Impact on Prognosis of the Surgical Route, Laparoscopy or Laparotomy, for the Surgical Staging of Early Stage Ovarian Cancer—A Study from the FRANCOGYN Group
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Treatment and Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Characteristics
3.3. Patients’ Management
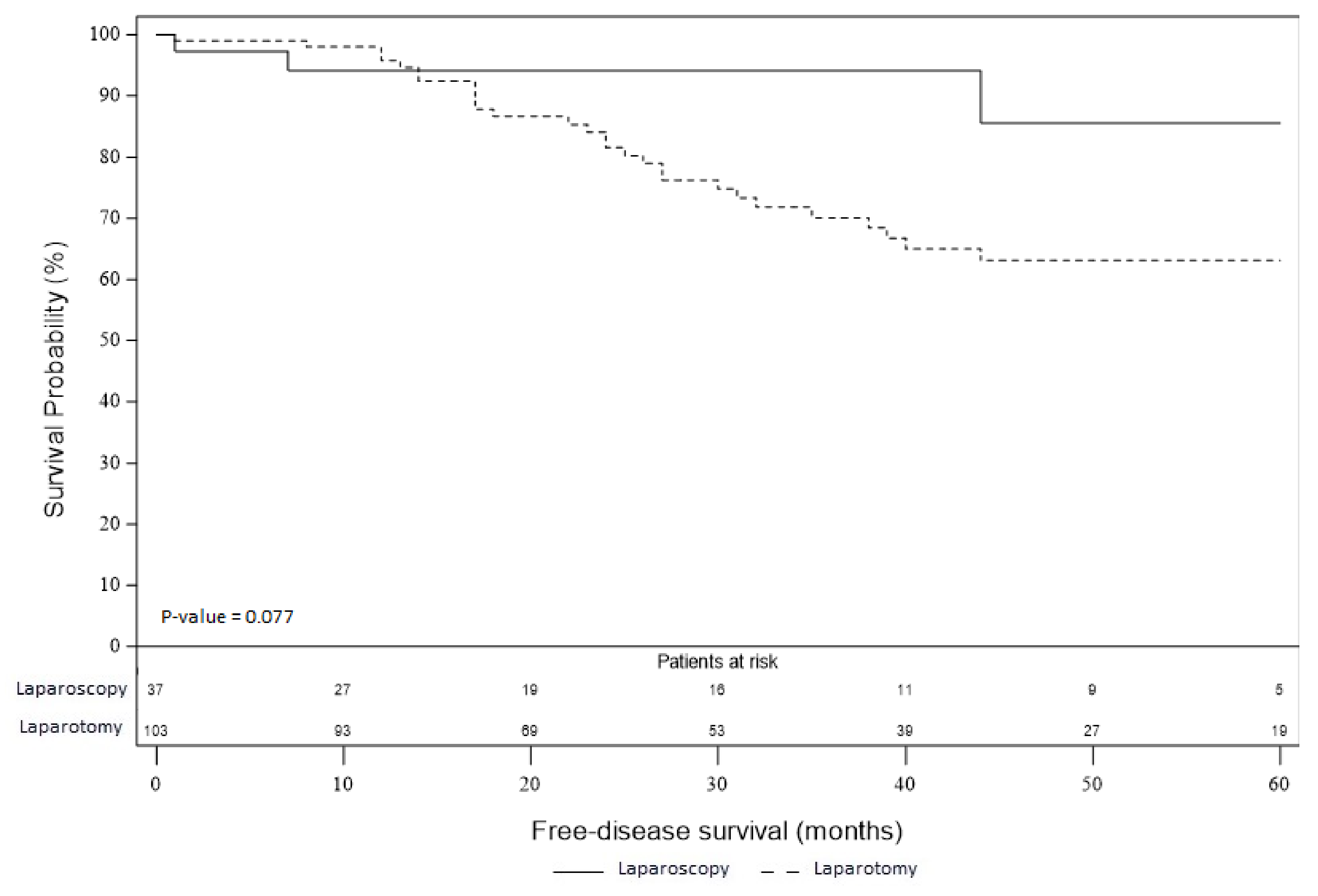
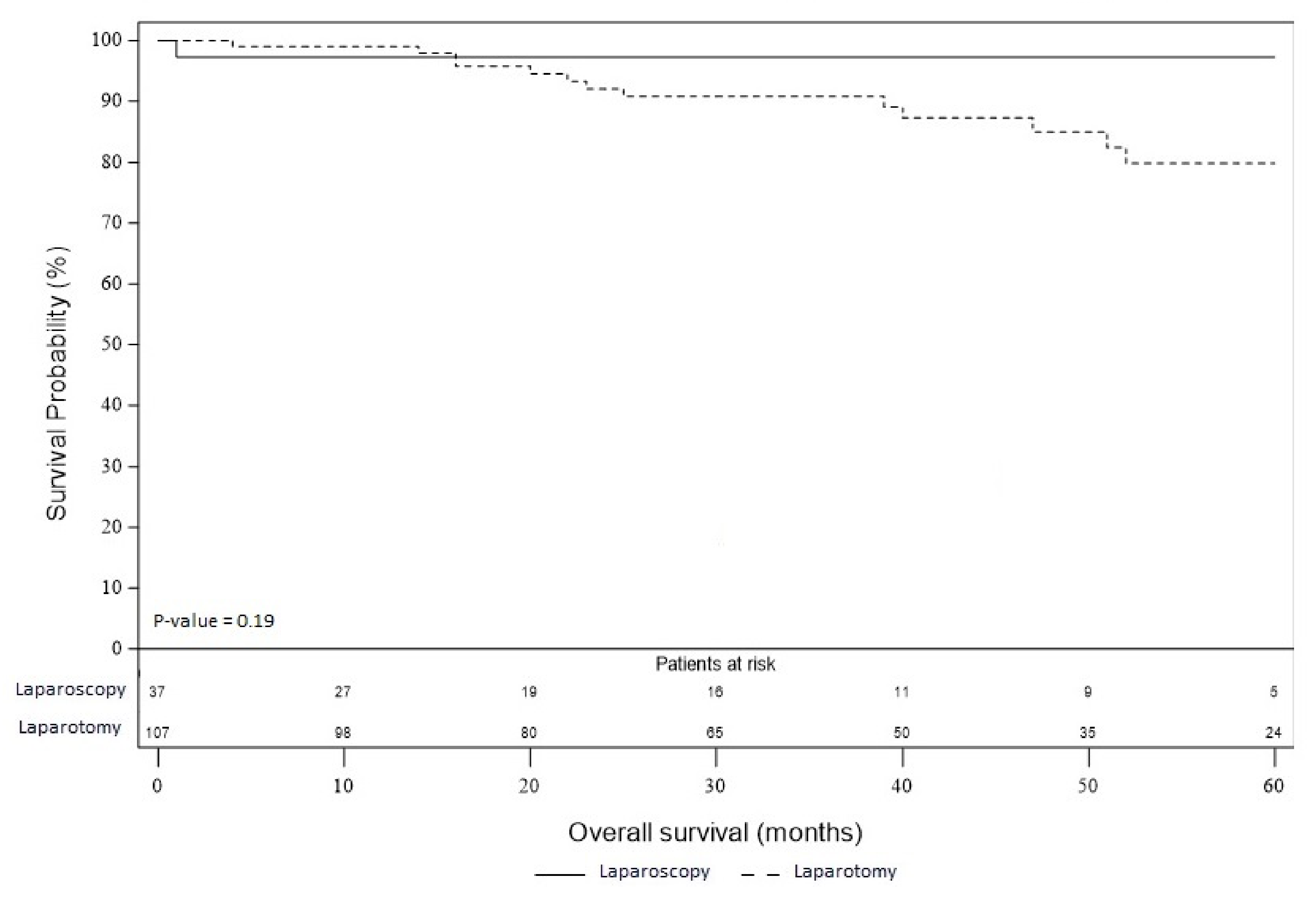
3.4. Overall and Recurrence-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lavoué, V.; Huchon, C.; Akladios, C.; Alfonsi, P.; Bakrin, N.; Ballester, M.; Bendifallah, S.; Bolze, P.; Bonnet, F.; Bourgin, C.; et al. Management of epithelial cancer of the ovary, fallopian tube, and primary peritoneum. Long text of the Joint French Clinical Practice Guidelines issued by FRANCOGYN, CNGOF, SFOG, and GINECO-ARCAGY, and endorsed by INCa. Part 1: Diagnostic exploration and staging, surgery, perioperative care, and pathology. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 369–378. [Google Scholar] [PubMed]
- Lu, Q.; Qu, H.; Liu, C.; Wang, S.; Zhang, Z.; Zhang, Z. Comparison of Laparoscopy and Laparotomy in Surgical Staging of Apparent Early Ovarian Cancer. Medicine 2016, 95, e3655. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bolze, P.-A.; Collinet, P.; Golfier, F.; Bourgin, C. Chirurgie des stades précoces des cancers ovariens. Article rédigé sur la base de la recommandation nationale de bonnes pratiques cliniques en cancérologie intitulée «Conduites à tenir initiales devant des patientes atteintes d’un cancer épithélial de l’ovaire» élaborée par FRANCOGYN, CNGOF, SFOG, GINECO-ARCAGY sous l’égide du CNGOF et labellisée par l’INCa. Gynécologie Obstétrique Fertilité Sénologie 2019, 47, 168–179. [Google Scholar]
- Leblanc, É.; Querleu, D.; Castelain, B.; Lanvin, D.; Chevalier, A.; Lesoin, A.; Gougeon, É.; Cabaret, V.; Taïeb, S. Bilan de la cœliochirurgie en oncologie gynécologique en 2000. Bull. Cancer 2000, 87, 76–83. [Google Scholar] [PubMed]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Spiegel, G.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Laparoscopy Compared With Laparotomy for Comprehensive Surgical Staging of Uterine Cancer: Gynecologic Oncology Group Study LAP2. J. Clin. Oncol. 2009, 27, 5331–5336. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Deng, L.; Xu, H.-C.; Zhang, Y.; Liang, Z.-Q. Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Weber, S.; McCann, C.K.; Boruta, D.M.; Schorge, J.; Growdon, W.B. Laparoscopic Surgical Staging of Early Ovarian Cancer. Rev. Obstet. Gynecol. 2011, 4, 117–122. [Google Scholar]
- Ghezzi, F.; Cromi, A.; Uccella, S.; Bergamini, V.; Tomera, S.; Franchi, M.; Bolis, P. Laparoscopy versus laparotomy for the surgical management of apparent early stage ovarian cancer. Gynecol. Oncol. 2007, 105, 409–413. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, D.-Y.; Suh, D.-S.; Kim, J.-H.; Kim, Y.-M.; Kim, Y.-T.; Nam, J.-H. Comparison of Laparoscopy and Laparotomy in Surgical Staging of Early-Stage Ovarian and Fallopian Tubal Cancer. Ann. Surg. Oncol. 2008, 15, 2012–2019. [Google Scholar] [CrossRef]
- Nezhat, F.; Ezzati, M.; Chuang, L.; Shamshirsaz, A.A.; Rahaman, J.; Gretz, H. Laparoscopic management of early ovarian and fallopian tube cancers: Surgical and survival outcome. Am. J. Obstet. Gynecol. 2009, 200, e1–e6. [Google Scholar] [CrossRef]
- Lee, C.-L.; Kusunoki, S.; Huang, C.-Y.; Wu, K.-Y.; Lee, P.-S.; Huang, K.-G. Surgical and survival outcomes of laparoscopic staging surgery for patients with stage I ovarian cancer. Taiwan J. Obstet. Gynecol. 2018, 57, 7–12. [Google Scholar] [CrossRef]
- Falcetta, F.S.; Lawrie, T.A.; Medeiros, L.R.; da Rosa, M.I.; Edelweiss, M.I.; Stein, A.T.; Zelmanowicz, A.; Moraes, A.B.; Zanini, R.R.; Rosa, D.D. Laparoscopy versus laparotomy for FIGO stage I ovarian cancer. Cochrane Database Syst. Rev. 2016, 10, CD005344. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseaset. Ann. Oncol. J. Eur. Soc. Med. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Prat, J. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obs. 2014, 124, 1–5. [Google Scholar] [CrossRef]
- Schoenfeld, D.A. Sample-Size Formula for the Proportional-Hazards Regression Model. Biometrics 1983, 39, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Jiang, L.; Tsiatis, A.A. Multiple Imputation Approaches for the Analysis of Dichotomized Responses in Longitudinal Studies with Missing Data. Biometrics 2010, 66, 1202–1208. [Google Scholar] [CrossRef]
- Minig, L.; Saadi, J.; Patrono, M.G.; Giavedoni, M.E.; Cárdenas-Rebollo, J.M.; Perrotta, M.; Patrono, G. Laparoscopic surgical staging in women with early stage epithelial ovarian cancer performed by recently certified gynecologic oncologists. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 201, 94–100. [Google Scholar] [CrossRef]
- Koo, Y.-J.; Kim, J.-E.; Kim, Y.-H.; Hahn, H.-S.; Lee, I.-H.; Kim, T.-J.; Lee, K.-H.; Shim, J.-U.; Lim, K.-T. Comparison of laparoscopy and laparotomy for the management of early-stage ovarian cancer: Surgical and oncological outcomes. J. Gynecol. Oncol. 2014, 25, 111–117. [Google Scholar] [CrossRef]
- Park, D.; Yun, J.; Kim, S.W.; Lee, S.H. Surgical and clinical safety and effectiveness of robot-assisted laparoscopic hysterectomy compared to conventional laparoscopy and laparotomy for cervical cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 994–1002. [Google Scholar] [CrossRef]
- Chi, D.S.; Abu-Rustum, N.R.; Sonoda, Y.; Ivy, J.; Rhee, E.H.; Moore, K.; Levine, U.A.; Barakat, R.R. The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers. Am. J. Obstet. Gynecol. 2005, 192, 1614–1619. [Google Scholar] [CrossRef]
- Vergote, I.; De Brabanter, J.; Fyles, A.; Bertelsen, K.; Einhorn, N.; Sevelda, P.; Gore, M.; Kærn, J.; Verrelst, H.; Sjövall, K.; et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 2001, 357, 176–182. [Google Scholar] [CrossRef]
- Bentivegna, E.; Gouy, S.; Maulard, A.; Pautier, P.; Leary, A.; Colombo, N.; Morice, P. Fertility-sparing surgery in epithelial ovarian cancer: A systematic review of oncological issues. Ann. Oncol. 2016, 27, 1994–2004. [Google Scholar] [CrossRef]
- Gallotta, V.; Petrillo, M.; Conte, C.; Vizzielli, G.; Fagotti, A.; Ferrandina, G.; Fanfani, F.; Costantini, B.; Carbone, V.; Scambia, G. Laparoscopic Versus Laparotomic Surgical Staging for Early-Stage Ovarian Cancer: A Case-Control Study. J. Minim. Invasive Gynecol. 2016, 23, 769–774. [Google Scholar] [CrossRef]
- Sjövall, K.; Nilsson, B.; Einhorn, N. Different types of rupture of the tumor capsule and the impact on survival in early ovarian carcinoma. Int. J. Gynecol. Cancer 1994, 4, 333–336. [Google Scholar] [CrossRef]
- Suh, D.H.; Park, J.-Y.; Lee, J.-Y.; Kim, B.-G.; Lim, M.C.; Kim, B.-G.; Bae, D.-S.; Park, S.-Y.; Nam, J.-H.; Kim, K.; et al. The clinical value of surgeons’ efforts of preventing intraoperative tumor rupture in stage I clear cell carcinoma of the ovary: A Korean multicenter study. Gynecol. Oncol. 2015, 137, 412–417. [Google Scholar] [CrossRef]
- Zivanovic, O.; Sonoda, Y.; Diaz, J.P.; Levine, D.A.; Brown, C.L.; Chi, D.S.; Barakat, R.R.; Abu-Rustum, N.R. The rate of port-site metastases after 2251 laparoscopic procedures in women with underlying malignant disease. Gynecol. Oncol. 2008, 111, 431–437. [Google Scholar] [CrossRef]
- Bogani, G.; Borghi, C.; Maggiore, U.L.R.; Ditto, A.; Signorelli, M.; Martinelli, F.; Chiappa, V.; Lopez, C.; Sabatucci, I.; Scaffa, C.; et al. Minimally Invasive Surgical Staging in Early-stage Ovarian Carcinoma: A Systematic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2017, 24, 552–562. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Xiang, Y.; Duan, H.; Sun, L. Comparison of the prognosis and recurrence of apparent early-stage ovarian tumors treated with laparoscopy and laparotomy: A meta-analysis of clinical studies. BMC Cancer 2015, 15, 597. [Google Scholar] [CrossRef]
- Krivak, T.C.; Elkas, J.C.; Rose, G.S.; Sundborg, M.; Winter, W.E.; Carlson, J.; MacKoul, P.J. The utility of hand-assisted laparoscopy in ovarian cancer. Gynecol. Oncol. 2005, 96, 72–76. [Google Scholar] [CrossRef]
- Gadducci, A.; Sartori, E.; Maggino, T.; Zola, P.; Landoni, F.; Fanucchi, A.; Stegher, C.; Alessi, C.; Buttitta, F. Analysis of failures in patients with stage I ovarian cancer: An Italian multicenter study. Int. J. Gynecol. Cancer 1997, 7, 445–450. [Google Scholar] [CrossRef]
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; Del Carmen, M.G.; Yang, J.; Seagle, B.-L.L.; Alexander, A.; Barber, E.L.; Rice, L.W.; et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N. Engl. J. Med. 2018, 379, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Canlorbe, G.; Levêque, J.; Koskas, M. Les résultats de l’essai LACC doivent-ils modifier les pratiques françaises pour le choix de la voie d’abord dans le traitement chirurgical du cancer du col de stade précoce ? Le point de vue de la commission de cancérologie du CNGOF. Bull. Cancer 2019, 106, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.; Hertel, H.; Herrmann, J.; Marnitz, S.; Mallmann, P.; Favero, G.; Plaikner, A.; Martus, P.; Gajda, M.; Schneider, A. Laparoscopic radical hysterectomy with transvaginal closure of vaginal cuff—A multicenter analysis. Int. J. Gynecol. Cancer 2019, 29, 845–850. [Google Scholar] [CrossRef]

| Characteristic | Laparoscopic (n = 37) | Laparotomy (n = 107) | P Value |
|---|---|---|---|
| Age (y) | 56.3 (±16.8) | 56.2 (±14.7) | 0.98 |
| BMI (Kg/m2) 1 | 23.8 (±5.0) | 25.4(±5.0) | 0.13 |
| Hormonal status | 0.56 | ||
| Menopausal | 31/36 (86.1) | 93/104 (89.4) | |
| Nonmenopausal | 5/36 (13.9) | 11/104 (10.6) | |
| Family history of gynecological cancer | 10/34 (29.4) | 24/100 (24.0) | 0.53 |
| Prior abdominal surgery | 13/30 (43.3) | 16/41 (39.0) | 0.72 |
| Surgical FIGO stage | 0.24 | ||
| IA | 18/37 (48.7) | 51/107 (47.7) | |
| IB | 1/37 (2.7) | 10/107 (9.4) | |
| IC | 12/37 (32.4) | 38/107 (35.5) | |
| IIA | 6/37 (16.2) | 7/107 (6.5) | |
| Grade | 0.014 | ||
| high | 17/22 (77.3) | 28/60 (46.7) | |
| Low | 5/22 (22.7) | 32/60 (53.3) | |
| Histology | 0.42 | ||
| Serous | 17/37 (46.0) | 35/107 (32.7) | |
| Mucinous | 7/37 (18.9) | 18/107 (16.8) | |
| Endometrioid | 8/37 (21.6) | 31/107 (29.0) | |
| Clear cell Mixed | 5/37 (13.5) 0/37 (0.0) | 18/107 (16.8) 5/107 (4.7) |
| Variables | Laparoscopy (n = 37) | Laparotomy (n = 107) | p Value |
|---|---|---|---|
| Pelvic lymphadenectomy | 28/37 (75.7) | 67/105 (63.8) | 0.19 |
| Para-aortic lymphadenectomy | 27/37 (73.0) | 67/107 (62.6) | 0.25 |
| Pelvic node removed | 12 (6 to18) | 7 (0 to13) | 0.026 |
| Para-aortic node removed | 14 (0 to23) | 9 (0 to20) | 0.27 |
| Intra operative complications Tumor rupture Organ damage | 3/22 (13.6) 0 3 | 6/105 (5.7) 4 2 | 0.19 NA NA |
| Postoperative complications | 7/34 (20.6) | 11/99 (11.1) | 0.24 |
| Chemotherapy | 34/37 (91.9) | 96/102 (94.1) | 0.70 |
| First Author | Year | Surgical Approach (n) | Recurrence n (%) | Odds Ratio, 95% CI | Death of Disease n (%) | Odds Ratio, 95% CI | Follow-up (Months) |
|---|---|---|---|---|---|---|---|
| Ditto | 2016 | MIS (n = 50) OPEN (n = 50) | 7 (14.0) 11 (22.0) | 0.79 (0.31–2.01) | 2 (4.0) 5 (10.0) | 0.87 (0.08–9.19) | 49.5 (+/−64) 52.6 (+/−31.7) p = 0.01 ** |
| Gallota | 2016 | MIS (n = 60) OPEN (n = 120) | 5 (8.3) 16 (13.3) | NR * p = 0.651 | 5 (8.0) 11 (9.0) | NR * p = 0.72 | 38 (24–48) 38 (24–48) |
| Lu | 2016 | MIS (n = 42) OPEN (n = 50) | 5 (13.0) 6 (13.0) | NR * p = NS | 3 (7.1) 5 (10.0) | NR * p = 0.63 | 82 (16–152) 82 (16–152) |
| Melamed | 2016 | MIS (n = 1096) OPEN (n = 1096) | NR NR | Not estimable | 55 (5.1) 71 (6.5) | 0.77 (0.54–1.09) | 28.7 (20.4–38.9) 29.3 (20.6–39 |
| Minig | 2016 | MIS (n = 50) OPEN (n = 58) | 6 (12.0) 7 (12.0) | 0.50 (0.21–1.21) | NR NR | NR * p = 0.42 | 25.9 (11.2–38.5) 34.3 (28.4–47.8) p = 0.004 ** |
| Bogani | 2014 | MIS (n = 35) OPEN (n = 32) | 4 (11.4) 9 (28.1) | 0.33 (0.09–1.20) | 2 (5.7) 4 (12.5) | NR * p = 0.26 | 64 (37–106) 100 (61–278) p <0.001 ** |
| Liu *** | 2014 | MIS (n = 35) OPEN (n = 40) | 3 (8.6) 2 (5.0) | 1.78 (0.28–11.33) | 1 (2.9) 1 (2.5) | 1.15 (0.07–19.05) | NR (36–84) |
| Koo | 2013 | MIS (n = 24) OPEN (n = 53) | 2 (8.3) 2 (3.8) | NR * p = 0.59 | 1 (4.2) 0 (0.0) | NR * p = 0.23 | 31.7 (+/−20.7) 31.1 (+/−19.1) |
| Lee *** | 2011 | MIS (n = 26) OPEN (n = 87) | 0 (0.0) 0 (0.0) | Not estimable | 0 (0) 3 (3.4) | 0.46 (0.02–9.11) | 12 (1–42) 25 (1–74) |
| Park (2) *** | 2008 | MIS (n = 19) OPEN (n = 33) | 0 (0.0) 0 (0.0) | 6.29 (0.28–140.86) | 0 (0) 0 (0) | 3.55 (0.14–93.01) | 17 (2–40) 23 (1–44) |
| Park (1) *** | 2008 | MIS (n = 17) OPEN (n = 19) | 2 (12.0) 0 (0.0) | Not estimable | 1 (5.9) 0 (0) | Not estimable | 19 (5–56) 14 (5–61) |
| Ghezzi *** | 2007 | MIS (n = 15) OPEN (n = 19) | 0 (0.0) 4 (7.1) | NR | 0 (0.0) 0 (0.0) | NR | 16 (4–33) 60 (32–108) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merlier, M.; Kerbage, Y.; Pierache, A.; Ramdane, N.; Canlorbe, G.; Bolze, P.-A.; Ballester, M.; Bendifallah, S.; Ouldamer, L.; Touboul, C.; et al. Impact on Prognosis of the Surgical Route, Laparoscopy or Laparotomy, for the Surgical Staging of Early Stage Ovarian Cancer—A Study from the FRANCOGYN Group. J. Clin. Med. 2020, 9, 3528. https://doi.org/10.3390/jcm9113528
Merlier M, Kerbage Y, Pierache A, Ramdane N, Canlorbe G, Bolze P-A, Ballester M, Bendifallah S, Ouldamer L, Touboul C, et al. Impact on Prognosis of the Surgical Route, Laparoscopy or Laparotomy, for the Surgical Staging of Early Stage Ovarian Cancer—A Study from the FRANCOGYN Group. Journal of Clinical Medicine. 2020; 9(11):3528. https://doi.org/10.3390/jcm9113528
Chicago/Turabian StyleMerlier, Margaux, Yohan Kerbage, Adeline Pierache, Nassima Ramdane, Geoffroy Canlorbe, Pierre-Adrien Bolze, Marcos Ballester, Sofiane Bendifallah, Lobna Ouldamer, Cyril Touboul, and et al. 2020. "Impact on Prognosis of the Surgical Route, Laparoscopy or Laparotomy, for the Surgical Staging of Early Stage Ovarian Cancer—A Study from the FRANCOGYN Group" Journal of Clinical Medicine 9, no. 11: 3528. https://doi.org/10.3390/jcm9113528
APA StyleMerlier, M., Kerbage, Y., Pierache, A., Ramdane, N., Canlorbe, G., Bolze, P.-A., Ballester, M., Bendifallah, S., Ouldamer, L., Touboul, C., Huchon, C., Lavoue, V., Dabi, Y., Akladios, C., Coutant, C., Raimond, E., Bricou, A., Phalippou, J., Collinet, P., & Azaïs, H. (2020). Impact on Prognosis of the Surgical Route, Laparoscopy or Laparotomy, for the Surgical Staging of Early Stage Ovarian Cancer—A Study from the FRANCOGYN Group. Journal of Clinical Medicine, 9(11), 3528. https://doi.org/10.3390/jcm9113528







