Immediate Effects of Transcutaneous Spinal Cord Stimulation on Motor Function in Chronic, Sensorimotor Incomplete Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Protocol
2.3. EMG and Kinematic Data
2.4. Transcutaneous Spinal Cord Stimulation
2.5. Data Analysis and Statistics
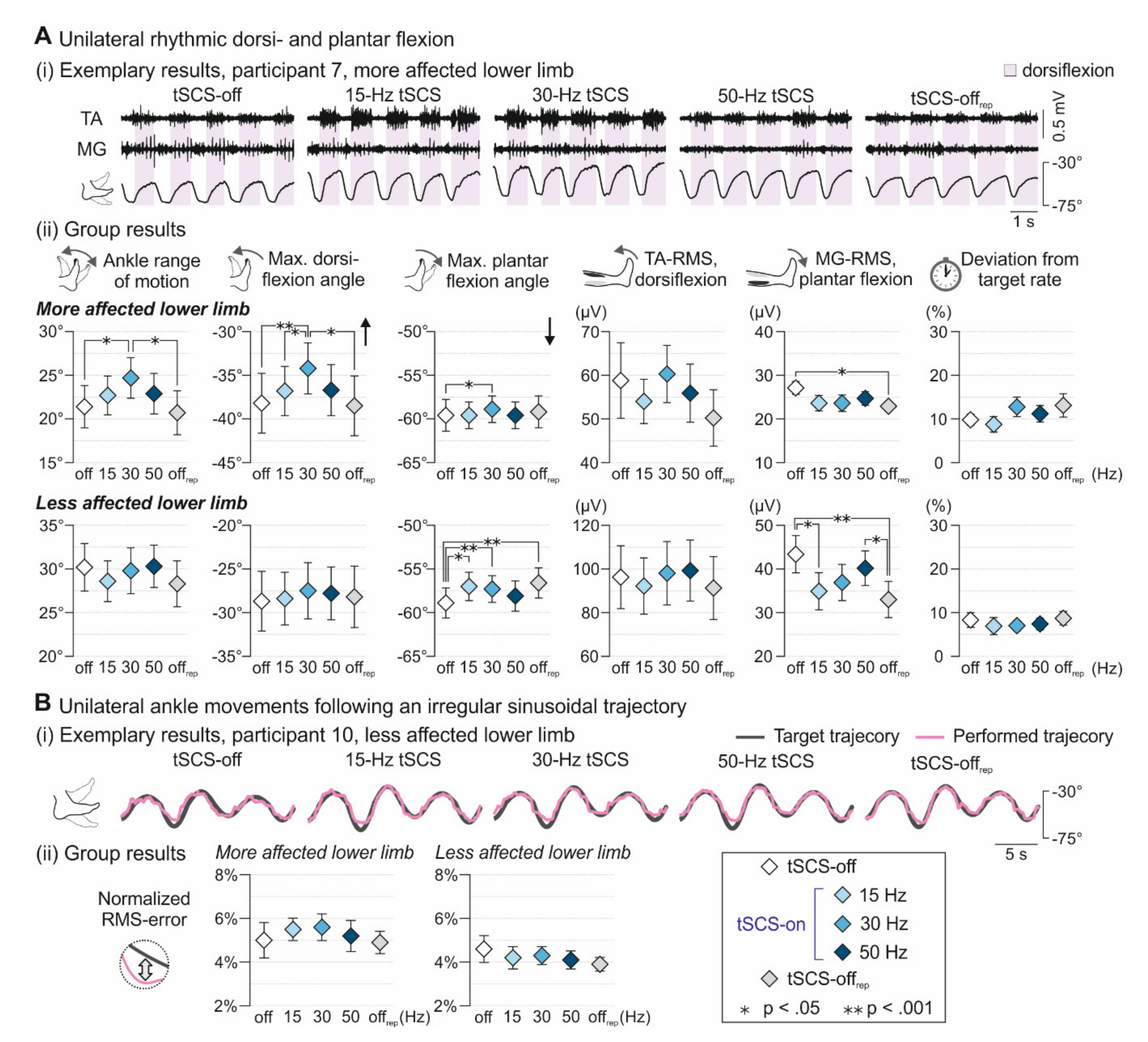
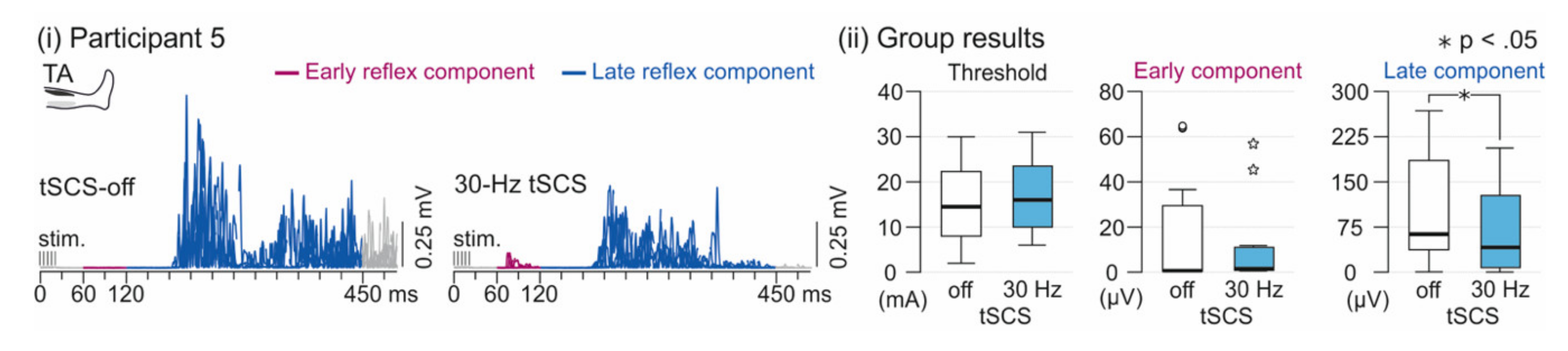
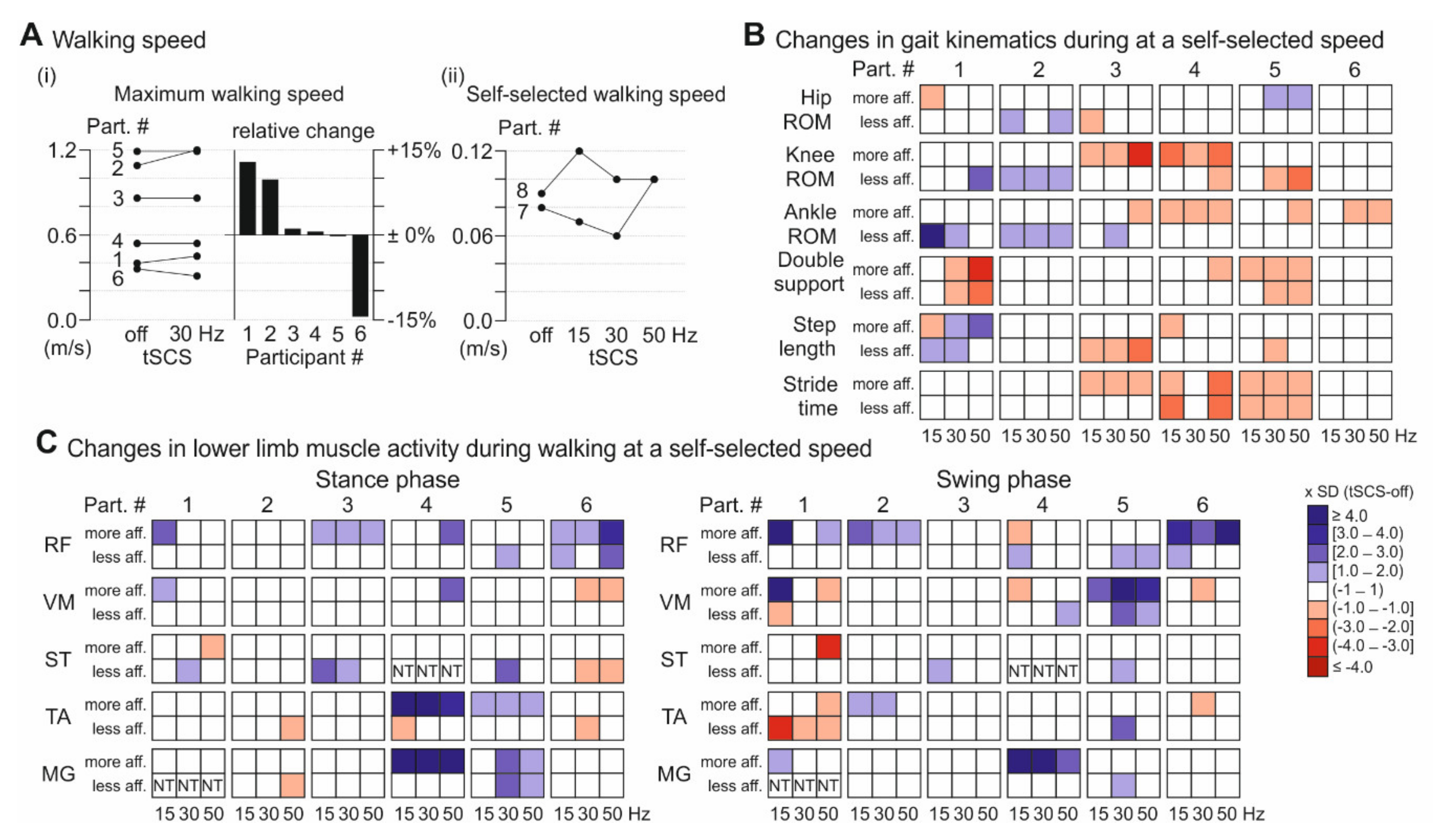
3. Results
3.1. Voluntary Ankle Control
3.2. Spinal Reflex Activity
3.3. Walking Performance
3.4. Subjective Reports
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grillner, S. Control of Locomotion in Bipeds, Tetrapods, and Fish. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2011; ISBN 9780470650714. [Google Scholar]
- Drew, T.; Marigold, D.S. Taking the next step: Cortical contributions to the control of locomotion. Curr. Opin. Neurobiol. 2015, 33, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Curt, A.; Jensen, L.; Dietz, V. Corticospinal input in human gait: Modulation of magnetically evoked motor responses. Exp. Brain Res. 1997, 115, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Capaday, C.; Lavoie, B.A.; Barbeau, H.; Schneider, C.; Bonnard, M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J. Neurophysiol. 1999, 81, 129–139. [Google Scholar] [CrossRef]
- Jayaraman, A.; Gregory, C.M.; Bowden, M.; Stevens, J.E.; Shah, P.; Behrman, A.L.; Vandenborne, K. Lower extremity skeletal muscle function in persons with incomplete spinal cord injury. Spinal Cord 2006, 44, 680–687. [Google Scholar] [CrossRef]
- Hansen, N.L.; Conway, B.A.; Halliday, D.M.; Hansen, S.; Pyndt, H.S.; Biering-Sørensen, F.; Nielsen, J.B. Reduction of common synaptic drive to ankle dorsiflexor motoneurons during walking in patients with spinal cord lesion. J. Neurophysiol. 2005, 94, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.M.; Hicks, A.L. Spasticity after spinal cord injury. Spinal Cord 2005, 43, 577–586. [Google Scholar] [CrossRef]
- Barbeau, H.; Pépin, A.; Norman, K.E.; Ladouceur, M.; Leroux, A. Walking after spinal cord injury: Control and recovery. Neuroscientist 1998, 4, 14–24. [Google Scholar] [CrossRef]
- Wirth, B.; Van Hedel, H.J.A.; Curt, A. Ankle paresis in incomplete spinal cord injury: Relation to corticospinal conductivity and ambulatory capacity. J. Clin. Neurophysiol. 2008, 25, 210–217. [Google Scholar] [CrossRef]
- Anderson, K.D. Targeting recovery: Priorities of the spinal cord-injured population. J. Neurotrauma 2004, 21, 1371–1383. [Google Scholar] [CrossRef]
- Ditunno, P.L.; Patrick, M.; Stineman, M.; Ditunno, J.F. Who wants to walk? Preferences for recovery after SCI: A longitudinal and cross-sectional study. Spinal Cord 2008, 46, 500–506. [Google Scholar] [CrossRef]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of over-ground walking after chronic motor complete spinal cord injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef]
- Wagner, F.B.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–93. [Google Scholar] [CrossRef]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137, 1394–1409. [Google Scholar] [CrossRef]
- Minassian, K.; Persy, I.; Rattay, F.; Pinter, M.M.; Kern, H.; Dimitrijevic, M.R. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum. Mov. Sci. 2007, 26, 275–295. [Google Scholar] [CrossRef]
- Murg, M.; Binder, H.; Dimitrijevic, M.R. Epidural electric stimulation of posterior structures of the human lumbar spinal cord: 1. Muscle twitches—A functional method to define the site of stimulation. Spinal Cord 2000, 38, 394–402. [Google Scholar] [CrossRef]
- Rattay, F.; Minassian, K.; Dimitrijevic, M.R. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. Quantitative analysis by computer modeling. Spinal Cord 2000, 38, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Ladenbauer, J.; Minassian, K.; Hofstoetter, U.S.; Dimitrijevic, M.R.; Rattay, F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: A computer simulation study. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 637–645. [Google Scholar] [CrossRef]
- Minassian, K.; Jilge, B.; Rattay, F.; Pinter, M.M.; Binder, H.; Gerstenbrand, F.; Dimitrijevic, M.R. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: Electromyographic study of compound muscle action potentials. Spinal Cord 2004, 42, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Jilge, B.; Minassian, K.; Rattay, F.; Pinter, M.M.; Gerstenbrand, F.; Binder, H.; Dimitrijevic, M.R. Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp. Brain Res. 2004, 154, 308–326. [Google Scholar] [CrossRef]
- Dimitrijevic, M.R.; Gerasimenko, Y.; Pinter, M.M. Evidence for a spinal central pattern generator in humans. Ann. N. Y. Acad. Sci. 1998, 860, 360–376. [Google Scholar] [CrossRef]
- Danner, S.M.; Hofstoetter, U.S.; Freundl, B.; Binder, H.; Mayr, W.; Rattay, F.; Minassian, K. Human spinal locomotor control is based on flexibly organized burst generators. Brain 2015, 138, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Minassian, K.; Persy, I.; Rattay, F.; Dimitrijevic, M. Effect of peripheral afferent and central afferent input to the human lumbar spinal cord isolated from brain control. Biocybern. Biomed. Eng. 2005, 25, 11–29. [Google Scholar]
- Pinter, M.M.; Gerstenbrand, F.; Dimitrijevic, M.R. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 3. Control of spasticity. Spinal Cord 2000, 38, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Minassian, K.; Persy, I.; Rattay, F.; Dimitrijevic, M.R.; Hofer, C.; Kern, H. Posterior root-muscle preflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 2007, 35, 327–336. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: Elicitation of posterior root-muscle reflexes. PLoS ONE 2018, 13, e0192013. [Google Scholar] [CrossRef]
- Danner, S.M.; Hofstoetter, U.S.; Ladenbauer, J.; Rattay, F.; Minassian, K. Can the Human Lumbar Posterior Columns Be Stimulated by Transcutaneous Spinal Cord Stimulation? A Modeling Study. Artif. Organs 2011, 35, 257–262. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Rath, M.; Ferguson, A.R.; Burdick, J.W.; Havton, L.A.; Edgerton, V.R.; Gerasimenko, Y.P. Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J. Neurotrauma 2019, 36, 1435–1450. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Spinal cord stimulation as a neuromodulatory intervention for altered motor control following spinal cord injury. In Biosystems and Biorobotics; Sandrini, G., Homberg, V., Saltuari, L., Smania, N., Pedrocchi, A., Eds.; Springer Verlag GmbH: Berlin/Heidelberg, Germany, 2018; Volume 19, pp. 501–521. [Google Scholar]
- Hofstoetter, U.S.; McKay, W.B.; Tansey, K.E.; Mayr, W.; Kern, H.; Minassian, K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J. Spinal Cord Med. 2014, 37, 202–211. [Google Scholar] [CrossRef]
- Estes, S.P.; Iddings, J.A.; Field-Fote, E.C. Priming neural circuits to modulate spinal reflex excitability. Front. Neurol. 2017, 8, 17. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Danner, S.M.; Krenn, M.J.; Mayr, W.; Binder, H.; Minassian, K. Transcutaneous Spinal Cord Stimulation Induces Temporary Attenuation of Spasticity in Individuals with Spinal Cord Injury. J. Neurotrauma 2020, 37, 481–493. [Google Scholar] [CrossRef]
- Gad, P.; Gerasimenko, Y.; Zdunowski, S.; Turner, A.; Sayenko, D.; Lu, D.C.; Edgerton, V.R. Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete paraplegia. Front. Neurosci. 2017, 11, 333. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Krenn, M.; Danner, S.M.; Hofer, C.; Kern, H.; Mckay, W.B.; Mayr, W.; Minassian, K. Augmentation of Voluntary Locomotor Activity by Transcutaneous Spinal Cord Stimulation in Motor-Incomplete Spinal Cord-Injured Individuals. Artif. Organs 2015, 39, E176–E186. [Google Scholar] [CrossRef]
- Gerasimenko, Y.P.; Lu, D.C.; Modaber, M.; Zdunowski, S.; Gad, P.; Sayenko, D.G.; Morikawa, E.; Haakana, P.; Ferguson, A.R.; Roy, R.R.; et al. Noninvasive reactivation of motor descending control after paralysis. J. Neurotrauma 2015, 32, 1968–1980. [Google Scholar] [CrossRef]
- Van den Brand, R.; Mignardot, J.B.; von Zitzewitz, J.; Le Goff, C.; Fumeaux, N.; Wagner, F.; Capogrosso, M.; Martin Moraud, E.; Micera, S.; Schurch, B.; et al. Neuroprosthetic technologies to augment the impact of neurorehabilitation after spinal cord injury. Ann. Phys. Rehabil. Med. 2015, 58, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Minassian, K.; Hofstoetter, U.S. Spinal Cord Stimulation and Augmentative Control Strategies for Leg Movement after Spinal Paralysis in Humans. CNS Neurosci. Ther. 2016, 22, 262–270. [Google Scholar] [CrossRef]
- Dimitrijevic, M.R.; Illis, L.S.; Nakajima, K.; Sharkey, P.C.; Sherwood, A.M. Spinal Cord Stimulation for the Control of Spasticity in Patients with Chronic Spinal Cord Injury: II. Neurophysiologic Observations. Cent. Nerv. Syst. Trauma 1986, 3, 145–152. [Google Scholar] [CrossRef]
- Dietz, V.; Grillner, S.; Trepp, A.; Hubli, M.; Bolliger, M. Changes in spinal reflex and locomotor activity after a complete spinal cord injury: A common mechanism. Brain 2009, 132, 2196–2205. [Google Scholar] [CrossRef]
- Hubli, M.; Dietz, V.; Bolliger, M. Spinal reflex activity: A marker for neuronal functionality after spinal cord injury. Neurorehabil. Neural Repair 2012, 26, 188–196. [Google Scholar] [CrossRef]
- Hubli, M.; Dietz, V.; Bolliger, M. Influence of spinal reflexes on the locomotor pattern after spinal cord injury. Gait Posture 2011, 34, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V. Behavior of spinal neurons deprived of supraspinal input. Nat. Rev. Neurol. 2010, 6, 167–174. [Google Scholar] [CrossRef]
- Hubli, M.; Bolliger, M.; Dietz, V. Neuronal dysfunction in chronic spinal cord injury. Spinal Cord 2011, 49, 582–587. [Google Scholar] [CrossRef]
- Kirshblum, S.; Waring, W. Updates for the international standards for neurological classification of Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 505–517. [Google Scholar] [CrossRef]
- Morganti, B.; Scivoletto, G.; Ditunno, P.; Ditunno, J.F.; Molinari, M. Walking index for spinal cord injury (WISCI): Criterion validation. Spinal Cord 2005, 43, 27–33. [Google Scholar] [CrossRef]
- Vallery, H.; Lutz, P.; Von Zitzewitz, J.; Rauter, G.; Fritschi, M.; Everarts, C.; Ronsse, R.; Curt, A.; Bolliger, M. Multidirectional transparent support for overground gait training. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, Seattle, WA, USA, 24–26 June 2013. [Google Scholar]
- Wirth, B.; Van Hedel, H.; Curt, A. Foot control in incomplete SCI: Distinction between paresis and dexterity. Neurol. Res. 2008, 30, 52–60. [Google Scholar] [CrossRef][Green Version]
- Marcus, J. Effects of stimulus intensity on the habituation of flexor withdrawal activity mediated by the functionally transected human spinal cord. Physiol. Psychol. 1977, 5, 321–326. [Google Scholar]
- Willer, J.C. Anticipation of pain-produced stress: Electrophysiological study in man. Physiol. Behav. 1980, 25, 49–51. [Google Scholar] [CrossRef]
- Willer, J.C.; Albe-Fessard, D. Electrophysiological evidence for a release of endogenous opiates in stress-induced ‘Analgesia’ in man. Brain Res. 1980, 198, 419–426. [Google Scholar] [CrossRef]
- Bannwart, M.; Bolliger, M.; Lutz, P.; Gantner, M.; Rauter, G. Systematic analysis of transparency in the gait rehabilitation device the FLOAT. In Proceedings of the 2016 14th International Conference on Control, Automation, Robotics and Vision, ICARCV 2016, Phuket, Thailand, 13–15 November 2016. [Google Scholar]
- Easthope, C.S.; Traini, L.R.; Awai, L.; Franz, M.; Rauter, G.; Curt, A.; Bolliger, M. Overground walking patterns after chronic incomplete spinal cord injury show distinct response patterns to unloading 11 Medical and Health Sciences 1103 Clinical Sciences. J. Neuroeng. Rehabil. 2018, 15, 102. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Vicon. Plug-in-Gait modelling instructions. In Plug-in-Gait Manual; Oxford Metrics plc: Oxford, UK, 2002; p. 612. [Google Scholar]
- Meyer, C.; Killeen, T.; Easthope, C.S.; Curt, A.; Bolliger, M.; Linnebank, M.; Zörner, B.; Filli, L. Familiarization with treadmill walking: How much is enough? Sci. Rep. 2019, 9, 5232. [Google Scholar] [CrossRef]
- Schmidt, K.; Duarte, J.E.; Grimmer, M.; Sancho-Puchades, A.; Wei, H.; Easthope, C.S.; Riener, R. The myosuit: Bi-articular anti-gravity exosuit that reduces hip extensor activity in sitting transfers. Front. Neurorobot. 2017, 11, 57. [Google Scholar] [CrossRef]
- Minassian, K.; Hofstoetter, U.; Rattay, F. Transcutaneous Lumbar Posterior Root Stimulation for Motor Control Studies and Modification of Motor Activity after Spinal Cord Injury. In Restorative Neurology of Spinal Cord Injury; Dimitrijevic, M., Kakulas, B., McKay, W., Vrbova, G., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 226–255. ISBN 9780199918768. [Google Scholar]
- Minassian, K.; McKay, W.B.; Binder, H.; Hofstoetter, U.S. Targeting Lumbar Spinal Neural Circuitry by Epidural Stimulation to Restore Motor Function After Spinal Cord Injury. Neurotherapeutics 2016, 13, 284–294. [Google Scholar] [CrossRef]
- Andrews, J.C.; Stein, R.B.; Roy, F.D. Reduced postactivation depression of soleus h reflex and root evoked potential after transcranial magnetic stimulation. J. Neurophysiol. 2015, 114, 485–492. [Google Scholar] [CrossRef][Green Version]
- Hultborn, H.; Illert, M.; Nielsen, J.; Paul, A.; Ballegaard, M.; Wiese, H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp. Brain Res. 1996, 108, 450–462. [Google Scholar] [CrossRef]
- Pierrot-Deseilligny, E.; Burke, D. The Circuitry of the Human Spinal Cord: SPINAL and Corticospinal Mechanisms of Movement; Cambridge University Press: Cambridge, UK, 2012; ISBN 9781139026727. [Google Scholar]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Recovery cycles of posterior root-muscle reflexes evoked by transcutaneous spinal cord stimulation and of the H reflex in individuals with intact and injured spinal cord. PLoS ONE 2019, 14, e0227057. [Google Scholar] [CrossRef]
- Barolat, G.; Myklebust, J.B.; Wenninger, W. Effects of spinal cord stimulation on spasticity and spasms secondary to myelopathy. Appl. Neurophysiol. 1988, 51, 29–44. [Google Scholar] [CrossRef]
- Zörner, B.; Filli, L.; Reuter, K.; Kapitza, S.; Lörincz, L.; Sutter, T.; Weller, D.; Farkas, M.; Easthope, C.S.; Czaplinski, A.; et al. Prolonged-release fampridine in multiple sclerosis: Improved ambulation effected by changes in walking pattern. Mult. Scler. 2016, 22, 1463–1475. [Google Scholar] [CrossRef]
- Meyer, C.; Filli, L.; Stalder, S.A.; Awai Easthope, C.; Killeen, T.; von Tscharner, V.; Curt, A.; Zörner, B.; Bolliger, M. Targeted Walking in Incomplete Spinal Cord Injury: Role of Corticospinal Control. J. Neurotrauma 2020. [Google Scholar] [CrossRef] [PubMed]
- Filli, L.; Sutter, T.; Easthope, C.S.; Killeen, T.; Meyer, C.; Reuter, K.; Lörincz, L.; Bolliger, M.; Weller, M.; Curt, A.; et al. Profiling walking dysfunction in multiple sclerosis: Characterisation, classification and progression over time. Sci. Rep. 2018, 8, 4984. [Google Scholar] [CrossRef]
- Capogrosso, M.; Wenger, N.; Raspopovic, S.; Musienko, P.; Beauparlant, J.; Luciani, L.B.; Courtine, G.; Micera, S. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J. Neurosci. 2013, 33, 19326–19340. [Google Scholar] [CrossRef]
- Formento, E.; Minassian, K.; Wagner, F.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Rowald, A.; Bloch, J.; Micera, S.; Capogrosso, M.; Courtine, G. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat. Neurosci. 2018, 21, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Varoqui, D.; Niu, X.; Mirbagheri, M.M. Ankle voluntary movement enhancement following robotic-assisted locomotor training in spinal cord injury. J. Neuroeng. Rehabil. 2014, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Wirth, B.; Van Hedel, H.J.A.; Curt, A. Ankle dexterity is less impaired than muscle strength in incomplete spinal cord lesion. J. Neurol. 2008, 255, 273–279. [Google Scholar] [CrossRef][Green Version]
- Van Hedel, H.J.A.; Wirth, B.; Curt, A. Ankle motor skill is intact in spinal cord injury, unlike stroke: Implications for rehabilitation. Neurology 2010, 74, 1271–1278. [Google Scholar] [CrossRef]
- Bolliger, M.; Trepp, A.; Zörner, B.; Dietz, V. Modulation of spinal reflex by assisted locomotion in humans with chronic complete spinal cord injury. Clin. Neurophysiol. 2010, 121, 2152–2158. [Google Scholar] [CrossRef]
- Smith, A.C.; Mummidisetty, C.K.; Rymer, W.Z.; Knikou, M. Locomotor training alters the behavior of flexor reflexes during walking in human spinal cord injury. J. Neurophysiol. 2014, 112, 2164–2175. [Google Scholar] [CrossRef]
- Gómez-Soriano, J.; Bravo-Esteban, E.; Pérez-Rizo, E.; Ávila-Martín, G.; Galán-Arriero, I.; Simón-Martinez, C.; Taylor, J. Abnormal cutaneous flexor reflex activity during controlled isometric plantarflexion in human spinal cord injury spasticity syndrome. Spinal Cord 2016, 54, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.J.; Dimitrijevic, M.R.; Sharkey, P.C.; Sherwood, A.M. Epidural spinal cord stimulation in spastic spinal cord injury patients. Stereotact. Funct. Neurosurg. 1987, 50, 453–454. [Google Scholar] [CrossRef]
- Musselman, K.E. Clinical significance testing in rehabilitation research: What, why, and how? Phys. Ther. Rev. 2007, 12, 287–296. [Google Scholar] [CrossRef]
- Field-Fote, E.C.; Tepavac, D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys. Ther. 2002, 82, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Minassian, K.; Hofstoetter, U.S.; Danner, S.M.; Mayr, W.; Bruce, J.A.; McKay, W.B.; Tansey, K.E. Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord-injured individuals. Neurorehabil. Neural Repair 2016, 30, 233–243. [Google Scholar] [CrossRef]
- Danner, S.M.; Krenn, M.; Hofstoetter, U.S.; Toth, A.; Mayr, W.; Minassian, K. Body position influences which neural structures are recruited by lumbar transcutaneous spinal cord stimulation. PLoS ONE 2016, 11, e0147479. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Danner, S.M.; Minassian, K. Paraspinal Magnetic and Transcutaneous Electrical Stimulation. In Encyclopedia of Computational Neuroscience; Jaeger, D., Jung, R., Eds.; Springer New York: New York, NY, USA, 2014; pp. 1–21. ISBN 978-1-4614-6676-5. [Google Scholar]
- Estes, S.; Iddings, J.A.; Ray, S.; Kirk-Sanchez, N.J.; Field-Fote, E.C. Comparison of Single-Session Dose Response Effects of Whole Body Vibration on Spasticity and Walking Speed in Persons with Spinal Cord Injury. Neurotherapeutics 2018, 15, 684–696. [Google Scholar] [CrossRef]
| Nr. | Sex | Age (y) | Neurol. Level of SCI | Time Post-SCI (y) | LEMS Total (L/R) | PP Sensory Score Total (L/R) | LT Sensory Score Total (L/R) | WISCI II Score | FLOAT-BWS (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 28 | C5 | 8 | 5/25 | 32/32 | 32/32 | 13 | 7 |
| 2 | M | 53 | C3 | 38 | 25/17 | 29/30 | 30/30 | 20 | 7 |
| 3 | M | 65 | T10 | 15 | 16/20 | 47/49 | 45/45 | 20 | 7 |
| 4 | M | 40 | C6 | 4 | 21/20 | 20/18 | 26/25 | 16 | 10 |
| 5 | M | 45 | C7 | 15 | 14/15 | 29/26 | 28/27 | 20 | 6 |
| 6 | M | 48 | C5 | 8 | 20/22 | 31/31 | 31/31 | 20 | 6 |
| 7 | M | 31 | T4 | 13 | 19/19 | 40/36 | 40/40 | 13 | 45 |
| 8 | M | 40 | C7 | 5 | 17/21 | 22/22 | 39/39 | 13 | 6 |
| 9 | F | 40 | C4 | 4 | 23/21 | 31/31 | 31/31 | 9 | NT |
| 10 | M | 64 | T3 | 6 | 25/23 | 39/37 | 44/45 | 13 | NT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, C.; Hofstoetter, U.S.; Hubli, M.; Hassani, R.H.; Rinaldo, C.; Curt, A.; Bolliger, M. Immediate Effects of Transcutaneous Spinal Cord Stimulation on Motor Function in Chronic, Sensorimotor Incomplete Spinal Cord Injury. J. Clin. Med. 2020, 9, 3541. https://doi.org/10.3390/jcm9113541
Meyer C, Hofstoetter US, Hubli M, Hassani RH, Rinaldo C, Curt A, Bolliger M. Immediate Effects of Transcutaneous Spinal Cord Stimulation on Motor Function in Chronic, Sensorimotor Incomplete Spinal Cord Injury. Journal of Clinical Medicine. 2020; 9(11):3541. https://doi.org/10.3390/jcm9113541
Chicago/Turabian StyleMeyer, Christian, Ursula S. Hofstoetter, Michèle Hubli, Roushanak H. Hassani, Carmen Rinaldo, Armin Curt, and Marc Bolliger. 2020. "Immediate Effects of Transcutaneous Spinal Cord Stimulation on Motor Function in Chronic, Sensorimotor Incomplete Spinal Cord Injury" Journal of Clinical Medicine 9, no. 11: 3541. https://doi.org/10.3390/jcm9113541
APA StyleMeyer, C., Hofstoetter, U. S., Hubli, M., Hassani, R. H., Rinaldo, C., Curt, A., & Bolliger, M. (2020). Immediate Effects of Transcutaneous Spinal Cord Stimulation on Motor Function in Chronic, Sensorimotor Incomplete Spinal Cord Injury. Journal of Clinical Medicine, 9(11), 3541. https://doi.org/10.3390/jcm9113541






