Balloon Pulmonary Angioplasty in Patients with Chronic Thromboembolic Pulmonary Hypertension: Impact on Clinical and Hemodynamic Parameters, Quality of Life and Risk Profile
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Patient Population
2.3. Assessed Endpoints
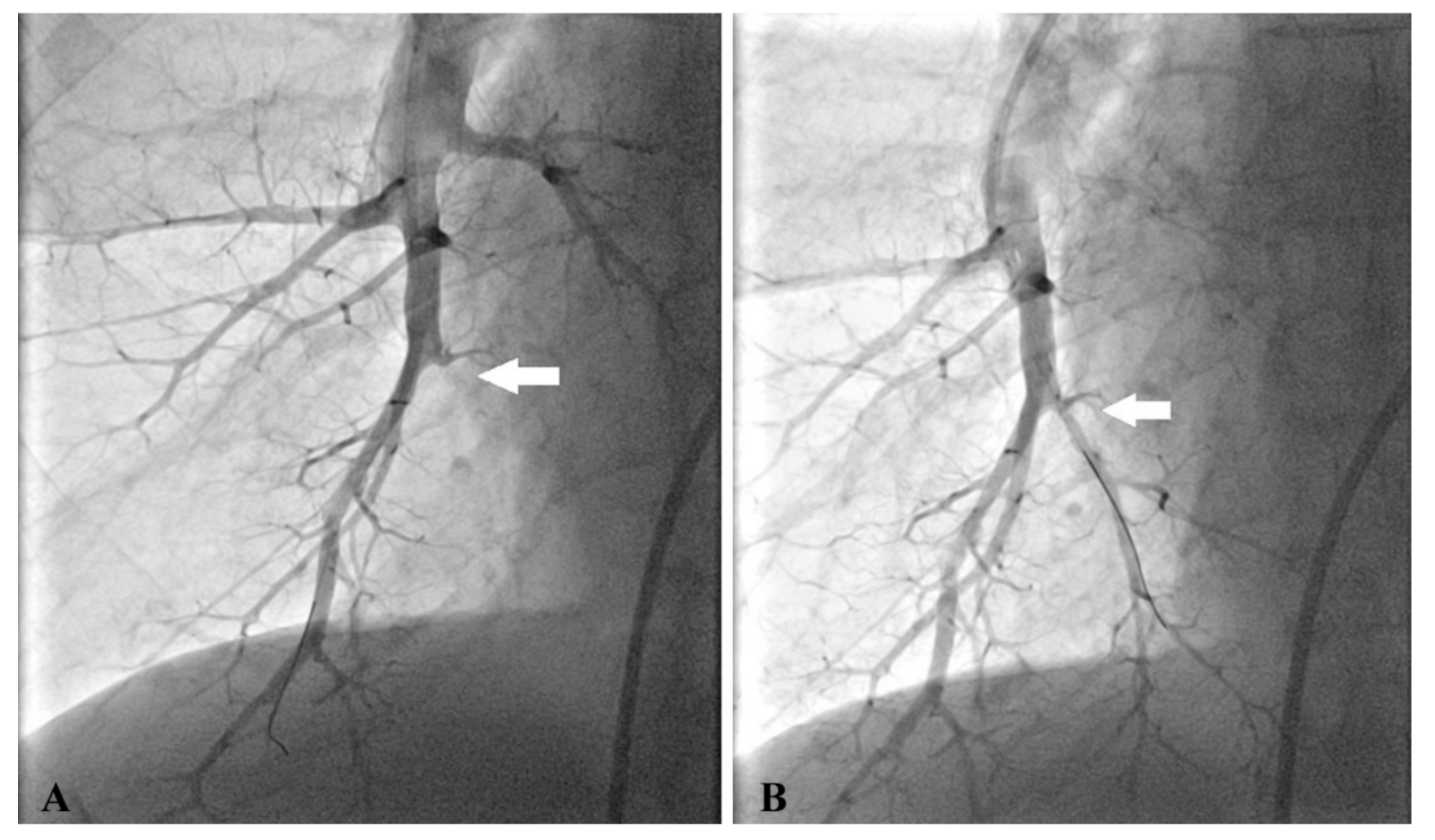
2.4. BPA Procedure
2.5. Statistical Analysis
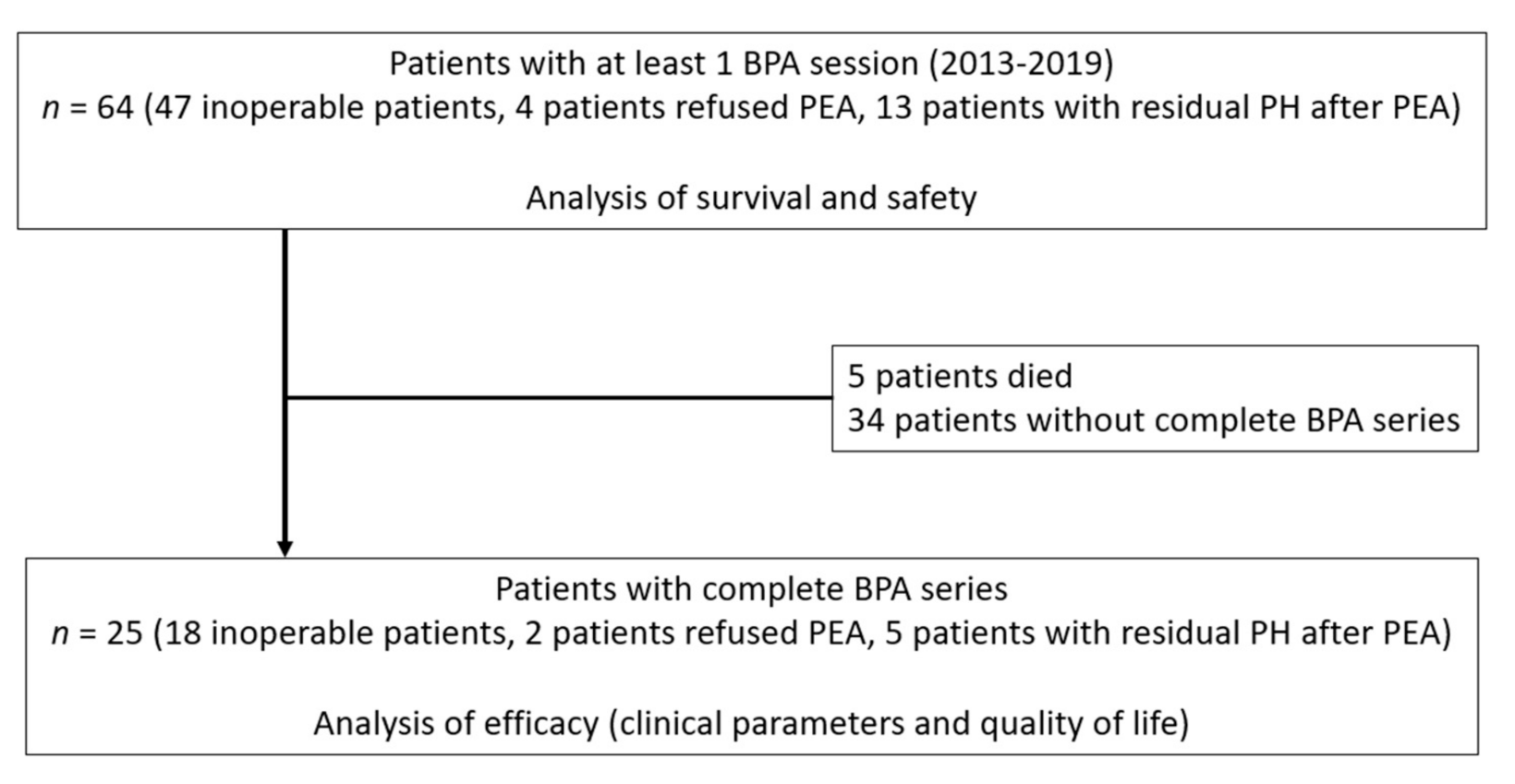
3. Results
3.1. Patient Characteristics
3.2. Effect of BPA on Clinical Outcome
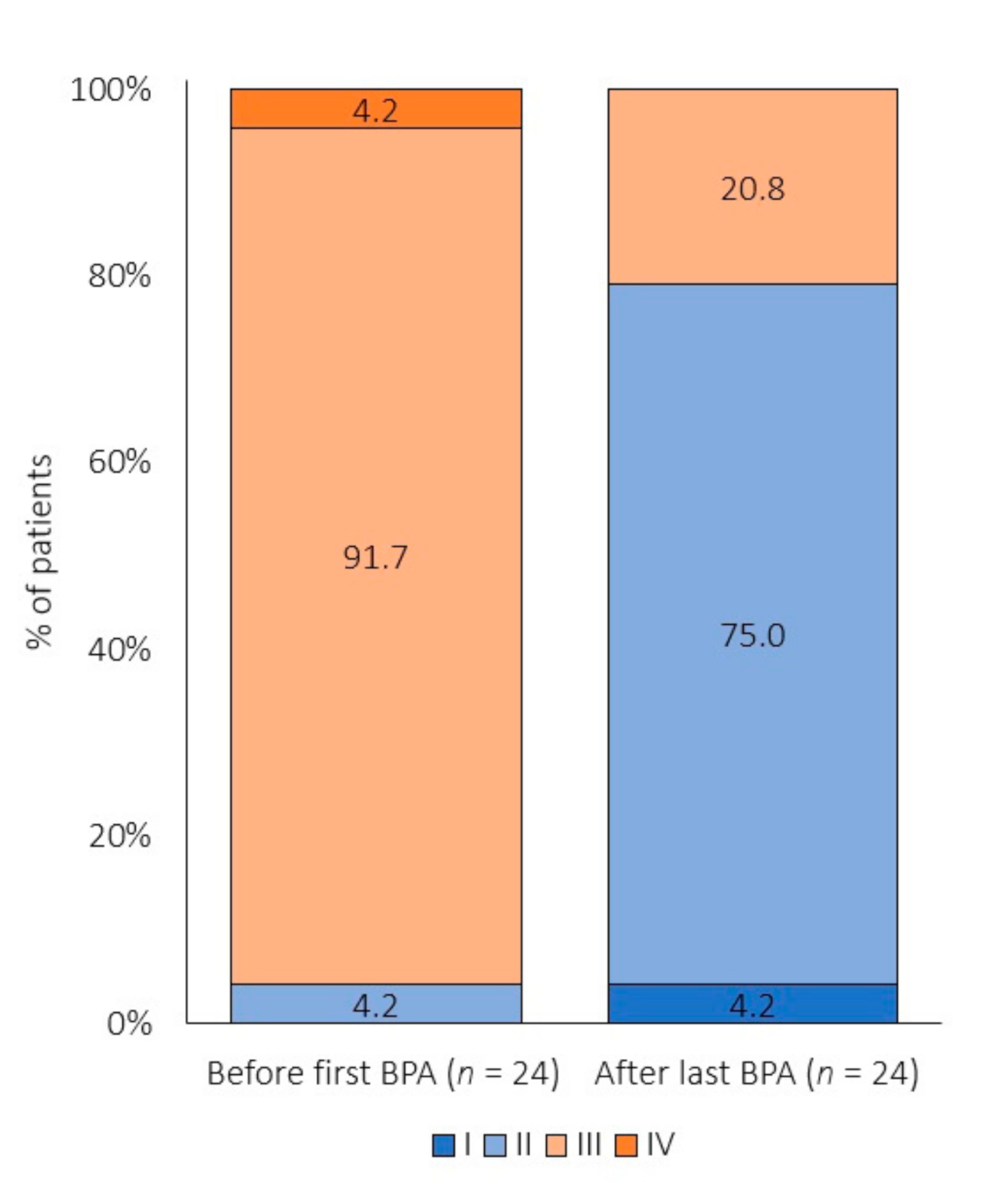
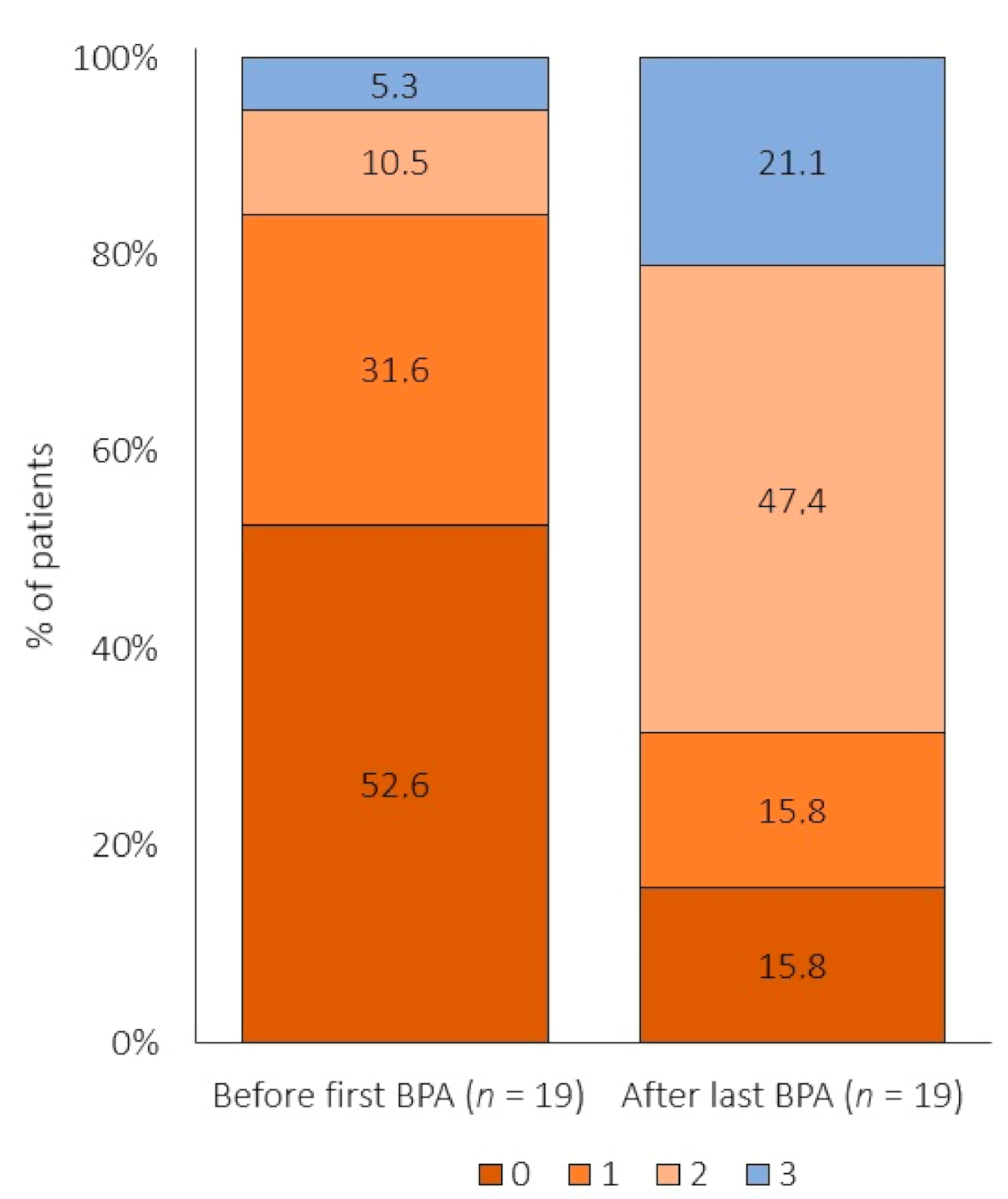
3.3. Effect of BPA on Risk Profile
3.4. Effect of BPA on Quality of Life
3.5. Safety
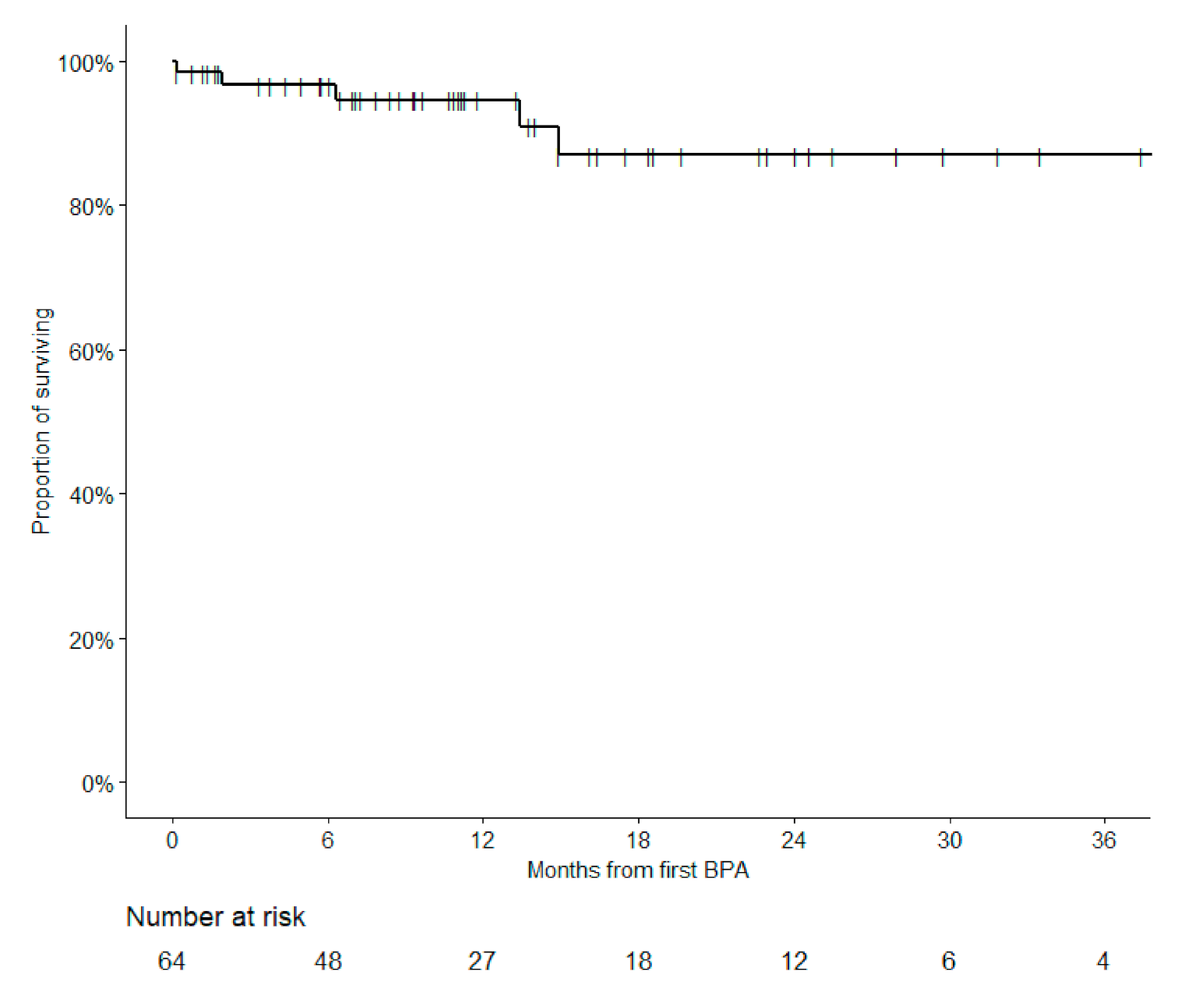
3.6. Survival
4. Discussion
4.1. Effect of BPA on Clinical Outcome
4.2. Effect of BPA on Risk Profile
4.3. Effect of BPA on Quality of Life
4.4. Safety
4.5. Survival
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicky, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef] [PubMed]
- Gall, H.; Hoeper, M.M.; Richter, M.J.; Cacheris, W.; Hinzmann, B.; Mayer, E. An epidemiological analysis of the burden of chronic thromboembolic pulmonary hypertension in the USA, Europe and Japan. Eur. Respir. Rev. 2017, 26. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.C.; Jansa, P.; Huang, W.C.; Nižnanský, M.; Omara, M.; Lindner, J. Residual pulmonary hypertension after pulmonary endarterectomy: A meta-analysis. J. Thorac. Cardiovasc. Surg. 2018, 156, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.M.; Jansa, P.; Kim, N.H.; Mayer, E.; Simonneau, G.; Wilkikns, M.R.; Fritsch, A.; et al. CHEST-1 Study Group. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Delcroix, M.; Jais, X.; Madani, M.M.; Matsubara, H.; Mayer, E.; Ogo, T.; Tapson, V.F.; Ghofrani, H.A.; Jenkins, D.P. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Ogawa, A.; Miyaji, K.; Mizoguchi, H.; Shimokawahara, H.; Naito, T.; Oka, T.; Yunoki, K.; Munemasa, M.; Matsubara, H. Novel Angiographic Classification of Each Vascular Lesion in Chronic Thromboembolic Pulmonary Hypertension Based on Selective Angiogram and Results of Balloon Pulmonary Angioplasty. Circ. Cardiovasc. Interv. 2016, 9. [Google Scholar] [CrossRef]
- Feinstein, J.A.; Goldhaber, S.Z.; Lock, J.E.; Ferndandes, S.M.; Landzberg, M.J. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001, 103, 10–13. [Google Scholar] [CrossRef]
- Kimura, M.; Kohno, T.; Kawakami, T.; Kataoka, M.; Inohara, T.; Takei, M.; Tsugu, T.; Murata, M.; Maekawa, Y.; Fukuda, K. Balloon pulmonary angioplasty attenuates ongoing myocardial damage in patients with chronic thromboembolic pulmonary hypertension. Int. J. Cardiol. 2016, 207, 387–389. [Google Scholar] [CrossRef]
- Ogo, T.; Fukuda, T.; Tsuji, A.; Fukui, S.; Ueda, J.; Sanda, Y.; Morita, Y.; Asano, R.; Konagai, N.; Yasuda, S. Efficacy and safety of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension guided by cone-beam computed tomography and electrocardiogram-gated area detector computed tomography. Eur. J. Radiol. 2017, 89, 270–276. [Google Scholar] [CrossRef]
- Ogawa, A.; Satoh, T.; Fukuda, T.; Sugimura, K.; Fukumoto, Y.; Emoto, N.; Yamada, N.; Yao, A.; Ando, M.; Ogino, H.; et al. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: Results of a Multicenter Registry. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e004029. [Google Scholar] [CrossRef]
- Andreassen, A.K.; Ragnarsson, A.; Gude, E.; Geiran, O.; Andersen, R. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013, 99, 1415–1420. [Google Scholar] [CrossRef]
- Kurzyna, M.; Darocha, S.; Koteja, A.; Pietura, R.; Torbicki, A. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Postepy Kardiol. Interwencyjnej. 2015, 11, 1–4. [Google Scholar] [CrossRef]
- Olsson, K.M.; Wiedenroth, C.B.; Kamp, J.C.; Breithecker, A.; Fuge, J.; Krombach, G.A.; Haas, M.; Hamm, C.; Kramm, T.; Guth, S. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: The initial German experience. Eur. Respir. J. 2017, 49, 1602409. [Google Scholar] [CrossRef]
- Brenot, P.; Jaïs, X.; Taniguchi, Y.; Garcia Alonso, C.; Gerardin, B.; Mussot, S.; Mercier, O.; Fabre, D.; Parent, F.; Jevnikar, M.; et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef] [PubMed]
- Hoole, S.P.; Coghlan, J.G.; Cannon, J.E.; Taboada, D.; Toshner, M.; Sheares, K.; Fletcher, A.J.; Martinez, G.; Ruggiero, A.; Screaton, N.; et al. Balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension: The UK experience. Open Heart 2020, 7, e001144. [Google Scholar] [CrossRef]
- Anand, V.; Frantz, R.P.; DuBrock, H.; Kane, G.C.; Krowka, M.; Yanagisawa, R.; Sandhu, G.S. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: Initial Single-Center Experience. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 311–318. [Google Scholar] [CrossRef]
- Lindner, J.; Jansa, P.; Kunstyr, J.; Mayer, E.; Blaha, J.; Palecek, T.; Aschermann, M.; Grus, T.; Ambroz, D.; Tosovský, J.; et al. Implementation of a new programme for the surgical treatment of CTEPH in the Czech Republic--Pulmonary endarterectomy. Thorac. Cardiovasc. Surg. 2006, 54, 528–531. [Google Scholar] [CrossRef]
- Boucly, A.; Weatherald, J.; Savale, L.; Jaïs, X.; Cottin, V.; Prevot, G.; Picard, F.; de Groote, P.; Jevnikar, M.; Bergot, E.; et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- EQ-5D-5L User Guide; EuroQol Research Foundation: Rotterdam, The Netherlands, 2015.
- Chausheva, S.; Naito, A.; Ogawa, A.; Seidl, V.; Winter, M.P.; Sharma, S.; Sadushi-Kolici, R.; Campean, I.A.; Taghavi, S.; Moser, B.; et al. Chronic thromboembolic pulmonary hypertension in Austria and Japan. J. Thorac. Cardiovasc. Surg. 2019, 158, 604–614.e2. [Google Scholar] [CrossRef]
- Tokunaga, N.; Ogawa, A.; Ito, H.; Matsubara, H. Rapid and high-dose titration of epoprostenol improves pulmonary hemodynamics and clinical outcomes in patients with idiopathic and heritable pulmonary arterial hypertension. J. Cardiol. 2016, 68, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Badagliacca, R.; Poscia, R.; Pezzuto, B.; Papa, S.; Reali, M.; Pesce, F.; Manzi, G.; Gianfrilli, D.; Ciciarello, F.; Sciomer, S.; et al. Prognostic relevance of right heart reverse remodeling in idiopathic pulmonary arterial hypertension. J. Heart Lung Transplant. 2017. [Google Scholar] [CrossRef] [PubMed]
- Badagliacca, R.; Raina, A.; Ghio, S.; D’Alto, M.; Confalonieri, M.; Correale, M.; Corda, M.; Paciocco, G.; Lombardi, C.; Mulè, M.; et al. Influence of various therapeutic strategies on right ventricular morphology, function and hemodynamics in pulmonary arterial hypertension. J. Heart Lung Transplant. 2018, 37, 365–375. [Google Scholar] [CrossRef]
- Delcroix, M.; Staehler, G.; Gall, H.; Grünig, E.; Held, M.; Halank, M.; Klose, H.; Vonk-Noordegraaf, A.; Rosenkranz, S.; Pepke-Zaba, J.; et al. Risk assessment in medically treated chronic thromboembolic pulmonary hypertension patients. Eur. Respir. J. 2018, 52. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Santos, M.; Vicente, M.; Furtado, I.; Cruz, C.; Melo, A.; Carvalho, L.; Gonçalves, F.; Sa-Couto, P.; Almeida, L. Health-Related Quality of Life in Pulmonary Hypertension and Its Clinical Correlates: A Cross-Sectional Study. BioMed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.; Meyer, B.C.; Ogo, T.; Matsubara, H.; Kurzyna, M.; Ghofrani, H.A.; Mayer, E.; Brenot, P. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26. [Google Scholar] [CrossRef]
- Kriechbaum, S.D.; Wiedenroth, C.B.; Hesse, M.L.; Ajnwojner, R.; Keller, T.; Sebastian Wolter, J.; Haas, M.; Roller, F.C.; Breithecker, A.; Rieth, A.J.; et al. Development of renal function during staged balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension. Scand. J. Clin. Lab. Investig. 2019, 79, 268–275. [Google Scholar] [CrossRef]
- Riedel, M.; Stanek, V.; Widimsky, J.; Prerovsky, I. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982, 81, 151–158. [Google Scholar] [CrossRef]
- Delcroix, M.; Lang, I.; Pepke-Zaba, J.; Jansa, P.; D’Armini, A.M.; Snijder, R.; Bresser, P.; Torbicki, A.; Mellemkjaer, S.; Lewczuk, J.; et al. Long-Term Outcome of Patients With Chronic Thromboembolic Pulmonary Hypertension: Results From an International Prospective Registry. Circulation 2016, 133, 859–871. [Google Scholar] [CrossRef]
| Parameter | n (%) | Valid n | Mean (SD) | Median (5th; 95th Percentile) | |
|---|---|---|---|---|---|
| Sex | Males | 32 (50.0%) | |||
| Females | 32 (50.0%) | ||||
| Age (years) | n = 63 | 61.4 (12.1) | 62.0 (46.0; 78.0) | ||
| Height (cm) | n = 60 | 171.3 (10.4) | 171.0 (154.5; 186.0) | ||
| Weight (kg) | n = 61 | 84.4 (16.9) | 84.0 (60.0; 112.0) | ||
| BMI (kg/m²) | n = 59 | 29.0 (5.4) | 28.4 (21.1; 39.6) | ||
| PE history | 52 (81.3%) | ||||
| Hereditary thrombophilia | 12 (18.8%) | ||||
| Diuretics | 33 (51.6%) | ||||
| Anticoagulation * | Warfarin | 41 (68.3%) | |||
| NOAC | 19 (31.7%) | ||||
| FEV1 (% of predicted) | n = 54 | 89.3 (19.1) | 89.5 (57.0; 119.0) | ||
| FVC (% of predicted) | n = 54 | 97.6 (19.3) | 98.6 (57.0; 129.0) | ||
| 6MWD (m) | n = 52 | 385.5 (110.1) | 387.5 (207.0; 597.0) | ||
| Borg dyspnoea index | n = 51 | 5.8 (1.7) | 6.0 (3.0; 8.0) | ||
| NYHA * | I | 0 (0.0%) | |||
| II | 16 (25.8%) | ||||
| III | 44 (71.0%) | ||||
| IV | 2 (3.2%) | ||||
| RAMP (mmHg) | n = 63 | 9.4 (5.1) | 9.0 (2.0; 19.0) | ||
| PASP (mmHg) | n = 64 | 75.8 (18.8) | 77.5 (45.0; 104.0) | ||
| PADP (mmHg) | n = 64 | 27.0 (9.2) | 26.0 (13.0; 40.0) | ||
| PAMP(mmHg) | n = 64 | 44.0 (12.7) | 44.0 (26.0; 62.0) | ||
| PCW (mmHg) | n = 64 | 9.9 (3.6) | 10.0 (4.0; 15.0) | ||
| CO (L/min) | n = 63 | 4.5 (1.1) | 4.5 (2.7; 5.8) | ||
| CI (L/min/m2) | n = 64 | 2.3 (0.5) | 2.2 (1.6; 3.1) | ||
| PVR (WU) | n = 63 | 8.3 (3.9) | 7.7 (3.3; 16.4) | ||
| HR (beats/min) | n = 61 | 71.3 (14.3) | 70.0 (54.0; 97.0) | ||
| SvO2 (%) | n = 63 | 65.0 (8.5) | 65.7 (51.5; 75.0) | ||
| TPR (WU) | n = 63 | 10.6 (4.5) | 9.9 (5.0; 18.6) | ||
| SV (L/beat) | n = 61 | 0.066 (0.023) | 0.065 (0.030; 0.098) | ||
| SVI (L/beat/m2) | n = 61 | 0.034 (0.010) | 0.033 (0.018; 0.048) | ||
| Cardiac efficiency (L/beat/mm Hg) | n = 61 | 0.0017 (0.0009) | 0.0015 (0.0006; 0.0033) | ||
| Compliance (L/beat/mm Hg) | n = 61 | 0.0014 (0.0007) | 0.0013 (0.0006; 0.0026) | ||
| PA elastance (mmHg/L/beat) | n = 61 | 1338.3 (679.3) | 1134.0 (567.5; 2490.0) | ||
| Creatinine (μmol/L) | n = 64 | 100.0 (80.4) | 89.0 (65.0; 124.0) | ||
| BNP (pg/mL) | n = 21 | 341.2 (571.3) | 159.0 (17.0; 619.0) | ||
| NTproBNP (pg/mL) | n = 31 | 1021.3 (1102.7) | 590.0 (50.0; 3,032.0) |
| Parameter | Valid n | Before the First BPA | Absolute Diference 1 | p-Value | Relative Diference 2 | |||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (5th; 95th Percentile) | Mean (SD) | Median (5th; 95th Percentile) | Mean (SD) | Median (5th; 95th Percentile) | |||
| NYHA | 25 | 3.0 (0.3) | 3.0 (3.0; 3.0) | −0.8 (0.6) | −1.0 (−2.0; 0.0) | <0.001 | 0.73 (0.18) | 0.67 (0.50; 1.00) |
| 6MWD (m) | 22 | 369.0 (116.3) | 381.5 (151.5; 527.0) | 54.3 (48.3) | 45.5 (−17.0; 146.5) | <0.001 | 1.19 (0.20) | 1.16 (0.96; 1.63) |
| Borg dyspnoea score | 22 | 6.3 (1.9) | 6.0 (2.5; 8.5) | −1.4 (1.7) | −1.5 (−4.5; 1.0) | 0.003 | 0.83 (0.27) | 0.81 (0.35; 1.35) |
| NTproBNP (pg/mL) | 24 | 1107.9 (1458.8) | 482.0 (90.0; 3 778.0) | −535.9 (949.4) | −54.0 (−2 384.0; 60.0) | 0.011 | 0.74 (0.38) | 0.70 (0.24; 1.34) |
| Creatinine (mmol/L) | 25 | 89.8 (21.1) | 89.0 (62.0; 116.0) | 0.5 (22.4) | 2.0 (−30.0; 39.0) | 0.554 | 1.03 (0.21) | 1.03 (0.71; 1.39) |
| RA (mmHg) | 24 | 7.3 (4.3) | 8.0 (1.0; 13.0) | −1.7 (5.2) | −1.0 (−10.0; 5.0) | 0.158 | 1.21 (1.36) | 0.75 (0.20; 5.00) |
| PASP (mmHg) | 25 | 69.8 (17.8) | 68.5 (43.0; 100.0) | −11.7 (12.3) | −14.0 (−25.0; 11.0) | <0.001 | 0.84 (0.17) | 0.78 (0.65; 1.15) |
| PADP (mmHg) | 25 | 24.4 (8.9) | 24.5 (12.0; 40.0) | −5.1 (7.0) | −6.0 (−17.0; 4.0) | 0.004 | 0.83 (0.24) | 0.80 (0.54; 1.17) |
| PAMP (mmHg) | 25 | 39.7 (11.1) | 37.0 (23.0; 58.0) | −7.4 (6.9) | −7.0 (−19.0; 5.0) | <0.001 | 0.82 (0.16) | 0.78 (0.57; 1.14) |
| PCW (mmHg) | 25 | 9.3 (4.0) | 10.0 (3.0; 14.0) | 0.5 (4.6) | 0.0 (−6.0; 7.0) | 0.542 | 1.39 (1.00) | 1.00 (0.50; 3.67) |
| CO (L/min) | 25 | 4.9 (1.2) | 4.9 (3.6; 6.9) | 0.4 (1.3) | 0.0 (−1.3; 2.1) | 0.187 | 1.11 (0.26) | 1.01 (0.81; 1.48) |
| CI (L/min/m2) | 25 | 2.5 (0.5) | 2.5 (1.8; 3.7) | 0.2 (0.6) | 0.0 (−0.6; 1.1) | 0.137 | 1.12 (0.26) | 1.00 (0.81; 1.49) |
| PVR (WU) | 25 | 6.6 (3.0) | 5.9 (3.5; 11.1) | −2.2 (1.6) | −1.7 (−5.1; −0.3) | <0.001 | 0.68 (0.19) | 0.71 (0.39; 0.95) |
| HR (bpm) | 25 | 72.9 (11.3) | 71.0 (54.0; 90.0) | −4.0 (10.2) | −3.0 (−23.0; 9.0) | 0.107 | 0.96 (0.12) | 0.95 (0.74; 1.14) |
| SvO2 (%) | 23 | 63.8 (8.8) | 65.0 (46.5; 75.0) | 3.6 (6.7) | 2.0 (−5.0; 17.0) | 0.041 | 1.07 (0.13) | 1.03 (0.93; 1.36) |
| SV (L/beat) | 25 | 0.069 (0.020) | 0.069 (0.041; 0.108) | 0.009 (0.015) | 0.006 (−0.013; 0.032) | 0.011 | 1.17 (0.26) | 1.09 (0.81; 1.57) |
| SVI (L/beat/m2) | 25 | 0.035 (0.008) | 0.035 (0.024; 0.049) | 0.005 (0.008) | 0.004 (−0.008; 0.018) | 0.005 | 1.17 (0.25) | 1.10 (0.79; 1.61) |
| Cardiac efficiency (L/beat/mmHg) | 25 | 0.0019 (0.0008) | 0.0018 (0.0008; 0.0033) | 0.0007 (0.0005) | 0.0006 (0.0002; 0.0013) | <0.001 | 1.46 (0.37) | 1.40 (1.06; 2.09) |
| Compliance (L/beat/mmHg) | 25 | 0.0016 (0.0006) | 0.0015 (0.0008; 0.0025) | 0.0006 (0.0004) | 0.0005 (−0.0001; 0.0012) | <0.001 | 1.38 (0.31) | 1.39 (0.97; 1.84) |
| PA elastance (mmHg/L/beat) | 25 | 1097.5 (462.5) | 1006.5 (580.1; 1 785.5) | −323.2 (265.9) | −225.8 (−786.5; −23.1) | <0.001 | 0.73 (0.15) | 0.73 (0.48; 0.97) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansa, P.; Heller, S.; Svoboda, M.; Pad’our, M.; Ambrož, D.; Dytrych, V.; Širanec, M.; Kovárník, T.; Felšőci, M.; Hutyra, M.; et al. Balloon Pulmonary Angioplasty in Patients with Chronic Thromboembolic Pulmonary Hypertension: Impact on Clinical and Hemodynamic Parameters, Quality of Life and Risk Profile. J. Clin. Med. 2020, 9, 3608. https://doi.org/10.3390/jcm9113608
Jansa P, Heller S, Svoboda M, Pad’our M, Ambrož D, Dytrych V, Širanec M, Kovárník T, Felšőci M, Hutyra M, et al. Balloon Pulmonary Angioplasty in Patients with Chronic Thromboembolic Pulmonary Hypertension: Impact on Clinical and Hemodynamic Parameters, Quality of Life and Risk Profile. Journal of Clinical Medicine. 2020; 9(11):3608. https://doi.org/10.3390/jcm9113608
Chicago/Turabian StyleJansa, Pavel, Samuel Heller, Michal Svoboda, Michal Pad’our, David Ambrož, Vladimír Dytrych, Michal Širanec, Tomáš Kovárník, Marián Felšőci, Martin Hutyra, and et al. 2020. "Balloon Pulmonary Angioplasty in Patients with Chronic Thromboembolic Pulmonary Hypertension: Impact on Clinical and Hemodynamic Parameters, Quality of Life and Risk Profile" Journal of Clinical Medicine 9, no. 11: 3608. https://doi.org/10.3390/jcm9113608
APA StyleJansa, P., Heller, S., Svoboda, M., Pad’our, M., Ambrož, D., Dytrych, V., Širanec, M., Kovárník, T., Felšőci, M., Hutyra, M., Linhart, A., Lindner, J., & Aschermann, M. (2020). Balloon Pulmonary Angioplasty in Patients with Chronic Thromboembolic Pulmonary Hypertension: Impact on Clinical and Hemodynamic Parameters, Quality of Life and Risk Profile. Journal of Clinical Medicine, 9(11), 3608. https://doi.org/10.3390/jcm9113608




