 c |
| BMI (kg/m2) | 335 | 26.2 (25.8–26.6) M,H | 341 | 25.4 (25.0–25.8) H | 266 | 24.6 (24.2–25.0) | <0.001 e | <0.001 a | <0.001 c |
| Total fat mass (kg) | 315 | 26.6 (25.7–27.5) M,H | 319 | 24.9 (24.0–25.9) H | 247 | 23.1 (22.0–24.2) | <0.001 e | <0.001 a | <0.001 c |
| Fat percentage (%) | 315 | 36.4 (35.6–37.1) H | 319 | 35.1 (34.3–35.9) H | 247 | 33.1 (32.1–34.0) | <0.001 e | <0.001 a | <0.001 c |
| Fat mass index (kg/m2) | 315 | 9.7 (9.4–10.1) M,H | 319 | 9.1 (8.8–9.4) H | 247 | 8.4 (8.0–8.8) | <0.001 e | <0.001 a | <0.001 c |
| Total lean mass (kg) | 315 | 42.3 (41.8–42.8) | 319 | 41.8 (41.4–42.3) | 247 | 42.3 (41.8–42.9) | 0.105 e | 0.099 a | 0.195 c |
| Lean mass index (kg/m2) | 315 | 15.5 (15.4–15.6) | 319 | 15.3 (15.2–15.5) | 247 | 15.3 (115.2–15.5) | 0.135 e | 0.032 a | 0.047 c |
| ALMI (kg/m2) | 315 | 6.7 (6.6–6.7) | 319 | 6.6 (6.5–6.7) | 247 | 6.6 (6.5–6.7) | 0.237 e | 0.075 a | 0.124 c |
| BMD | |||||||||
| FN BMD (g/cm2) | 315 | 0.97 (0.96–0.98) H | 319 | 0.96 (0.95–0.97) | 247 | 0.94 (0.93–0.96) | 0.034 e | 0.056 a | 0.056 c |
| FN T score < −1 | 315 | 24.2% (76) | 319 | 23.5% (75) | 247 | 29.6% (73) | 0.214 | ||
| FN T score ≤ −2.5 | 315 | 0.3% (1) | 319 | 1.3% (4) | 247 | 0.8% (2) | 0.404 | ||
| Physical performance | |||||||||
| Hand grip force (N) | 321 | 314.0 (307.2–320.8) | 324 | 315.0 (308.6–321.5) | 254 | 311.0 (304.0–318.1) | 0.715 e | 0.279 a | 0.195 c |
| Knee extension force (N) | 282 | 468.8 (457.7–480.0) | 271 | 457.8 (447.1–468.5) | 230 | 459.7 (446.6–472.7) | 0.344 e | 0.419 a | 0.442 c |
| Jumping height (cm) | 300 | 18.7 (18.3–19.2) H | 302 | 19.2 (18.7–19.7) | 243 | 19.8 (19.2–20.4) | 0.013 e | 0.019 a | 0.013 c |
| Walking speed (m/s) | 319 | 2.6 (2.6–2.7) | 324 | 2.7 (2.6–2.7) | 255 | 2.7 (2.6–2.7) | 0.443 e | 0.508 a | 0.507 c |
| Walking distance in 6 min (m) | 300 | 665.5 (658.5–672.5) | 293 | 669.8 (663.2–676.3) | 240 | 674.1 (666.0–682.1) | 0.259 e | 0.155 a | 0.093 c |
| Self-reported PA | |||||||||
| Leisure time PA (MET-h/d) | 391 | 4.4 (4.0–4.8) | 377 | 4.0 (3.6–4.3) | 313 | 4.2 (3.8–4.5) | 0.l78 f | 0.288 b | 0.285 d |
| Accelerometer-measured PA | |||||||||
| Leisure-time MVPA (min/d) | 282 | 42.4 (39.6–45.2) | 277 | 41.0 (38.4–43.6) | 225 | 46.9 (43.4–50.4) | 0.054 f | 0.053 b | 0.036 d |
| Leisure-time step count (steps/d) | 282 | 6812 (6495–7128) H | 277 | 6586 (6282–6892) H | 225 | 7365 (6986–7744) | 0.005 f | 0.025 b | 0.018 d |
| Total MVPA (min/d) | 282 | 48.6 (45.6–51.6) | 277 | 48.3 (45.6–51.1) | 225 | 53.6 (49.8–57.5) | 0.117 f | 0.117 b | 0.093 d |
| Total step count (steps/d) | 282 | 8541 (8214–8868) H | 277 | 8492 (8179–8804) H | 225 | 9066 (8677–9455) | 0.030 f | 0.166 b | 0.169 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravi, S.; Kujala, U.M.; Tammelin, T.H.; Hirvensalo, M.; Kovanen, V.; Valtonen, M.; Waller, B.; Aukee, P.; Sipilä, S.; Laakkonen, E.K. Adolescent Sport Participation and Age at Menarche in Relation to Midlife Body Composition, Bone Mineral Density, Fitness, and Physical Activity. J. Clin. Med. 2020, 9, 3797. https://doi.org/10.3390/jcm9123797
Ravi S, Kujala UM, Tammelin TH, Hirvensalo M, Kovanen V, Valtonen M, Waller B, Aukee P, Sipilä S, Laakkonen EK. Adolescent Sport Participation and Age at Menarche in Relation to Midlife Body Composition, Bone Mineral Density, Fitness, and Physical Activity. Journal of Clinical Medicine. 2020; 9(12):3797. https://doi.org/10.3390/jcm9123797
Chicago/Turabian StyleRavi, Suvi, Urho M. Kujala, Tuija H. Tammelin, Mirja Hirvensalo, Vuokko Kovanen, Maarit Valtonen, Benjamin Waller, Pauliina Aukee, Sarianna Sipilä, and Eija K. Laakkonen. 2020. "Adolescent Sport Participation and Age at Menarche in Relation to Midlife Body Composition, Bone Mineral Density, Fitness, and Physical Activity" Journal of Clinical Medicine 9, no. 12: 3797. https://doi.org/10.3390/jcm9123797
APA StyleRavi, S., Kujala, U. M., Tammelin, T. H., Hirvensalo, M., Kovanen, V., Valtonen, M., Waller, B., Aukee, P., Sipilä, S., & Laakkonen, E. K. (2020). Adolescent Sport Participation and Age at Menarche in Relation to Midlife Body Composition, Bone Mineral Density, Fitness, and Physical Activity. Journal of Clinical Medicine, 9(12), 3797. https://doi.org/10.3390/jcm9123797








