Impact of Air Pollution and Weather on Dry Eye
Abstract
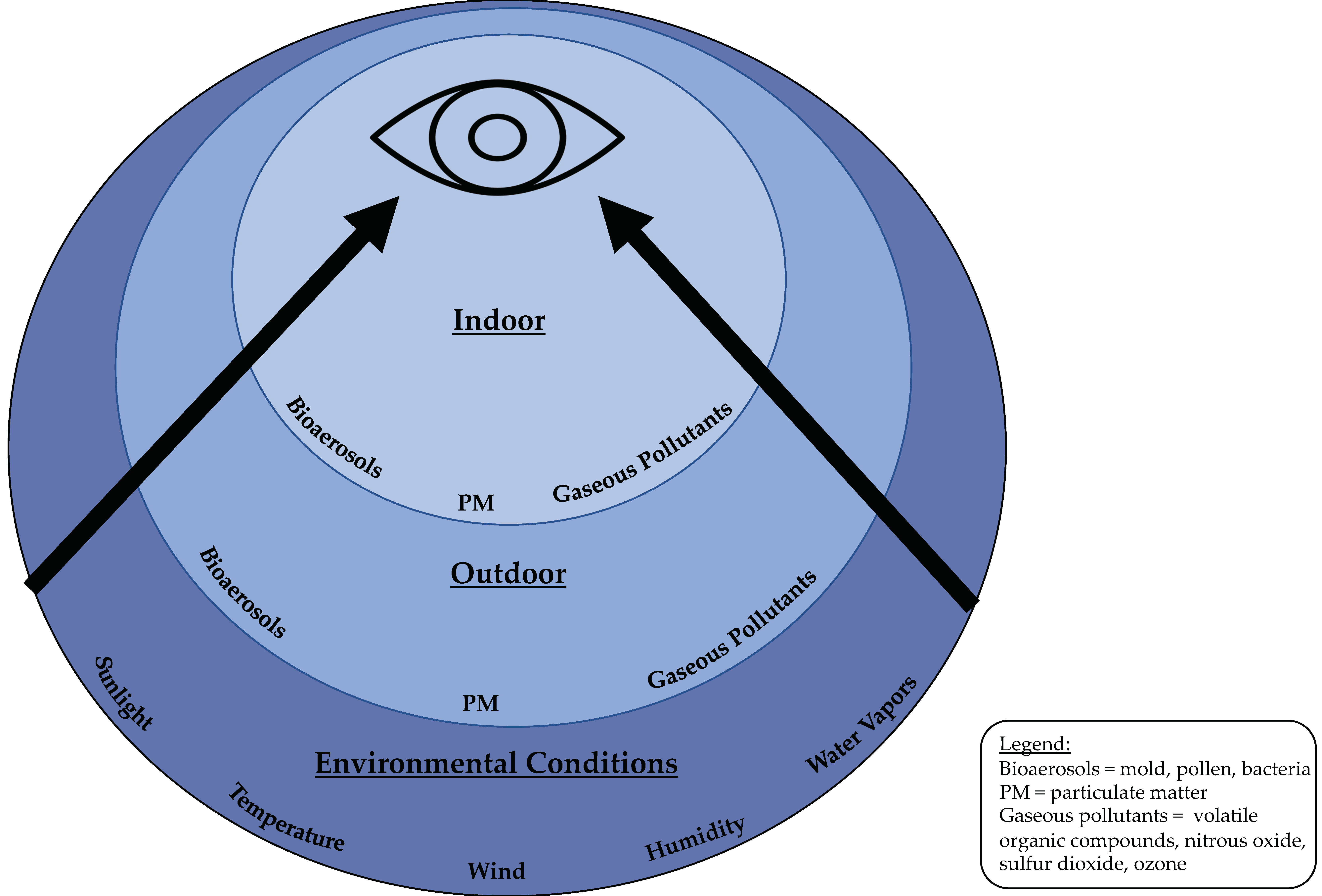
1. Introduction
2. Methods
3. Dry Eye as a Disease Model
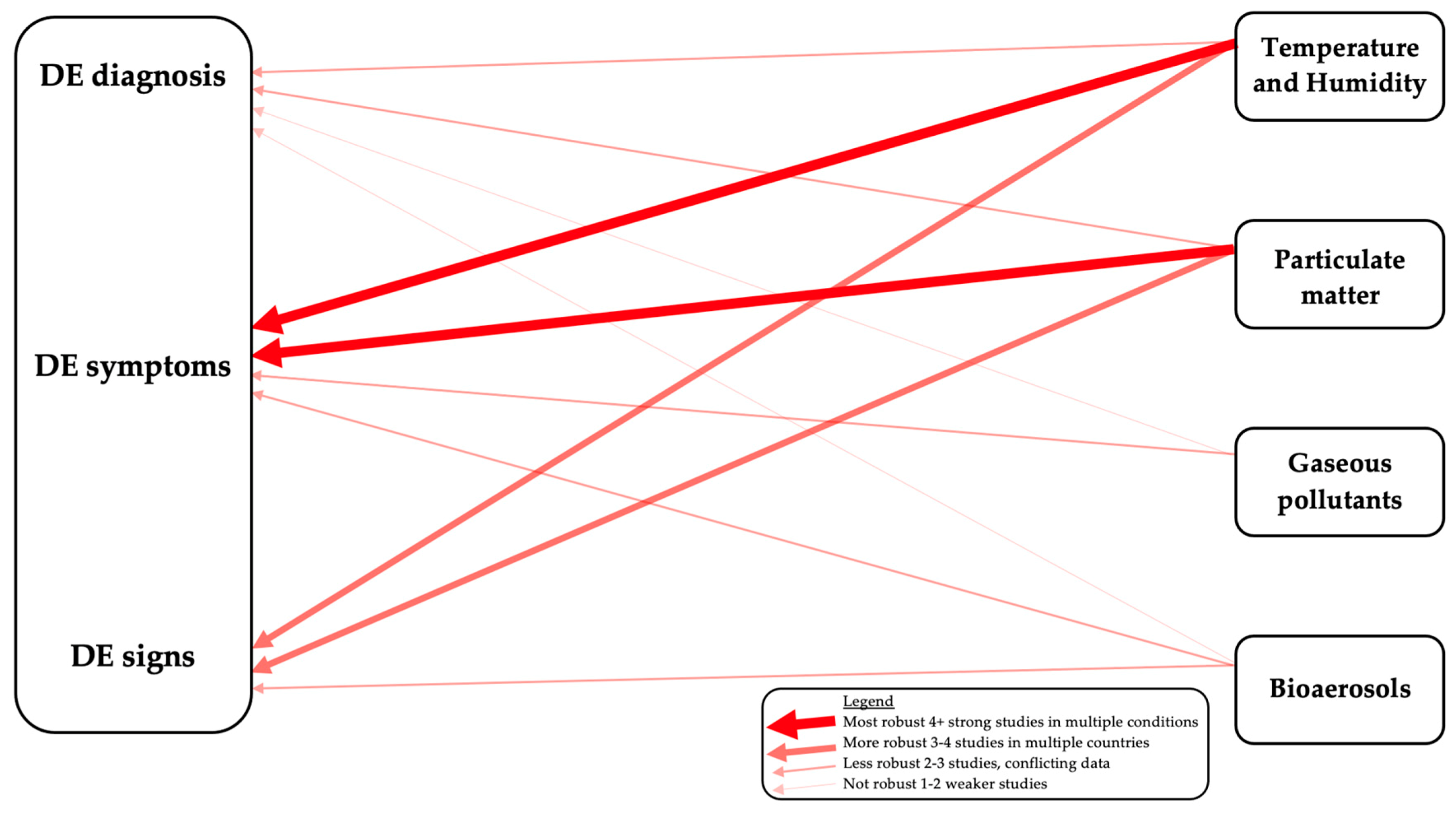
4. Effect of Particulate Matter (PM) on the Ocular Surface
4.1. Outdoor PM
4.1.1. The Effect of Outdoor PM on a Dry Eye Diagnosis
4.1.2. The Effect of Outdoor PM on Dry Eye Symptoms
4.1.3. The Effect of Outdoor PM on Dry Eye Signs
4.2. Indoor PM
4.2.1. The Effect of Indoor PM on Dry Eye Symptoms
4.2.2. The Effect of Indoor PM on Dry Eye Signs
4.3. Potential Pathophysiology
5. Effect of Gaseous Pollutants on the Ocular Surface
5.1. Outdoor Gaseous Pollutants
5.1.1. The Effect of Outdoor Gaseous Pollutants on a DE Diagnosis
5.1.2. The Effect of Outdoor Gaseous Pollutants on DE Symptoms
5.1.3. The Effect of Outdoor Gaseous Pollutants on DE Signs
5.2. Indoor Gaseous Pollutants
The Effect of Indoor Gaseous Pollutants DE Symptoms
5.3. Potential Pathophysiology
6. Effect of Weather on the Ocular Surface
6.1. Outdoor Weather Conditions
6.1.1. The Effect of Outdoor Weather Conditions on a Dry Eye Diagnosis
6.1.2. The Effect of Outdoor Weather Conditions on Dry Eye Symptoms
6.1.3. The Effect of Outdoor Weather Conditions on Dry Eye Signs
6.2. Indoor Temperature and Humidity
6.2.1. The Effect of Indoor Humidity and Temperature on Dry Eye Symptoms
6.2.2. The Effect of Indoor Humidity and Temperature on Dry Eye Signs
6.3. Potential Pathophysiology
7. Mitigation Strategies
7.1. Indoor Mitigation Strategies
7.2. Outdoor Mitigation Strategies
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shima, M. Health Effects of Air Pollution: A Historical Review and Present Status. Nihon Eiseigaku Zasshi 2017, 72, 159–165. [Google Scholar] [CrossRef]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C., Jr.; et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef]
- Pope, C.A.; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef]
- Conforti, A.; Mascia, M.; Cioffi, G.; De Angelis, C.; Coppola, G.; De Rosa, P.; Pivonello, R.; Alviggi, C.; De Placido, G. Air pollution and female fertility: A systematic review of literature. Reprod. Biol. Endocrinol. 2018, 16, 117. [Google Scholar] [CrossRef]
- Jurewicz, J.; Dziewirska, E.; Radwan, M.; Hanke, W. Air pollution from natural and anthropic sources and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 109. [Google Scholar] [CrossRef]
- Sass, V.; Kravitz-Wirtz, N.; Karceski, S.M.; Hajat, A.; Crowder, K.; Takeuchi, D. The effects of air pollution on individual psychological distress. Health Place 2017, 48, 72–79. [Google Scholar] [CrossRef]
- Calderon-Garciduenas, L.; Leray, E.; Heydarpour, P.; Torres-Jardon, R.; Reis, J. Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: The clinical impact on children and beyond. Rev. Neurol. 2016, 172, 69–80. [Google Scholar] [CrossRef]
- Johannson, K.A.; Balmes, J.R.; Collard, H.R. Air pollution exposure: A novel environmental risk factor for interstitial lung disease? Chest 2015, 147, 1161–1167. [Google Scholar] [CrossRef]
- Stanek, L.W.; Brown, J.S.; Stanek, J.; Gift, J.; Costa, D.L. Air pollution toxicology—A brief review of the role of the science in shaping the current understanding of air pollution health risks. Toxicol. Sci. 2011, 120 (Suppl. 1), S8–S27. [Google Scholar] [CrossRef]
- Wang, Q. Urbanization and Global Health: The Role of Air Pollution. Iran. J. Public Health 2018, 47, 1644–1652. [Google Scholar]
- Cincinelli, A.; Martellini, T. Indoor Air Quality and Health. Int. J Environ. Res. Public Health 2017, 14, 1286. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Kalangara, J.P.; Galor, A.; Levitt, R.C.; Covington, D.B.; McManus, K.T.; Sarantopoulos, C.D.; Felix, E.R. Characteristics of Ocular Pain Complaints in Patients With Idiopathic Dry Eye Symptoms. Eye Contact Lens 2017, 43, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.D.; Crews, L.A.; Messmer, E.M.; Foulks, G.N.; Nichols, K.K.; Baenninger, P.; Geerling, G.; Figueiredo, F.; Lemp, M.A. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: Clinical implications. Acta Ophthalmol. 2014, 92, 161–166. [Google Scholar] [CrossRef]
- Galor, A.; Feuer, W.; Lee, D.J.; Florez, H.; Venincasa, V.D.; Perez, V.L. Ocular surface parameters in older male veterans. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1426–1433. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benitez-del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef]
- Uchino, M.; Schaumberg, D.A. Dry Eye Disease: Impact on Quality of Life and Vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Kumar, N.; Feuer, W.; Lee, D.J. Environmental factors affect the risk of dry eye syndrome in a United States veteran population. Ophthalmology 2014, 121, 972–973.e971. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Feuer, W.; Lanza, N.L.; Galor, A. Seasonal Variation in Dry Eye. Ophthalmology 2015, 122, 1727–1729. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.Y.; Lee, Y.C.; Hsieh, C.J.; Tseng, C.C.; Yiin, L.M. Association between Dry Eye Disease, Air Pollution and Weather Changes in Taiwan. Int. J. Environ. Res. Public Health 2018, 15, 2269. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Fu, Q.; Lyu, D.; Zhang, L.; Qin, Z.; Tang, Q.; Yin, H.; Xu, P.; Wu, L.; Wang, X.; et al. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: A case-crossover study. Environ. Pollut. 2019, 246, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Deng, Q.; Wang, J.; Chang, X.; Wang, S.; Yang, R.; Yu, J.; Yu, J. Air Pollutants are associated with Dry Eye Disease in Urban Ophthalmic Outpatients: A Prevalence Study in China. J. Transl. Med. 2019, 17, 46. [Google Scholar] [CrossRef]
- Saxena, R.; Srivastava, S.; Trivedi, D.; Anand, E.; Joshi, S.; Gupta, S.K. Impact of environmental pollution on the eye. Acta. Ophthalmol. Scand. 2003, 81, 491–494. [Google Scholar] [CrossRef]
- Wiwatanadate, P. Acute air pollution-related symptoms among residents in Chiang Mai, Thailand. J. Environ. Health 2014, 76, 76–84. [Google Scholar]
- Hwang, S.H.; Choi, Y.H.; Paik, H.J.; Wee, W.R.; Kim, M.K.; Kim, D.H. Potential Importance of Ozone in the Association Between Outdoor Air Pollution and Dry Eye Disease in South Korea. JAMA Ophthalmol. 2016, 134, 503–510. [Google Scholar] [CrossRef]
- Torricelli, A.A.; Novaes, P.; Matsuda, M.; Braga, A.; Saldiva, P.H.; Alves, M.R.; Monteiro, M.L. Correlation between signs and symptoms of ocular surface dysfunction and tear osmolarity with ambient levels of air pollution in a large metropolitan area. Cornea 2013, 32, e11–e15. [Google Scholar] [CrossRef]
- Kiotseridis, H.; Cilio, C.M.; Bjermer, L.; Tunsater, A.; Jacobsson, H.; Dahl, A. Grass pollen allergy in children and adolescents-symptoms, health related quality of life and the value of pollen prognosis. Clin. Transl. Allergy 2013, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.L.A.; Lerner, J.E.C.; Giuliani, D.S.; Porta, A.A.; Andrinolo, D. Comparative study of tear lipid composition in two human populations with different exposure to particulate matter in La Plata, Argentina. Environ. Sci. Pollut. Res. Int. 2019, 26, 6948–6956. [Google Scholar] [CrossRef] [PubMed]
- Idarraga, M.A.; Guerrero, J.S.; Mosle, S.G.; Miralles, F.; Galor, A.; Kumar, N. Relationships Between Short-Term Exposure to an Indoor Environment and Dry Eye (DE) Symptoms. J. Clin. Med. 2020, 9, 1316. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Janecki, J.; Galor, A.; Rock, S.; Menendez, D.; Hackam, A.S.; Jeng, B.H.; Kumar, N. Association of the Indoor Environment With Dry Eye Metrics. JAMA Ophthalmol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L.; Boechat, J.L.; Gioda, A.; dos Santos, C.Y.; de Aquino Neto, F.R.; Silva, J.R.L. Symptoms prevalence among office workers of a sealed versus a non-sealed building: Associations to indoor air quality. Environ. Int. 2009, 35, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Norback, D.; Hashim, J.H.; Markowicz, P.; Cai, G.H.; Hashim, Z.; Ali, F.; Larsson, L. Endotoxin, ergosterol, muramic acid and fungal DNA in dust from schools in Johor Bahru, Malaysia—Associations with rhinitis and sick building syndrome (SBS) in junior high school students. Sci. Total Environ. 2016, 545, 95–103. [Google Scholar] [CrossRef]
- Lu, C.; Deng, Q.; Li, Y.; Sundell, J.; Norback, D. Outdoor air pollution, meteorological conditions and indoor factors in dwellings in relation to sick building syndrome (SBS) among adults in China. Sci. Total Environ. 2016, 560, 186–196. [Google Scholar] [CrossRef]
- Rock, S.; Galor, A.; Kumar, N. Indoor Airborne Microbial Concentration and Dry Eye. Am. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Norback, D.; Hashim, J.H.; Hashim, Z.; Sooria, V.; Ismail, S.A.; Wieslander, G. Ocular symptoms and tear film break up time (BUT) among junior high school students in Penang, Malaysia—Associations with fungal DNA in school dust. Int. J. Hyg. Environ. Health 2017, 220, 697–703. [Google Scholar] [CrossRef]
- Pan, Z.; Molhave, L.; Kjaergaard, S.K. Effects on eyes and nose in humans after experimental exposure to airborne office dust. Indoor Air 2000, 10, 237–245. [Google Scholar] [CrossRef]
- Berra, M.; Galperin, G.; Dawidowski, L.; Tau, J.; Marquez, I.; Berra, A. Impact of wildfire smoke in Buenos Aires, Argentina, on ocular surface. Arq. Bras. Oftalmol. 2015, 78, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Li, J.; Yang, Q.; Wu, A.; Qu, D.Y.; Wang, Y.; Ye, L.; Bao, J.; Shao, Y. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Sci. Rep. 2018, 8, 17828. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Jeong, I.Y.; Park, Y.G.; Yang, S.Y. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea 2007, 26, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shen, J.; Zhang, C.; Park, C.Y.; Kohanim, S.; Yew, M.; Parker, J.S.; Chuck, R.S. Inflammatory cytokine expression on the ocular surface in the Botulium toxin B induced murine dry eye model. Mol. Vis. 2009, 15, 250–258. [Google Scholar]
- Bossou, Y.M.; Serssar, Y.; Allou, A.; Vitry, S.; Momas, I.; Seta, N.; Menotti, J.; Achard, S. Impact of Mycotoxins Secreted by Aspergillus Molds on the Inflammatory Response of Human Corneal Epithelial Cells. Toxins 2017, 9, 197. [Google Scholar] [CrossRef]
- Xiang, P.; He, R.W.; Han, Y.H.; Sun, H.J.; Cui, X.Y.; Ma, L.Q. Mechanisms of housedust-induced toxicity in primary human corneal epithelial cells: Oxidative stress, proinflammatory response and mitochondrial dysfunction. Environ. Int. 2016, 89, 30–37. [Google Scholar] [CrossRef]
- Kim, Y.; Paik, H.J.; Kim, M.K.; Choi, Y.H.; Kim, D.H. Short-Term Effects of Ground-Level Ozone in Patients With Dry Eye Disease: A Prospective Clinical Study. Cornea 2019, 38, 1483–1488. [Google Scholar] [CrossRef]
- Yorifuji, T.; Noguchi, M.; Tsuda, T.; Suzuki, E.; Takao, S.; Kashima, S.; Yanagisawa, Y. Does open-air exposure to volatile organic compounds near a plastic recycling factory cause health effects? J. Occup. Health 2012, 54, 79–87. [Google Scholar] [CrossRef]
- Kongtip, P.; Singkaew, P.; Yoosook, W.; Chantanakul, S.; Sujiratat, D. Health effects of people living close to a petrochemical industrial estate in Thailand. J. Med. Assoc. Thai 2013, 96 (Suppl. S5), S64–S72. [Google Scholar]
- Novaes, P.; do Nascimento, P.H.S.; Kara-José, N.; Macchione, M.; Matsuda, M.; Racca, L.; Berra, A. Ambient levels of air pollution induce goblet-cell hyperplasia in human conjunctival epithelium. Environ. Health Perspect. 2007, 115, 1753–1756. [Google Scholar] [CrossRef]
- Beko, G.; Allen, J.G.; Weschler, C.J.; Vallarino, J.; Spengler, J.D. Impact of cabin ozone concentrations on passenger reported symptoms in commercial aircraft. PLoS ONE 2015, 10, e0128454. [Google Scholar] [CrossRef] [PubMed]
- Strom-Tejsen, P.; Weschler, C.J.; Wargocki, P.; Myskow, D.; Zarzycka, J. The influence of ozone on self-evaluation of symptoms in a simulated aircraft cabin. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Norbäck, D.; Hashim, J.H.; Hashim, Z.; Ali, F. Volatile organic compounds (VOC), formaldehyde and nitrogen dioxide (NO. Sci. Total Environ. 2017, 592, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.; Bruckner, T.; Triebig, G. Formaldehyde and chemosensory irritation in humans: A controlled human exposure study. Regul. Toxicol. Pharmacol. 2008, 50, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-W.; Park, H.-W.; Kim, W.J.; Kim, M.-G.; Lee, S.-J. Exposure to volatile organic compounds and airway inflammation. Environ. Health 2018, 17, 65. [Google Scholar] [CrossRef]
- Lu, C.Y.; Ma, Y.C.; Lin, J.M.; Li, C.Y.; Lin, R.S.; Sung, F.C. Oxidative stress associated with indoor air pollution and sick building syndrome-related symptoms among office workers in Taiwan. Inhal. Toxicol. 2007, 19, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Kishi, R.; Sata, F.; Katakura, Y.; Urashima, Y.; Hatakeyama, A.; Kobayashi, S.; Jin, K.; Kurahashi, N.; Kondo, T.; et al. Symptoms in relation to chemicals and dampness in newly built dwellings. Int. Arch. Occup. Environ. Health 2004, 77, 461–470. [Google Scholar] [CrossRef]
- Jung, S.J.; Mehta, J.S.; Tong, L. Effects of environment pollution on the ocular surface. Ocul. Surf. 2018, 16, 198–205. [Google Scholar] [CrossRef]
- Bessac, B.F.; Jordt, S.E. Sensory detection and responses to toxic gases: Mechanisms, health effects, and countermeasures. Proc. Am. Thorac. Soc. 2010, 7, 269–277. [Google Scholar] [CrossRef]
- Arsalane, K.; Gosset, P.; Vanhee, D.; Voisin, C.; Hamid, Q.; Tonnel, A.B.; Wallaert, B. Ozone stimulates synthesis of inflammatory cytokines by alveolar macrophages in vitro. Am. J. Respir. Cell Mol. Biol. 1995, 13, 60–68. [Google Scholar] [CrossRef]
- Koren, H.S.; Devlin, R.B.; Graham, D.E.; Mann, R.; McGee, M.P.; Horstman, D.H.; Kozumbo, W.J.; Becker, S.; House, D.E.; McDonnell, W.F.; et al. Ozone-induced inflammation in the lower airways of human subjects. Am. Rev. Respir. Dis. 1989, 139, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, E.K.; Kang, S.W.; Kim, J.H.; Hwang, H.J.; Kim, T.I. Effects of ozone exposure on the ocular surface. Free Radic. Biol. Med. 2013, 63, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Ezratty, V.; Guillossou, G.; Neukirch, C.; Dehoux, M.; Koscielny, S.; Bonay, M.; Cabanes, P.A.; Samet, J.M.; Mure, P.; Ropert, L.; et al. Repeated nitrogen dioxide exposures and eosinophilic airway inflammation in asthmatics: A randomized crossover study. Environ. Health Perspect. 2014, 122, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Pouli, A.E.; Hatzinikolaou, D.G.; Piperi, C.; Stavridou, A.; Psallidopoulos, M.C.; Stavrides, J.C. The cytotoxic effect of volatile organic compounds of the gas phase of cigarette smoke on lung epithelial cells. Free Radic. Biol. Med. 2003, 34, 345–355. [Google Scholar] [CrossRef]
- Um, S.B.; Kim, N.H.; Lee, H.K.; Song, J.S.; Kim, H.C. Spatial epidemiology of dry eye disease: Findings from South Korea. Int. J. Health Geogr. 2014, 13, 31. [Google Scholar] [CrossRef]
- Sahai, A.; Malik, P. Dry eye: Prevalence and attributable risk factors in a hospital-based population. Indian J. Ophthalmol. 2005, 53, 87–91. [Google Scholar] [CrossRef]
- Gupta, N.; Prasad, I.; Himashree, G.; D’Souza, P. Prevalence of dry eye at high altitude: A case controlled comparative study. High Alt. Med. Biol. 2008, 9, 327–334. [Google Scholar] [CrossRef]
- van Setten, G.; Labetoulle, M.; Baudouin, C.; Rolando, M. Evidence of seasonality and effects of psychrometry in dry eye disease. Acta Ophthalmol. 2016, 94, 499–506. [Google Scholar] [CrossRef]
- Berg, E.J.; Ying, G.S.; Maguire, M.G.; Sheffield, P.E.; Szczotka-Flynn, L.B.; Asbell, P.A.; Shen, J.F.; Group, D.S.R. Climatic and Environmental Correlates of Dry Eye Disease Severity: A Report From the Dry Eye Assessment and Management (DREAM) Study. Transl. Vis. Sci. Technol. 2020, 9, 25. [Google Scholar] [CrossRef]
- Reinikainen, L.M.; Jaakkola, J.J.; Seppanen, O. The effect of air humidification on symptoms and perception of indoor air quality in office workers: A six-period cross-over trial. Arch. Environ. Health 1992, 47, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Mendell, M.J.; Fisk, W.J.; Petersen, M.R.; Hines, C.J.; Dong, M.; Faulkner, D.; Deddens, J.A.; Ruder, A.M.; Sullivan, D.; Boeniger, M.F. Indoor particles and symptoms among office workers: Results from a double-blind cross-over study. Epidemiology 2002, 13, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ikeda, K.; Kagi, N.; Yanagi, U.; Osawa, H. Prevalence and risk factors associated with nonspecific building-related symptoms in office employees in Japan: Relationships between work environment, Indoor Air Quality, and occupational stress. Indoor Air 2015, 25, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, M.J.; Gonzalez-Saiz, A.; de la Fuente, B.; Morilla-Grasa, A.; Mayo-Iscar, A.; San-Jose, J.; Feijo, J.; Stern, M.E.; Calonge, M. Exposure to a controlled adverse environment impairs the ocular surface of subjects with minimally symptomatic dry eye. Invest. Ophthalmol. Vis. Sci. 2007, 48, 4026–4032. [Google Scholar] [CrossRef] [PubMed]
- Korb, D.R.; Blackie, C.A. Using goggles to increase periocular humidity and reduce dry eye symptoms. Eye Contact Lens 2013, 39, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Dogru, M.; Toriyama, N.; Yamaguchi, T.; Shimazaki, J.; Tsubota, K. Evaluation of the Effect of Moist Chamber Spectacles in Patients With Dry Eye Exposed to Adverse Environment Conditions. Eye Contact Lens 2018, 44, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Chan, E.; Ea, L.; Kam, C.; Lu, Y.; Misra, S.L.; Craig, J.P. Randomized Trial of Desktop Humidifier for Dry Eye Relief in Computer Users. Optom. Vis. Sci. 2017, 94, 1052–1057. [Google Scholar] [CrossRef]
- He, X.; Brem, B.T.; Bahk, Y.K.; Kuo, Y.-Y.; Wang, J. Effects of relative humidity and particle type on the performance and service life of automobile cabin air filters. Aerosol. Sci. Technol. 2016, 50, 542–554. [Google Scholar] [CrossRef]
- Madden, L.C.; Tomlinson, A.; Simmons, P.A. Effect of humidity variations in a controlled environment chamber on tear evaporation after dry eye therapy. Eye Contact Lens 2013, 39, 169–174. [Google Scholar] [CrossRef]
- Lopez-Miguel, A.; Teson, M.; Martin-Montanez, V.; Enriquez-de-Salamanca, A.; Stern, M.E.; Calonge, M.; Gonzalez-Garcia, M.J. Dry eye exacerbation in patients exposed to desiccating stress under controlled environmental conditions. Am. J. Ophthalmol. 2014, 157, 788–798.e782. [Google Scholar] [CrossRef]
- Abusharha, A.A.; Pearce, E.I.; Fagehi, R. Effect of Ambient Temperature on the Human Tear Film. Eye Contact Lens 2016, 42, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Mathews, J.; Leake, K.; Bell, J. Factors affecting evaporation rates of tear film components measured in vitro. Eye Contact Lens 2009, 35, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Keitelman, I.; Sabbione, F.; Trevani, A.S.; Giordano, M.N.; Galletti, J.G. Desiccating stress-induced disruption of ocular surface immune tolerance drives dry eye disease. Clin. Exp. Immunol. 2016, 184, 248–256. [Google Scholar] [CrossRef]
- Teson, M.; Gonzalez-Garcia, M.J.; Lopez-Miguel, A.; Enriquez-de-Salamanca, A.; Martin-Montanez, V.; Benito, M.J.; Mateo, M.E.; Stern, M.E.; Calonge, M. Influence of a controlled environment simulating an in-flight airplane cabin on dry eye disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Lee, C.H.; Ji, Y.W.; Yeo, A.; Noh, H.; Song, I.; Kim, E.K.; Lee, H.K. Activation of Dll4/Notch Signaling and Hypoxia-Inducible Factor-1 Alpha Facilitates Lymphangiogenesis in Lacrimal Glands in Dry Eye. PLoS ONE 2016, 11, e0147846. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.A.; Galor, A.; Arheart, K.L.; Musselman, D.L.; Venincasa, V.D.; Florez, H.J.; Lee, D.J. Dry eye syndrome, posttraumatic stress disorder, and depression in an older male veteran population. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3666–3672. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.J.; Crain, E.F.; Gruchalla, R.S.; O’Connor, G.T.; Kattan, M.; Evans, R.; Stout, J.; Malindzak, G.; Smartt, E.; Plaut, M.; et al. Results of a home-based environmental intervention among urban children with asthma. N. Engl. J. Med. 2004, 351, 1068–1080. [Google Scholar] [CrossRef]
- Mosaddegh, M.H.; Jafarian, A.; Ghasemi, A.; Mosaddegh, A. Phytoremediation of benzene, toluene, ethylbenzene and xylene contaminated air by D. deremensis and O. microdasys plants. J. Environ. Health Sci. Eng. 2014, 12, 39. [Google Scholar] [CrossRef]
- Smajlovic, S.K.; Kukec, A.; Dovjak, M. Association between Sick Building Syndrome and Indoor Environmental Quality in Slovenian Hospitals: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2019, 16, 3224. [Google Scholar] [CrossRef]
- Nowak, D.; Crane, D.; Stevens, J. Air Pollution Removal by Urban Trees and Shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Yeager, R.; Riggs, D.W.; DeJarnett, N.; Srivastava, S.; Lorkiewicz, P.; Xie, Z.; Krivokhizhina, T.; Keith, R.J.; Browning, M.; Zafar, N.; et al. Association between residential greenness and exposure to volatile organic compounds. Sci. Total Environ. 2020, 707, 135435. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandell, J.T.; Idarraga, M.; Kumar, N.; Galor, A. Impact of Air Pollution and Weather on Dry Eye. J. Clin. Med. 2020, 9, 3740. https://doi.org/10.3390/jcm9113740
Mandell JT, Idarraga M, Kumar N, Galor A. Impact of Air Pollution and Weather on Dry Eye. Journal of Clinical Medicine. 2020; 9(11):3740. https://doi.org/10.3390/jcm9113740
Chicago/Turabian StyleMandell, Jordan T., Maria Idarraga, Naresh Kumar, and Anat Galor. 2020. "Impact of Air Pollution and Weather on Dry Eye" Journal of Clinical Medicine 9, no. 11: 3740. https://doi.org/10.3390/jcm9113740
APA StyleMandell, J. T., Idarraga, M., Kumar, N., & Galor, A. (2020). Impact of Air Pollution and Weather on Dry Eye. Journal of Clinical Medicine, 9(11), 3740. https://doi.org/10.3390/jcm9113740




