The Activity of N-acetyl-β-hexosaminidase in the Blood, Urine, Cerebrospinal Fluid and Vitreous Humor Died People Due to Alcohol Intoxication
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Ethics
2.3. Procedures
- the femoral vein (blood)
- the bulb of the eye (vitreous humor)
- the lateral ventricle of the brain (cerebrospinal fluid)
- the urinary bladder (urine)
2.4. Assays
2.5. Statistics
2.6. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Opitz, A.; Hubert, J.; Beste, C.; Stock, A.K. Alcohol Hangover Slightly Impairs Response Selection but not Response Inhibition. J. Clin. Med. 2019, 8, 1317. [Google Scholar] [CrossRef]
- Van Lawick van Pabst, A.E.; Devenney, L.E.; Verster, J.C. Sex Differences in the Presence and Severity of Alcohol Hangover Symptoms. J. Clin. Med. 2019, 8, 867. [Google Scholar]
- Waszkiewicz, N.; Konarzewska, B.; Waszkiewicz, M.; Popławska, R.; Szajda, S.D.; Zalewska, A.; Markowski, T.; Szulc, A. Biomarkers of alcohol abuse. Part, I. Traditional biomarkers and their interpretation. Psychiatr. Pol. 2010, 44, 127–136. [Google Scholar] [PubMed]
- Waszkiewicz, N.; Popławska, R.; Konarzewska, B.; Szajda, S.D.; Galińska, B.; Rutkowski, P.; Leśniak, R.; Szulc, A. Biomarkers of alkohol abuse. Part II. New biomarkers and their interpretation. Psychiatr. Pol. 2010, 44, 137–146. [Google Scholar]
- Sharpe, P.C. Biochemical detection and monitoring of alcohol abuse and abstinence. Ann. Clin. Biochem. 2001, 38, 652–664. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Alcohol and Health 2018. 2018. Available online: https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/ (accessed on 1 January 2019).
- Waszkiewicz, N.; Szajda, S.D.; Szulc, A.; Zwierz, K. Binge drinking and tuberculosis prevention. Tuberculosis 2015, 95, 89–90. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Galińska-Skok, B.; Nestsiarovich, A.; Kułak-Bejda, A.; Wilczyńska, K.; Simonienko, K.; Kwiatkowski, M.; Konarzewska, B. Neurobiological Effects of Binge Drinking Help in Its Detection and Differential Diagnosis from Alcohol Dependence. Dis. Markers 2018. [Google Scholar] [CrossRef]
- Verster, J.C.; Arnoldy, L.; van de Loo, A.J.A.E.; Benson, S.; Scholey, A.; Stock, A.K. The Impact of Mood and Subjective Intoxication on Hangover Severity. J. Clin. Med. 2020, 9, 2462. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Szulc, A.; Zwierz, K. Binge drinking—Induced subtle myocardial injury. Alcohol. Clin. Exp. Res. 2013, 37, 1261–1263. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Galińska-Skok, B.; Zalewska, A.; Szajda, S.D.; Zwierz, K.; Więdłocha, M.; Szulc, A. Salivary immune proteins monitoring can help detection of binge and chronic alcohol drinkers: Preliminary findings. Drug Alcohol Depend. 2018, 183, 13–18. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Szajda, S.D.; Kępka, A.; Szulc, A.; Zwierz, K. Glycoconjugates in the detection of alcohol abuse. Biochem. Soc. Trans. 2011, 39, 365–369. [Google Scholar] [CrossRef]
- Winchester, B. Lysosomal metabolism of glycoproteins. Glycobiology 2005, 15, 1R–15R. [Google Scholar] [CrossRef]
- Suzuki, K.; Sango, K.; Proia, R.L.; Langaman, C. Mice deficient of all forms of lysosomal b-hexosaminidase show mucopolysaccharidosis-like pathology. J. Neuropathol. Exp. Neurol. 1997, 56, 693–703. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Szajda, S.D.; Zalewska, A.; Szulc, A.; Kępka, A.; Minarowska, A.; Wojewódzka-Żelezniakowicz, M.; Konarzewska, B.; Chojnowska, S.; Ladny, J.R.; et al. Alcohol abuse and glycoconjugate metabolism. Folia Histochem. Cytobiol. 2012, 50, 1–11. [Google Scholar] [CrossRef][Green Version]
- Borzym-Kluczyk, M.; Radziejewska, I.; Olszewska, E.; Szajda, S.; Knaś, M.; Zwierz, K. Statistical evaluation of the isoform patterns od N-acetyl-beta-hexosaminidase from human renal cancer tissue separate by isoelectrofocusing. Clin. Biochem. 2007, 40, 403–406. [Google Scholar] [CrossRef]
- Elsafi, M.E.; Hultberg, B.; Isaksson, A.; Hägerstrand, I.; Prytz, H.; Stenram, U. Lysosomes and human liver disease: A biochemical and immunohistochemical study of beta-hexosaminidase Eur. J. Clin. Chem. Clin. Biochem. 1994, 32, 669–673. [Google Scholar] [CrossRef][Green Version]
- Zwierz, K.; Gindzieński, A.; Ostrowska, L.; Stankiewicz-Choroszucha, B. Metabolism of glycoconjungates in human gastrin mucosa. A review. Acta Med. Hung. 1989, 46, 275–288. [Google Scholar]
- Baritussio, A.; Marzini, S.; Agostini, M.; Alberti, A.; Cimenti, C.; Bruttomesso, D.; Manzato, E.; Quaglino, D.; Pettenazzo, A. Amiodarone inhibits lung degradation of SP-A and terturbs the distribution of lysosomal enzyms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, 1189–1199. [Google Scholar] [CrossRef]
- Szajda, S.D.; Snarska, J.; Jankowska, A.; Puchalski, Z.; Zwierz, K. Isoenzymes A and B of N-acetyl-β-D-hexosaminidase in serum and urine of patients with pancreatic cancer. Hepatogastroenterology 2008, 55, 695–698. [Google Scholar]
- Szajda, S.D.; Borzym-Kluczyk, M.; Snarska, J.; Puchalski, Z.; Zwierz, K. N-acetyl-β-D-hexosaminidase and its isoenzymes A and B in blood serum and urine, as a potential colon cancer markers. Hepatogastroenterology 2009, 56, 1287–1298. [Google Scholar] [PubMed]
- Karkkainen, P. Serum and urinary beta-hexosaminidase as markers of heavy drinking. Alcohol Alcohol. 1990, 25, 365–369. [Google Scholar] [PubMed]
- Isaksson, A.; Hultberg, B. Serum beta-hexosaminidase isoenzymes are precursor forms. Scand. J. Clin. Lab. Investig. 1995, 55, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.F.; Martinez, M.I.; Ribeiro, H.M.; Pinto, R.A.; Miranda, M.C.; Tutor, J.C. Characterization of the isoenzyme profile of beta-N-acetylhexosaminidase in the urine of newborns. Clin. Chem. Lab. Med. 1999, 37, 765–769. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.S.; Okada, S.; Chen, A.; Fillerup, D.L. Tay-Sachs disease: Detection by serum hexosaminidase assay. N. Engl. J. Med. 1970, 283, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ikonne, J.U.; Ellis, R.B. N-acetyl-β-D-hexosaminidase component A. Different forms in human tissues and fluids. Biochem. J. 1973, 135, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Gabrylewska, A.; Knaś, M.; Dudzik, D.; Zwierz, K. Activity of N-acetyl-beta glucosaminidase in dudenal aspirates and serum of patients with Giardia lamblia. Diagn. Lab. 2006, 42, 85–101. [Google Scholar]
- Choromańska, B.; Luto, M.; Szajda, S.D.; Waszkiewicz, N.; Kępka, A.; Janica, J.; Ladny, J.R.; Dadan, J.; Myśliwiec, P.; Zwierz, K. Activity of N-acetyl-β-hexosaminidase and its isoenzymes A and B in cancer. Postepy Hig. Med. Dosw. 2011, 65, 752–758. [Google Scholar]
- Zalewska-Szajda, B.; Szajda, S.D.; Waszkiewicz, N. Activity of N-acetyl-beta-D-hexosaminidase in the saliva of children with type 1 diabetes. Postepy Hig. Med. Dosw. 2013, 67, 996–999. [Google Scholar] [CrossRef]
- Ostrowska, L.; Zwierz, K.; Koniusz, Z.; Gindzieński, A. Function, properties and clinical significance of n-acetyl-beta-D-hexosaminidase. Postepy Hig. Med. Dosw. 1993, 47, 67–79. [Google Scholar]
- Zwierz, K.; Zalewska, A.; Zoch-Zwierz, W. Isoenzymes of N-acetyl-hexosaminidase. Acta Biochim. Pol. 1999, 46, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Wehr, H.; Czartoryska, B.; Górska, D.; Matsumoto, H. Serum beta-hexosaminidase and alfa-mannosidase activities as markers of alcohol abuse. Alcohol. Clin. Exp. Res. 1991, 15, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Wehr, H.; Habrat, B.; Czartoryska, B.; Górska, D.; Poźniak, M. Zastosowanie oznaczania beta-heksozoaminidazy w moczu do diagnostyki nadużywania alkoholu. Alkohol. Narkom. 1994, 2, 163–168. [Google Scholar]
- Waszkiewicz, N.; Olański, W.; Chojnowska, S.; Kołakowska, U.; Plewa, K.; Mielech, W.; Bagniuk-Plewa, A.; Wasilewska, A.; Szulc, A.; Szajda, S.D.; et al. Serum Exoglycosidases in Children and Adolescents with Harmful Alcohol Use. J. Addict. Med. 2018, 12, 329–335. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Kratz, E.M.; Chojnowska, S.; Zalewska, A.; Zwierz, K.; Szulc, A.; Szajda, S.D.; Nestsiarovich, A.; Kapitau, A.; Kępka, A.; et al. Long-term changes of salivary exoglycosidases and their applicability as chronic alcohol-drinking and dependence markers. World J. Biol. Psychiatry 2018, 20, 64–75. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Szajda, S.D.; Jankowska, A.; Kepka, A.; Dobryniewski, J.; Szulc, A.; Zwierz, K. The effect of the binge drinking session on the activity of salivary, serum and urinary β-hexosaminidase: Preliminary data. Alcohol Alcohol. 2008, 43, 446–450. [Google Scholar] [CrossRef]
- Pott, G.; Chi-Boesler, D.; Gerlach, U. Isoenzymes of N-acetyl-β-hexosaminidase from human liver and serum: Separation by electrofocusing in thin layers of polyacrylamide gel. J. Clin. Chem. Clin. Biochem. 1978, 16, 15–18. [Google Scholar]
- Eiser, A.R. The effects of alcohol in renal function and excretion. Alcohol. Clin. Exp. Res. 1987, 11, 127–138. [Google Scholar] [CrossRef]
- Beratis, N.G.; Mavrommatis, T.; Hatiris, I.; Kavaliotis, J.; Tsagaropoulou-Stiga, H.; Syrogiannopoulos, G.A. Increased activity of lysosomal acid hydrolases in the cell-free cerebrospinal fluid of bacterial meningitis. Pediatr. Res. 1997, 41, 235–241. [Google Scholar] [CrossRef][Green Version]
- Epstein, M. Alcohol’s impact on kidney function. Alcohol Health Res. World 1997, 21, 84–92. [Google Scholar]
- Kępka, A.; Szajda, S.D.; Jankowska, A.; Waszkiewicz, N.; Chojnowska, S.; Zwierz, K. N-acetyl-beta-hexosaminidase-marker of damage to renal proximal tubules. Pol. Merkur. Lek. 2008, 25, 288–290. [Google Scholar]
- Lopez-Caneda, E.; Rodriguez Holguin, S.; Corral, M.; Doallo, S.; Cadaveira, F. Evolution of the binge drinking pattern on college students: Neurophysiological correlates. Alcohol 2014, 48, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Kerr, W.C.; Greenfield, T.K.; Tujague, J.; Brown, S.E. A drink is a drink? Variation in the amount of alcohol contained in beer, wine and spirits drinks in a US methodological sample. Alcohol. Clin. Exp. Res. 2005, 29, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Ochwanowska, E.; Witek, B.; Tymińska-Tkacz, T.; Sito, A.; Prokop, A.; Piotrowicz, M.; Liedke, P. Changes in the activity of basic biochemical parameters indicating alcohol abuse. Alcohol. Drug Addict. 2015, 28, 235–250. [Google Scholar] [CrossRef]
- Martines, D.; Moris, A.I.; Gilmore, I.T.; Ansari, M.A.; Patel, A.; Quayle, J.A.; Billington, D. Urinary enzyme output during detoxification of chronic alcoholic patients. Alcohol Alcohol. 1989, 24, 113–120. [Google Scholar] [CrossRef]
- Karkkainen, P.; Salaspuro, M. beta-Hexosaminidase in the detection of alcoholism and heavy drinking. Alcohol Alcohol. 1991, 1, 459–464. [Google Scholar]
- Hultberg, B.; Isaksson, A.; Berglund, M.; Alling, C. Increases and time-course variations in beta-hexosaminidase isoenzyme B and carbohydrate-deficient transferrin in serum from alcoholics are similar. Alcohol. Clin. Exp. Res. 1995, 19, 452–456. [Google Scholar] [CrossRef]
- Stowell, L.; Stowell, A.; Garrett, N.; Robinson, G. Comparison of serum β-hexosaminidase isoenzyme B activity with serum carbohydrate-deficient transferring and other markers of alcohol abuse. Alcohol Alcohol. 1997, 32, 703–714. [Google Scholar] [CrossRef][Green Version]
- Markowski, T.; Ferens-Sieczkowska, M.; Zwierz, A.; Wojtulewska, A. The activity of N-acetyl-betahexosaminidase and gamma-glutamyltransferase in the serum of alcohol dependent people hospitalised after a long-lasting drinking period. Psychiatr. Pol. 2003, 37, 495–502. [Google Scholar]
- Varga, Z.V.; Matyas, C.; Paloczi, J.; Pacher, P. Alcohol Misuse and Kidney Injury: Epidemiological Evidence and Potential Mechanisms. Alcohol Res. 2017, 38, 283–288. [Google Scholar]
- Chojnowska, S.; Kępka, A.; Szajda, S.D.; Kołodziejczyk, Z.P.; Zwierz, K.; Waszkiewicz, N. Determination of N-acetyl-β-hexosaminidase in hemolysed blood. Clin. Biochem. 2016, 49, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F. Chromatographic methods for the determination of markers of chronic and acute alcohol consumption. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 781, 457–480. [Google Scholar] [CrossRef]
- Niemela, O. Biomarkers in alcoholism. Clin. Chim. Acta 2007, 377, 39–49. [Google Scholar] [CrossRef]
- Lenz, B.; Köllner, M.G.; Mühle, C.; Weinland, C.; Kornhuber, J. Basic Human Body Dimensions Relate to Alcohol Dependence and Predict Hospital Readmission. J. Clin. Med. 2019, 8, 2076. [Google Scholar] [CrossRef]
- Helander, A.; Eriksson, C.J.P. Laboratory tests for acute alcohol consumption: Results of the WHO/ISBRA Study on State and Trait Markers of Alcohol Use and Dependence. Alcohol. Clin. Exp. Res. 2002, 26, 1070–1077. [Google Scholar] [CrossRef]
- Sommers, M.S. Measurement of alcohol consumption: Issues and challenges. Ann. Rev. Nurs. Res. 2005, 23, 27–64. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Chojnowska, S.; Zalewska, A.; Zwierz, K.; Szulc, A.; Szajda, S.D. Salivary hexosaminidase in smoking alcoholics with bad periodontal and dental states. Drug Alcohol Depend. 2013, 129, 33–40. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Chojnowska, S.; Zalewska, A.; Zwierz, K.; Szulc, A.; Szajda, S.D. Salivary exoglycosidases as marker of alcohol dependence. Alcohol Alcohol. 2014, 49, 409–416. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Zalewska-Szajda, B.; Zalewska, A.; Waszkiewicz, M. Serum and urinary α-mannosidase in acute alcohol intoxication. E&C Hepatol. 2013, 9, 5–8. [Google Scholar]
- Gmel, G.; Kuntsche, E.; Rehm, J. Risky single-occasion drinking: Bingeing is not bingeing. Addiction 2011, 106, 1037–1045. [Google Scholar] [CrossRef]
- van den Berg, E.H.; Gruppen, E.G.; Blokzijl, H.; Bakker, S.J.; Dullaart, R.P. Higher Sodium Intake Assessed by 24 Hour Urinary Sodium Excretion Is Associated with Non-Alcoholic Fatty Liver Disease: The PREVEND Cohort Study. J. Clin. Med. 2019, 8, 2157. [Google Scholar] [CrossRef]
- Katz, G.G.; Shear, N.H.; Malkiewicz, I.M.; Valentino, K.; Neuman, M.G. Signaling for ethanol-induced apoptosis and repair in vitro. Clin. Biochem. 2001, 34, 219–227. [Google Scholar] [CrossRef]
- Marlatt, G.A.; Witkiewicz, L. Harm reduction approaches to alcohol use: Health, promotion, prevention and treatment. Addict. Behav. 2002, 27, 867–886. [Google Scholar] [CrossRef]
- Jelski, W.; Chrostek, L.; Szmitkowski, M. Biochemical basic of alcoholic liver injury. Pol. Merkur. Lek. 2006, 21, 376–380. [Google Scholar]
- Lis, K. Influence of ethyl alcohol intake on results of laboratory diagnostics. Alkohol. Narkom. 2009, 22, 65–73. [Google Scholar]
- Jankowski, M.M.; Ignatowska-Jankowska, B.; Kumański, K.; Witek, B.; Świergiel, A.H. Wpływ alkoholu na układ odpornościowy—Przegląd badań. Alkohol. Narkom. 2013, 26, 37–53. [Google Scholar]
- Rubini, M.E.; Kleeman, C.R.; Lamdin, E. Studies on alcohol diuresis. The effect of ethyl alcohol igestion on water, electrolyte and acid-base matabolism. J. Clin. Investig. 1955, 34, 439–447. [Google Scholar] [CrossRef]
- Nicholson, W.M.; Taylor, H.M. Blood volume studies in acute alcoholism. Q. J. Stud. Alcohol 1940, 1, 472–482. [Google Scholar] [CrossRef]
- Hultberg, B.; Isaksson, A.; Berglund, M.; Moberg, A.L. Serum β-hexosaminidase isoenzyme: A sensitive marker for alcohol abuse. Alcohol. Clin. Exp. Res. 1991, 15, 549–552. [Google Scholar] [CrossRef]
- Knaś, M.; Karczewska, K.; Szajda, S.D.; Zarzycki, W.; Dudzik, D.; Zwierz, K. Saliva of patients with type I diabetes: Effect of smoking on activity of lysosomal exoglycosidases. Oral Dis. 2006, 12, 278–282. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Jelski, W.; Zalewska, A.; Szulc, A.; Szmitkowski, M.; Zwierz, K.; Szajda, S.D. Salivary alcohol dehydrogenase in non-smoking and smoking alcohol-dependent persons. Alcohol 2014, 48, 611–616. [Google Scholar] [CrossRef]
- Waszkiewicz, N. Mentally Sick or Not—(Bio)Markers of Psychiatric Disorders Needed. J. Clin. Med. 2020, 9, 2375. [Google Scholar] [CrossRef]
| Variable | Material | B | A | p |
|---|---|---|---|---|
| Age | 54 (15–83) | 46 (26–82) | 0.416 | |
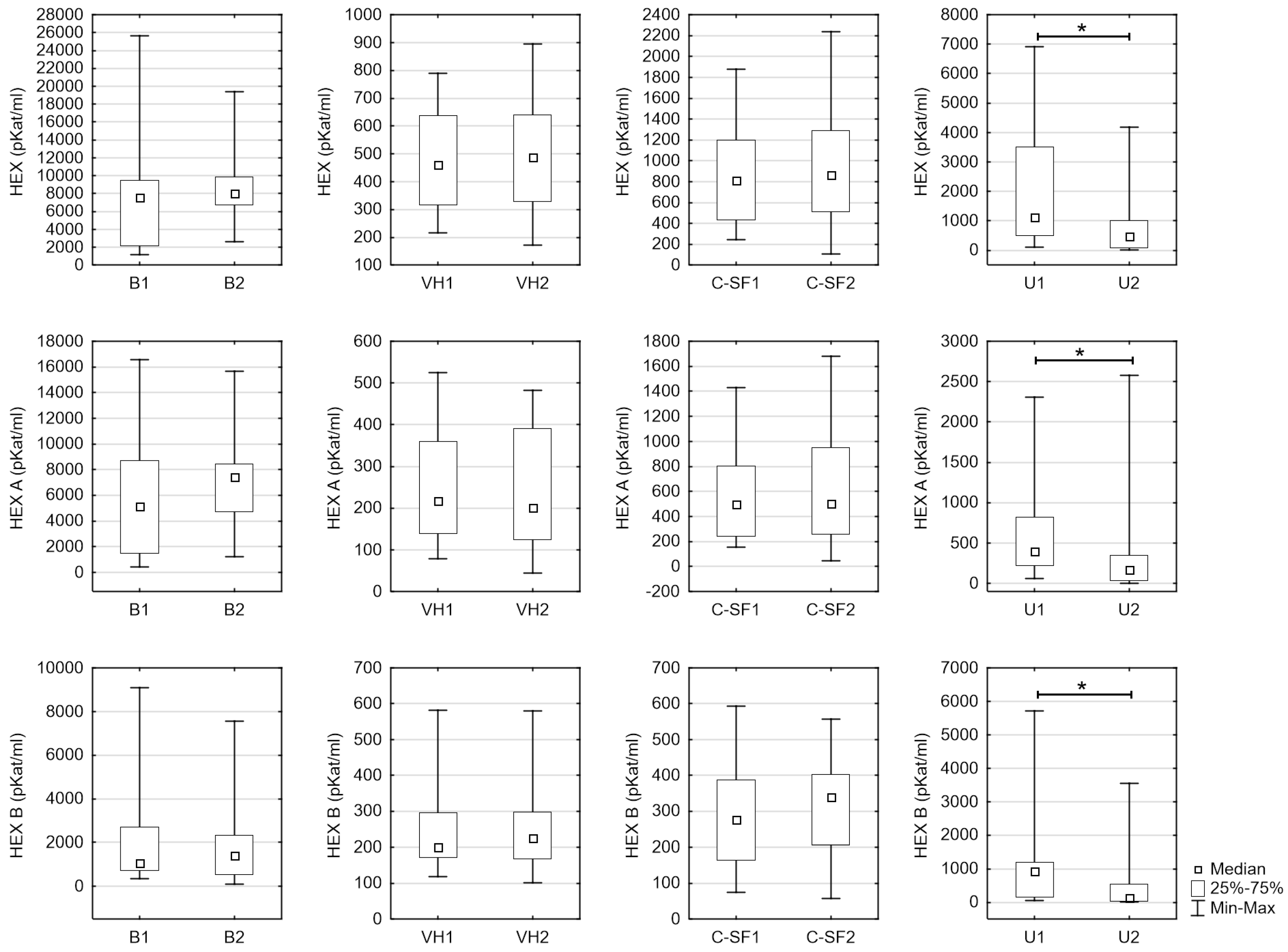
| HEX [pKat/mL] | Blood serum | 7535.58 (1162.25–25,679.50) | 8035.68 (2638.80–19,378.00) | 0.198 |
| Vitreous humor | 460.57 (215.26–788.23) | 488.11 (170.57–895.26) | 0.919 | |
| Cerebrospinal fluid | 813.72 (246.31–1878.45) | 488.11 (104.93–2235.65) | 0.756 | |
| Urine | 1124.60 (106.23–6923.41) | 472.51 (13.86–4179.39) | 0.015 * | |
| HEX A [pKat/mL] | Blood serum | 5132.45 (439.20–16,588.20) | 7436.98 (1221.60–15,665.60) | 0.162 |
| Vitreous humor | 217.00 (78.43–524.30) | 200.92 (44.79–482.48) | 0.788 | |
| Cerebrospinal fluid | 501.02 (152.11–1429.15) | 501.10 (48.10–1678.73) | 0.934 | |
| Urine | 394.89 (60.54–2311.70) | 175.56 (2.99–2581.15) | 0.011 * | |
| HEX B [pKat/mL] | Blood serum | 1071.46 (364.22–9091.30) | 1408.80 (102.91–7562.05) | 0.875 |
| Vitreous humour | 201.11 (118.23–581.93) | 225.26 (100.64–580.41) | 0.399 | |
| Cerebrospinal fluid | 277.69 (74.16–594.25) | 339.97 (56.84–556.92) | 0.321 | |
| Urine | 937.56 (45.69–5711.90) | 133.85 (7.77–3542.55) | 0.015 * |
| B Group Blood Serum | A Group Blood Serum | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mate-Rial | HEX | HEX A | HEX B | Variable | HEX | HEX A | HEX B | Blood Ethanol Concentration (BAC) |
| HEX | Blood serum | - | 0.975 *** | 0.542 ** | HEX | - | 0.938 *** | 0.345 | 0.407 |
| Vitreous humor | 0.503 ** | 0.589 *** | −0.160 | 0.285 | 0.299 | −0.460 * | 0.031 | ||
| Cerebro-spinal fluid | 0.204 | 0.174 | 0.357 | 0.103 | −0.061 | 0.654 *** | 0.316 | ||
| Urine | 0.229 | 0.283 | 0.138 | −0.010 | 0.073 | −0.166 | −0.204 | ||
| HEX A | Blood serum | 0.975 *** | - | 0.415 * | HEX A | 0.938 *** | - | 0.186 | 0.293 |
| Vitreous humor | 0.318 | 0.404 * | −0.237 | 0.046 | 0.028 | −0.404 | −0.003 | ||
| Cerebro-spinal fluid | 0.066 | 0.044 | 0.247 | −0.015 | −0.178 | 0.651 ** | 0.297 | ||
| Urine | 0.151 | 0.230 | −0.056 | 0.055 | 0.082 | −0.108 | −0.040 | ||
| HEX B | Blood serum | 0.542 ** | 0.415 * | - | HEX B | 0.345 | 0.184 | - | 0.495 * |
| Vitreous humor | 0.426 * | 0.478 ** | −0.073 | 0.415 | 0.392 | −0.286 | 0.130 | ||
| Cerebro-spinal fluid | 0.495 * | 0.500 ** | 0.403 * | 0.300 | 0.179 | 0.391 | 0.367 | ||
| Urine | 0.200 | 0.247 | 0.199 | −0.031 | 0.061 | −0.149 | −0.233 | ||
| HEX | HEX A | HEX B | |
|---|---|---|---|
| Blood serum | 0.407 | 0.293 | 0.495 * |
| Vitreous humor | 0.052 | −0.186 | 0.176 |
| Cerebrospinal fluid | 0.197 | 0.230 | 0.125 |
| Urine | 0.014 | 0.099 | −0.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptaszyńska-Sarosiek, I.; Chojnowska, S.; Szajda, S.D.; Szeremeta, M.; Wardaszka, Z.; Cwalina, U.; Niemcunowicz-Janica, A.; Waszkiewicz, N. The Activity of N-acetyl-β-hexosaminidase in the Blood, Urine, Cerebrospinal Fluid and Vitreous Humor Died People Due to Alcohol Intoxication. J. Clin. Med. 2020, 9, 3636. https://doi.org/10.3390/jcm9113636
Ptaszyńska-Sarosiek I, Chojnowska S, Szajda SD, Szeremeta M, Wardaszka Z, Cwalina U, Niemcunowicz-Janica A, Waszkiewicz N. The Activity of N-acetyl-β-hexosaminidase in the Blood, Urine, Cerebrospinal Fluid and Vitreous Humor Died People Due to Alcohol Intoxication. Journal of Clinical Medicine. 2020; 9(11):3636. https://doi.org/10.3390/jcm9113636
Chicago/Turabian StylePtaszyńska-Sarosiek, Iwona, Sylwia Chojnowska, Sławomir Dariusz Szajda, Michał Szeremeta, Zofia Wardaszka, Urszula Cwalina, Anna Niemcunowicz-Janica, and Napoleon Waszkiewicz. 2020. "The Activity of N-acetyl-β-hexosaminidase in the Blood, Urine, Cerebrospinal Fluid and Vitreous Humor Died People Due to Alcohol Intoxication" Journal of Clinical Medicine 9, no. 11: 3636. https://doi.org/10.3390/jcm9113636
APA StylePtaszyńska-Sarosiek, I., Chojnowska, S., Szajda, S. D., Szeremeta, M., Wardaszka, Z., Cwalina, U., Niemcunowicz-Janica, A., & Waszkiewicz, N. (2020). The Activity of N-acetyl-β-hexosaminidase in the Blood, Urine, Cerebrospinal Fluid and Vitreous Humor Died People Due to Alcohol Intoxication. Journal of Clinical Medicine, 9(11), 3636. https://doi.org/10.3390/jcm9113636






