Maintaining Blood Glucose Levels in Range (70–150 mg/dL) is Difficult in COVID-19 Compared to Non-COVID-19 ICU Patients—A Retrospective Analysis
Abstract
1. Introduction
Background
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Variables
2.4. Glucose Management
2.5. End Points/Outcomes
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
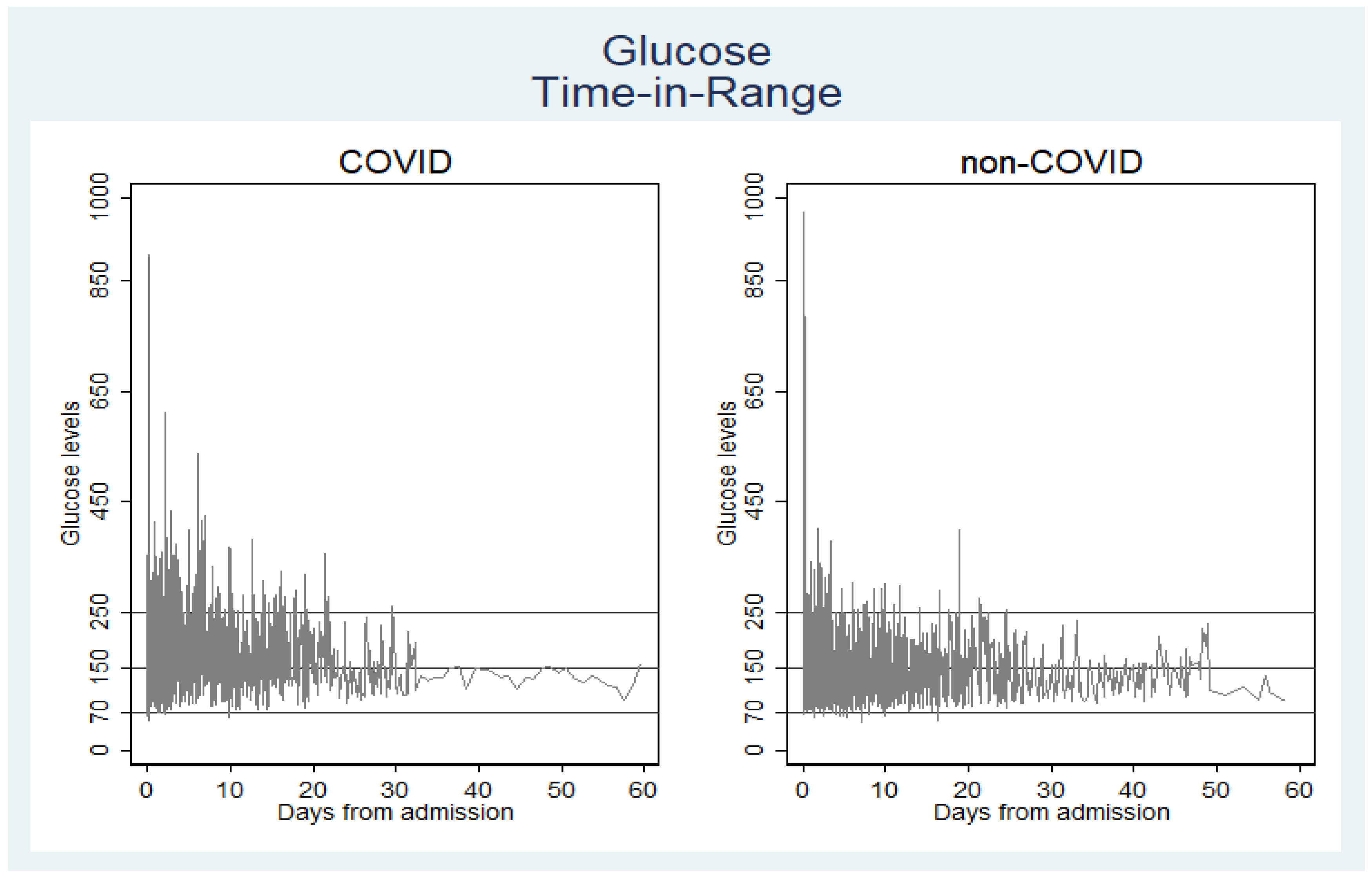
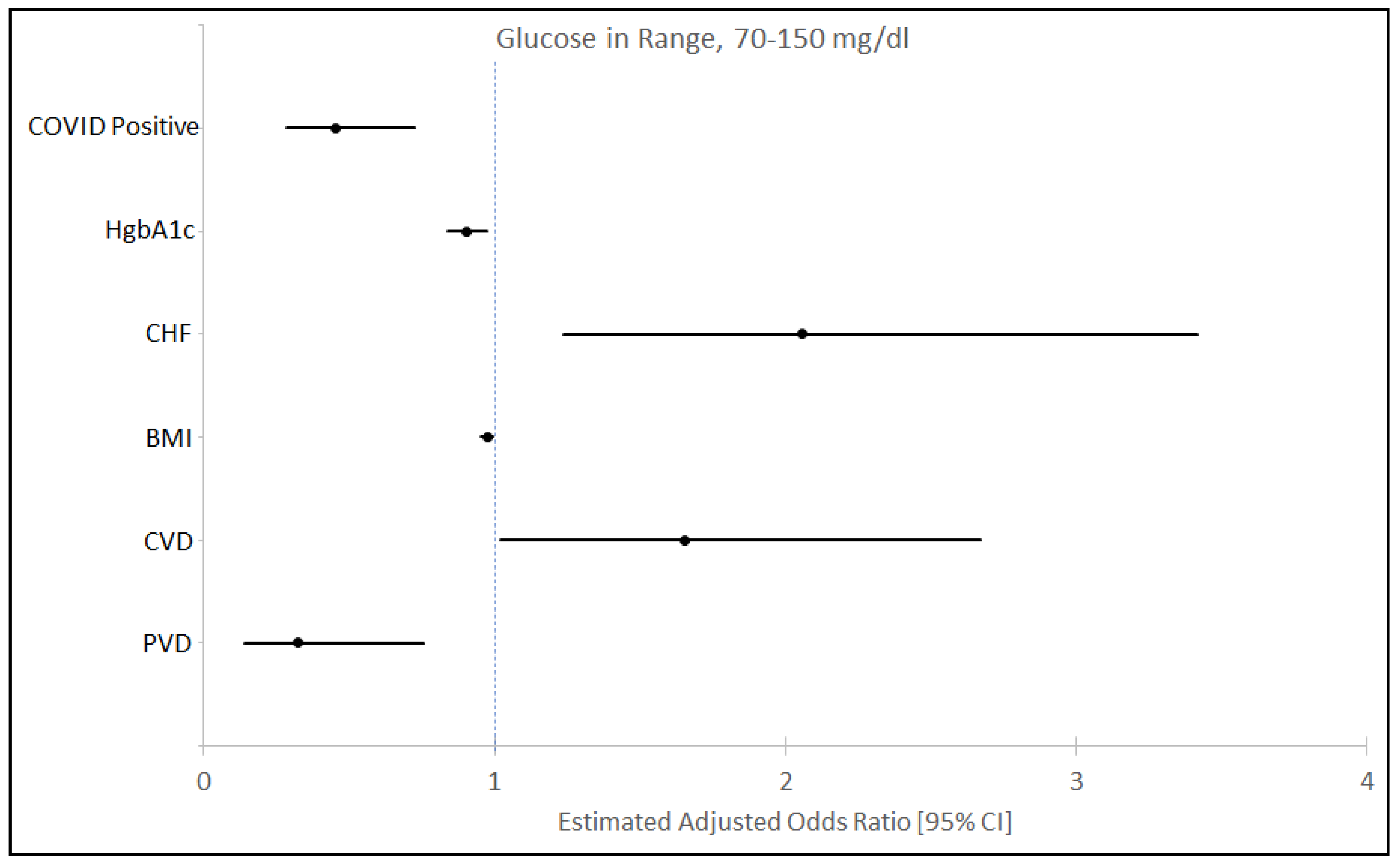
3.2. Time in Range of Blood Glucose Level and Insulin Utilization
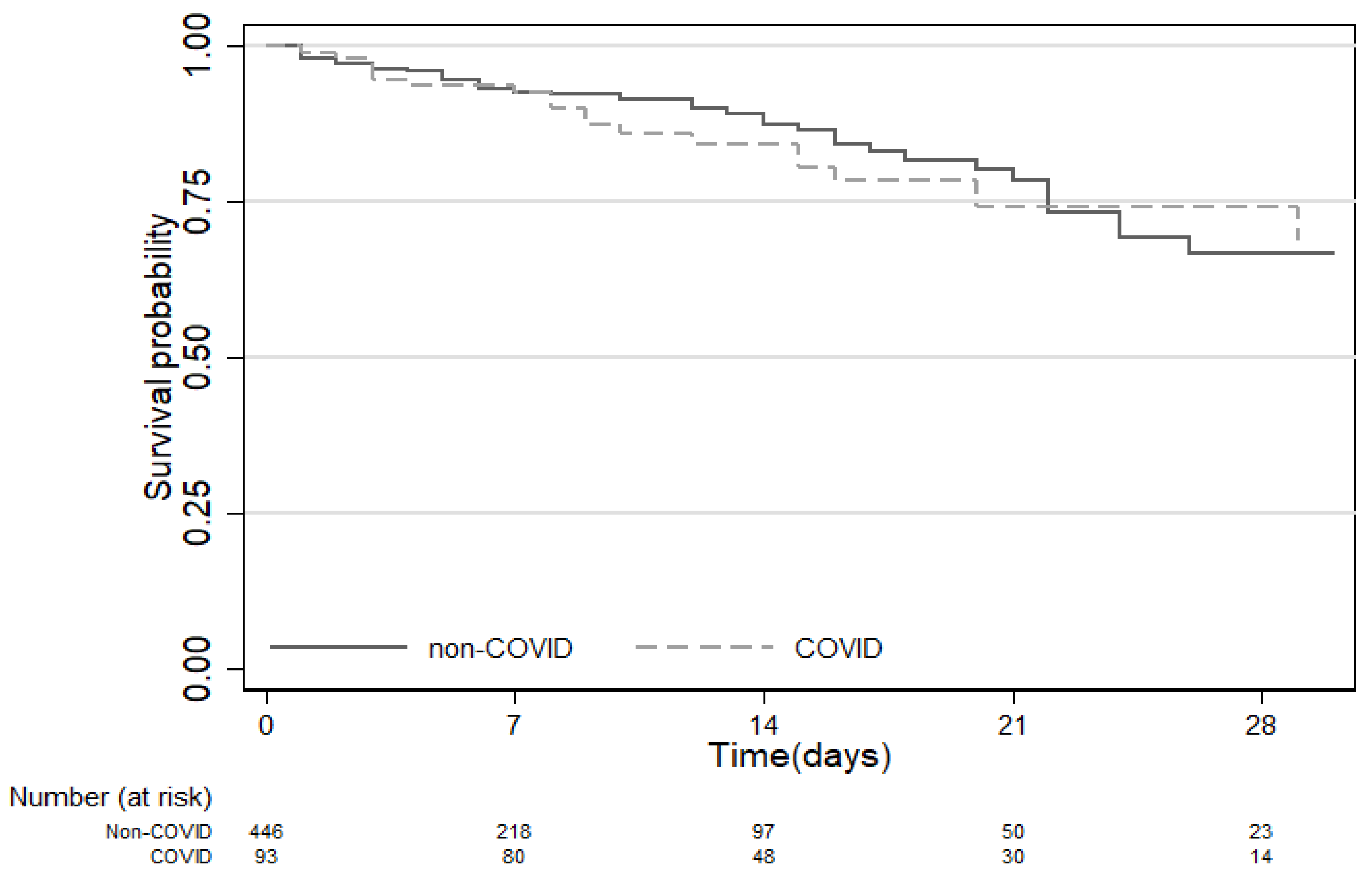
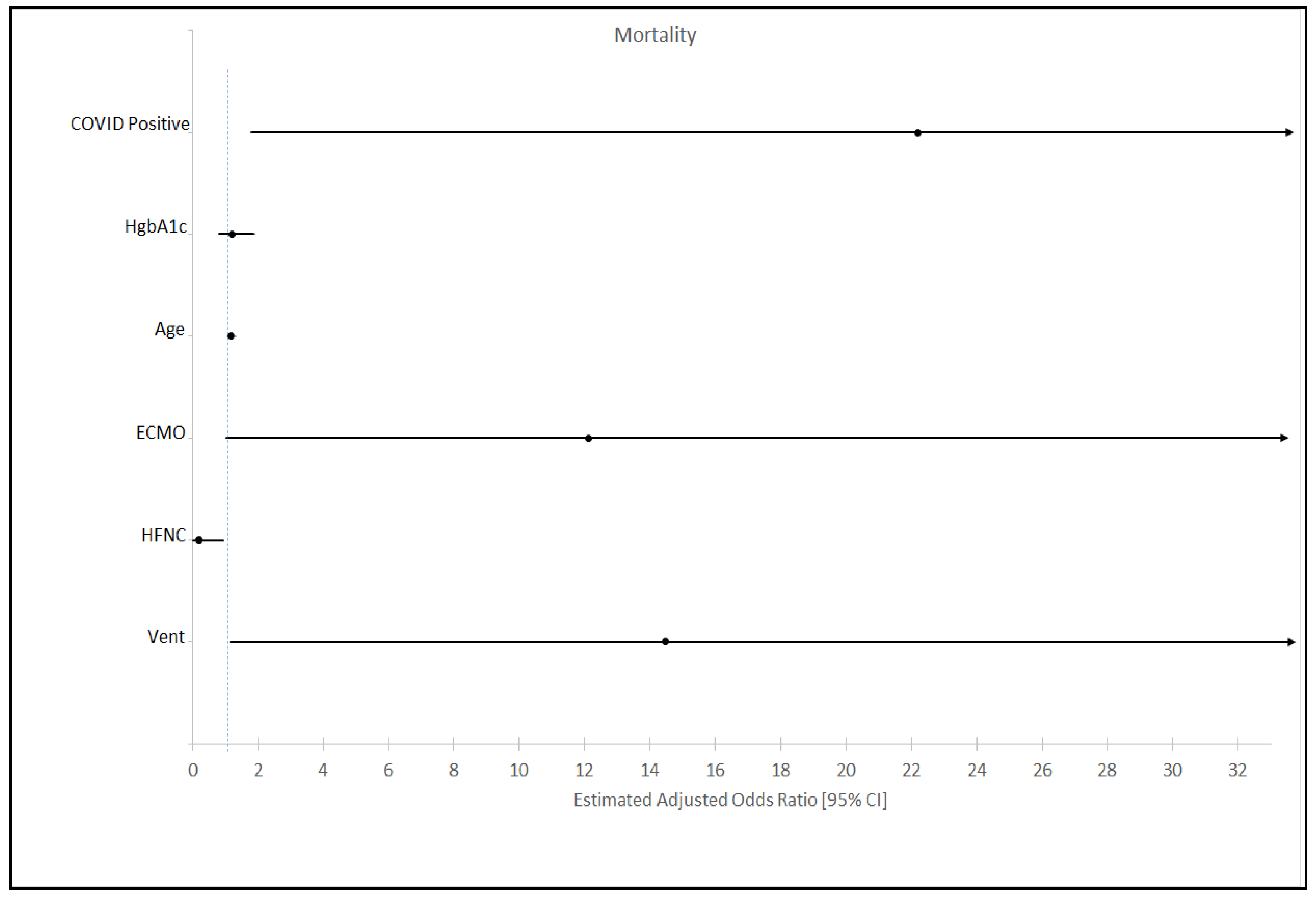
3.3. Mortality
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TIR | time in range; |
| COVID-19 | Coronavirus disease 2019; |
| non-COVID-19 | non-coronavirus-related disease 2019; |
| BG | blood glucose; ICU—intensive care unit; |
| CI | confidence interval; |
| OR | odds ratio; |
| ACE2 | angiotensin converting enzyme 2. |
References
- Capes, S.E.; Hunt, D.; Malmberg, K.; Pathak, P.; Gerstein, H.C. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: A systematic overview. Stroke 2001, 32, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Capes, S.E.; Hunt, D.; Malmberg, K.; Gerstein, H.C. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: A systematic overview. Lancet 2000, 355, 773–778. [Google Scholar] [CrossRef]
- Gale, S.C.; Sicoutris, C.; Reilly, P.M.; Schwab, C.W.; Gracias, V.H. Poor glycemic control is associated with increased mortality in critically ill trauma patients. Am. Surg. 2007, 73, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Krinsley, J.S. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin. Proc. 2003, 78, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, J.; Bircher, N.; Krinsley, J.; Agus, M.; Braithwaite, S.S.; Deutschman, C.; Freire, A.X.; Geehan, D.; Kohl, B.; Nasraway, S.A.; et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit. Care Med. 2012, 40, 3251–3276. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Wilmer, A.; Hermans, G.; Meersseman, W.; Wouters, P.J.; Milants, I.; Van Wijngaerden, E.; Bobbaers, H.; Bouillon, R. Intensive insulin therapy in the medical ICU. N. Engl. J. Med. 2006, 354, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.M.; Braithwaite, S.S.; Preiser, J.C. Stress hyperglycaemia. Lancet 2009, 373, 1798–1807. [Google Scholar] [CrossRef]
- Lanspa, M.J.; Krinsley, J.S.; Hersh, A.M.; Wilson, E.L.; Holmen, J.R.; Orme, J.F.; Morris, A.H.; Hirshberg, E.L. Percentage of Time in Range 70 to 139 mg/dL Is Associated with Reduced Mortality among Critically Ill Patients Receiving IV Insulin Infusion. Chest 2019, 156, 878–886. [Google Scholar] [CrossRef]
- Investigators, N.-S.S.; Finfer, S.; Chittock, D.R.; Su, S.Y.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P.; et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef]
- Preiser, J.C.; Devos, P.; Ruiz-Santana, S.; Melot, C.; Annane, D.; Groeneveld, J.; Iapichino, G.; Leverve, X.; Nitenberg, G.; Singer, P.; et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: The Glucontrol study. Intensive Care Med. 2009, 35, 1738–1748. [Google Scholar] [CrossRef]
- American Diabetes Association. 15. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S193–S202. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- Apicella, M.; Campopiano, M.C.; Mantuano, M.; Mazoni, L.; Coppelli, A.; Del Prato, S. COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Finucane, F.M.; Davenport, C. Coronavirus and Obesity: Could Insulin Resistance Mediate the Severity of Covid-19 Infection? Front. Public Health 2020, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.A.; Laviada-Molina, H.A.; Serres-Perales, J.M.; Rodriguez-Ayala, E.; Bastarrachea, R.A. The COVID-19 Pandemic during the Time of the Diabetes Pandemic: Likely Fraternal Twins? Pathogens 2020, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Juneja, R.; Golas, A.A.; Carroll, J.; Nelson, D.; Abad, V.J.; Roudebush, C.P.; Flanders, S.J. Safety and effectiveness of a computerized subcutaneous insulin program to treat inpatient hyperglycemia. J. Diabetes Sci. Technol. 2008, 2, 384–391. [Google Scholar] [CrossRef]
- Flanders, S.J.; Juneja, R.; Roudebush, C.P.; Carroll, J.; Golas, A.; Elias, B.L. Glycemic control and insulin safety: The impact of computerized intravenous insulin dosing. Am. J. Med. Qual. 2009, 24, 489–497. [Google Scholar] [CrossRef]
- Juneja, R.; Roudebush, C.P.; Nasraway, S.A.; Golas, A.A.; Jacobi, J.; Carroll, J.; Nelson, D.; Abad, V.J.; Flanders, S.J. Computerized intensive insulin dosing can mitigate hypoglycemia and achieve tight glycemic control when glucose measurement is performed frequently and on time. Crit. Care 2009, 13, R163. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, J.H.; Long, J.D.; Welch, G.W.; Reynolds, A.; Swearer, S.M. Fitting the Fractional Polynomial Model to Non-Gaussian Longitudinal Data. Front. Psychol. 2017, 8, 1431. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Altman, D.G. Regression Using Fractional Polynomials of Continuous Covariates: Parsimonious Parametric Modelling. J. R. Stat. Soc. Ser. C Appl. Stat. 1994, 43, 429–467. [Google Scholar] [CrossRef]
- StataCorp LP. StataCorp, 2015. Stata Statistical Software; StataCorp LP: College Station, TX, USA, 2015. [Google Scholar]
- Indiana; Marion County, Indiana. Available online: https://www.census.gov/quickfacts/fact/table/IN,marioncountyindiana/PST045219 (accessed on 11 July 2020).
- Indiana COVID-19 Data Report. Available online: https://www.coronavirus.in.gov (accessed on 11 July 2020).
- Mo, P.; Xing, Y.; Xiao, Y.; Deng, L.; Zhao, Q.; Wang, H.; Xiong, Y.; Cheng, Z.; Gao, S.; Liang, K.; et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Deng, S.Q.; Peng, H.J. Characteristics of and Public Health Responses to the Coronavirus Disease 2019 Outbreak in China. J. Clin. Med. 2020, 9, 575. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Zhu, L.; She, Z.G.; Cheng, X.; Qin, J.J.; Zhang, X.J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077.e3. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.; Walker, A.J.; Bhaskaran, K.J.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. OpenSAFELY: Factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Yamamoto, K.; Takemura, Y.; Takeshita, H.; Hongyo, K.; Kawai, T.; Hanasaki-Yamamoto, H.; Oguro, R.; Takami, Y.; Tatara, Y.; et al. Loss of ACE2 exaggerates high-calorie diet-induced insulin resistance by reduction of GLUT4 in mice. Diabetes 2013, 62, 223–233. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Philips, B.J.; Meguer, J.X.; Redman, J.; Baker, E.H. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003, 29, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; D’Onofrio, N.; Balestrieri, M.L.; Barbieri, M.; Rizzo, M.R.; Messina, V.; Maggi, P.; Coppola, N.; Paolisso, G.; Marfella, R. Outcomes in Patients with Hyperglycemia Affected by COVID-19: Can We Do More on Glycemic Control? Diabetes Care 2020, 43, 1408–1415. [Google Scholar] [CrossRef]
- Goldberg, P.A.; Siegel, M.D.; Russell, R.R.; Sherwin, R.S.; Halickman, J.I.; Cooper, D.A.; Dziura, J.D.; Inzucchi, S.E. Experience with the continuous glucose monitoring system in a medical intensive care unit. Diabetes Technol. Ther. 2004, 6, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hedo, R.; Rivera, A.; Rull, R.; Richardson, S.; Tu, X.M. Post hoc power analysis: Is it an informative and meaningful analysis? Gen. Psychiatry 2019, 32, e100069. [Google Scholar] [CrossRef]
- Walters, S.J. Consultants’ forum: Should post hoc sample size calculations be done? Pharm. Stat. 2009, 8, 163–169. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G.; Alberiche, M.; Formentini, G.; Calcaterra, F.; Lombardi, S.; Marini, F.; Poli, M.; Zenari, L.; Raffaelli, A.; et al. Predictors of insulin sensitivity in Type 2 diabetes mellitus. Diabet. Med. 2002, 19, 535–542. [Google Scholar] [CrossRef]
- Hrebicek, J.; Janout, V.; Malincikova, J.; Horakova, D.; Cizek, L. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J. Clin. Endocrinol. Metab. 2002, 87, 144–147. [Google Scholar] [CrossRef]
| Baseline Characteristics | In Sample (562) | COVID-19 (93) | Non-COVID-19 (469) | p-Value |
|---|---|---|---|---|
| Age (Years) median (IQR) a | 59.5 (47–69) | 61 (51–69) | 59 (47–69) | 0.2844 |
| Sex—Male (%) | 316 (56.23) | 50 (53.76) | 266 (56.72) | 0.6 |
| Body Mass Index kg/m2—Median (IQR) a | 29.70 (24.95–36) | 31.15 (26.8–36.9) | 29.55 (24.65–35.2) | 0.0253 |
| Race n(%) | ||||
| Caucasian | 334 (59.43) | 28 (30.11) | 306 (65.25) | |
| African American | 184 (32.74) | 49 (52.69) | 135 (28.78) | |
| Other | 14 (2.49) | 4 (4.30) | 10 (2.13) | <0.001 |
| Hispanic | 30 (5.34) | 12 (12.90) | 18 (3.84) | |
| Comorbidities n(%) | ||||
| Diabetes Mellitus | 192 | 37 (39.78) | 155 (33.05) | 0.211 |
| Hyperlipidemia | 65 (11.57) | 11 (11.83) | 54 (11.51) | 0.931 |
| Stroke/Cerebrovascular disease b | 57 (10.14) | 3 (3.23) | 54 (11.51) | 0.014 |
| Chronic Kidney Disease | 135 (24.02) | 30 (32.26) | 105 (22.39) | 0.042 |
| Coronary Artery Disease b | 15 (2.67) | 0 (0) | 15 (3.20) | 0.149 |
| Congestive Heart Failure | 77 (13.70) | 8 (8.60) | 69 (14.71) | 0.117 |
| Arrhythmia | 104 (18.51) | 12 (12.90) | 92 (19.62) | 0.128 |
| Chronic Lung disease | 139 (24.73) | 25 (26.88) | 114 (24.31) | 0.599 |
| Charlson Comorbidity Index Score | 0.666 | |||
| 0 | 166 (29.53) | 142 (30.28) | 24 (25.81) | |
| 1–3 | 317 (56.40) | 261 (55.65) | 56 (60.22) | |
| 4+ | 79 (14.03) | 66 (14.07) | 13 (13.98) | |
| DM Tx (home meds) | ||||
| Diet Control (%) | 43 (7.65) | 12 (12.90) | 31 (6.61) | 0.037 |
| Non-insulin Hypoglycemic Agents (%) | 65 (11.57) | 13 (13.98) | 52 (11.09) | 0.426 |
| Insulin (%) | 138 (24.56) | 22 (23.66) | 116 (24.73) | 0.825 |
| HbA1C (n = 403) median (IQR) a | 6.2 (5.7–7.2) | 6.8 (6–8) | 6.1 (5.6–7.1) | <0.001 |
| <7% n (%) | 288 (51.24) | 36 (38.70) | 252 (53.73) | |
| 7.1–8% n (%) | 47 (8.36) | 13 (13.97) | 34 (7.24) | |
| >8.1% n (%) | 68 (12.09) | 16 (17.20) | 52 (11.08) | |
| Respiratory Intervention n(%) | ||||
| Nasal Cannula | 385 (68.51) | 78 (83.87) | 307 (65.46) | <0.001 |
| High-Flow Nasal Cannula | 110 (19.57) | 50 (53.76) | 60 (12.79) | <0.001 |
| Non-Invasive Ventilation | 66 (11.74) | 6 (6.45) | 60 (12.79) | 0.083 |
| Ventilator | 264 (46.98) | 66 (70.97) | 198 (42.44) | <0.001 |
| Proning | 35 (6.23) | 25 (26.88) | 10 (2.13) | <0.001 |
| Paralytics | 112 (19.93) | 52 (55.91) | 60 (12.79) | <0.001 |
| ECMO | 13 (2.31) | 7 (7.53) | 6 (1.28) | <0.001 |
| Days of Ventilator Mean (SD) a | 4.81 (11.76) | 9.56 (9.98) | 3.87 (11.86) | <0.001 |
| Medications n(%) | ||||
| Steroids | 205 (36.48) | 57 (61.29) | 148 (31.56) | <0.001 |
| Pressors | 196 (34.88) | 51 (54.84) | 145 (30.92) | <0.001 |
| Remdesivir | 4 (0.71) | 4 (4.30) | 0 (0) | <0.001 |
| Tocilizumab b | 4 (0.71) | 4 (4.30) | 0 (0) | 0.001 |
| Hydroxychloroquine b | 76 (13.52) | 67 (72.04) | 9 (1.92) | <0.001 |
| Outcome | In Sample (562) | COVID-19 (93) | Non-COVID-19 (469) | p-Value |
|---|---|---|---|---|
| Insulin use (daily average) a | 7.63 (4.65) | 8.37 (4.08) | 6.17 (5.30) | <0.001 |
| Glucose Time in Range (%) | ||||
| <70 mg/dL | 0.44 | 0.44 | 0.44 | |
| 70–150 mg/dL | 60.13 | 44.42 | 68.52 | |
| 151–250 mg/dL | 33.31 | 43.48 | 27.88 | <0.001 |
| >250 mg/dL | 6.12 | 11.66 | 3.16 | |
| Glucose mg/dL | ||||
| Mean (SD) | 150.89 (60.51) | 170.59 (66.60) | 140.37 (54.13) | <0.001 |
| Median (IQR) a | 136 (112–174) | 157 (124–205) | 130 (107–159) | |
| Coefficient of Variation in Glucose level | 0.40 | 0.39 | 0.38 | |
| Peak Glucose mg/dL | ||||
| Mean (SD) | 190.31 (98.79) | 243.07 (122.62) | 179.18 (89.25) | <0.001 |
| Median (IQR) a | 164 (130–218.5) | 215 (146–323) | 160 (128–201.5) | |
| Mortality n (%) | 85 (15.12) | 20 (21.51) | 65 (13.86) | 0.06 |
| Outcome | In Sample COVID-19 n = 93(%) Non-COVID-19 n = 469(%) | >/=85% in Range: 70–150 mg/dL COVID-19 Non-COVID-19 | <85% in Range: 70–150 mg/dL COVID-19 NON-COVID-19 | p-Value |
|---|---|---|---|---|
| Mortality n (%) a | ||||
| COVID-19 | 20 (21.51) | 2 (10) | 18 (90) | 0.085 |
| Non-COVID-19 | 65 (13.86) | 21 (32.3) | 44 (67.69) | 0.046 |
| Days of Ventilator Mean (SD) b | ||||
| COVID-19 | 9.56 (9.98) | 1.84 (3.59) | 12.40 (10.09) | <0.001 |
| Non-COVID-19 | 3.87 (11.86) | 2.12 (5.48) | 5.22 (14.93) | <0.001 |
| High Flow Nasal Cannula n (%) | ||||
| COVID-19 | 50 (53.76) | 19 (38) | 31 (62) | 0.009 |
| Non-COVID-19 | 60 (12.79) | 24 (40) | 36 (60) | 0.535 |
| Ventilator n (%) | ||||
| COVID-19 | 66 (70.97) | 8 (12.12) | 58 (87.87) | <0.001 |
| Non-COVID-19 | 198 (42.22) | 72 (36.36) | 126 (63.63) | 0.006 |
| Proning n (%) a | ||||
| COVID-19 | 25 (26.88) | 6 (24) | 19 (76) | 0.704 |
| Non-COVID-19 | 10 (2.13) | 4 (40) | 6 (60) | >0.99 |
| Paralytics n (%) | ||||
| COVID-19 | 52 (55.91) | 6 (11.53) | 46 (88.46) | <0.001 |
| Non-COVID-19 | 60 (12.79) | 19 (31.66) | 41 (69.34) | 0.044 |
| ECMO n (%) a | ||||
| COVID-19 | 7 (7.53) | 0 (0) | 7 (100) | 0.183 |
| Non-COVID-19 | 6 (1.28) | 1 (16.67) | 5 (83.33) | 0.238 |
| HbA1C | In Sample COVID-19 n = 65(%) non-COVID-19 n = 338(%) | >/=85% in Range: 70–150 mg/dL COVID-19 non-COVID-19 | <85% in Range: 70–150 mg/dL COVID-19 non-COVID-19 | p-Value |
|---|---|---|---|---|
| <=7% | ||||
| COVID-19 n (%) | 36 (55.38) | 7 (19.44) | 29 (80.56) | 0.014 |
| Non-COVID-19 n (%) | 252 (74.56) | 103 (40.87) | 149 (59.12) | <0.001 |
| 7.1–8.0% | ||||
| COVID-19 n (%) | 13 (20.00) | 0 (0) | 13 (100) | 0.329 |
| Non-COVID-19 n (%) | 34 (10.06) | 3 (8.82) | 31 (91.18) | 0.002 |
| >=8.1% | ||||
| COVID-19 n (%) | 16 (24.62) | 0 (0) | 16 (100) | 0.18 |
| Non-COVID-19 n (%) | 52 (15.38) | 4 (7.69) | 48 (92.31) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapoor, R.; Timsina, L.R.; Gupta, N.; Kaur, H.; Vidger, A.J.; Pollander, A.M.; Jacobi, J.; Khare, S.; Rahman, O. Maintaining Blood Glucose Levels in Range (70–150 mg/dL) is Difficult in COVID-19 Compared to Non-COVID-19 ICU Patients—A Retrospective Analysis. J. Clin. Med. 2020, 9, 3635. https://doi.org/10.3390/jcm9113635
Kapoor R, Timsina LR, Gupta N, Kaur H, Vidger AJ, Pollander AM, Jacobi J, Khare S, Rahman O. Maintaining Blood Glucose Levels in Range (70–150 mg/dL) is Difficult in COVID-19 Compared to Non-COVID-19 ICU Patients—A Retrospective Analysis. Journal of Clinical Medicine. 2020; 9(11):3635. https://doi.org/10.3390/jcm9113635
Chicago/Turabian StyleKapoor, Rajat, Lava R. Timsina, Nupur Gupta, Harleen Kaur, Arianna J. Vidger, Abby M. Pollander, Judith Jacobi, Swapnil Khare, and Omar Rahman. 2020. "Maintaining Blood Glucose Levels in Range (70–150 mg/dL) is Difficult in COVID-19 Compared to Non-COVID-19 ICU Patients—A Retrospective Analysis" Journal of Clinical Medicine 9, no. 11: 3635. https://doi.org/10.3390/jcm9113635
APA StyleKapoor, R., Timsina, L. R., Gupta, N., Kaur, H., Vidger, A. J., Pollander, A. M., Jacobi, J., Khare, S., & Rahman, O. (2020). Maintaining Blood Glucose Levels in Range (70–150 mg/dL) is Difficult in COVID-19 Compared to Non-COVID-19 ICU Patients—A Retrospective Analysis. Journal of Clinical Medicine, 9(11), 3635. https://doi.org/10.3390/jcm9113635






