Obesity and Pancreatic Cancer: A Matched-Pair Survival Analysis
Abstract
1. Introduction
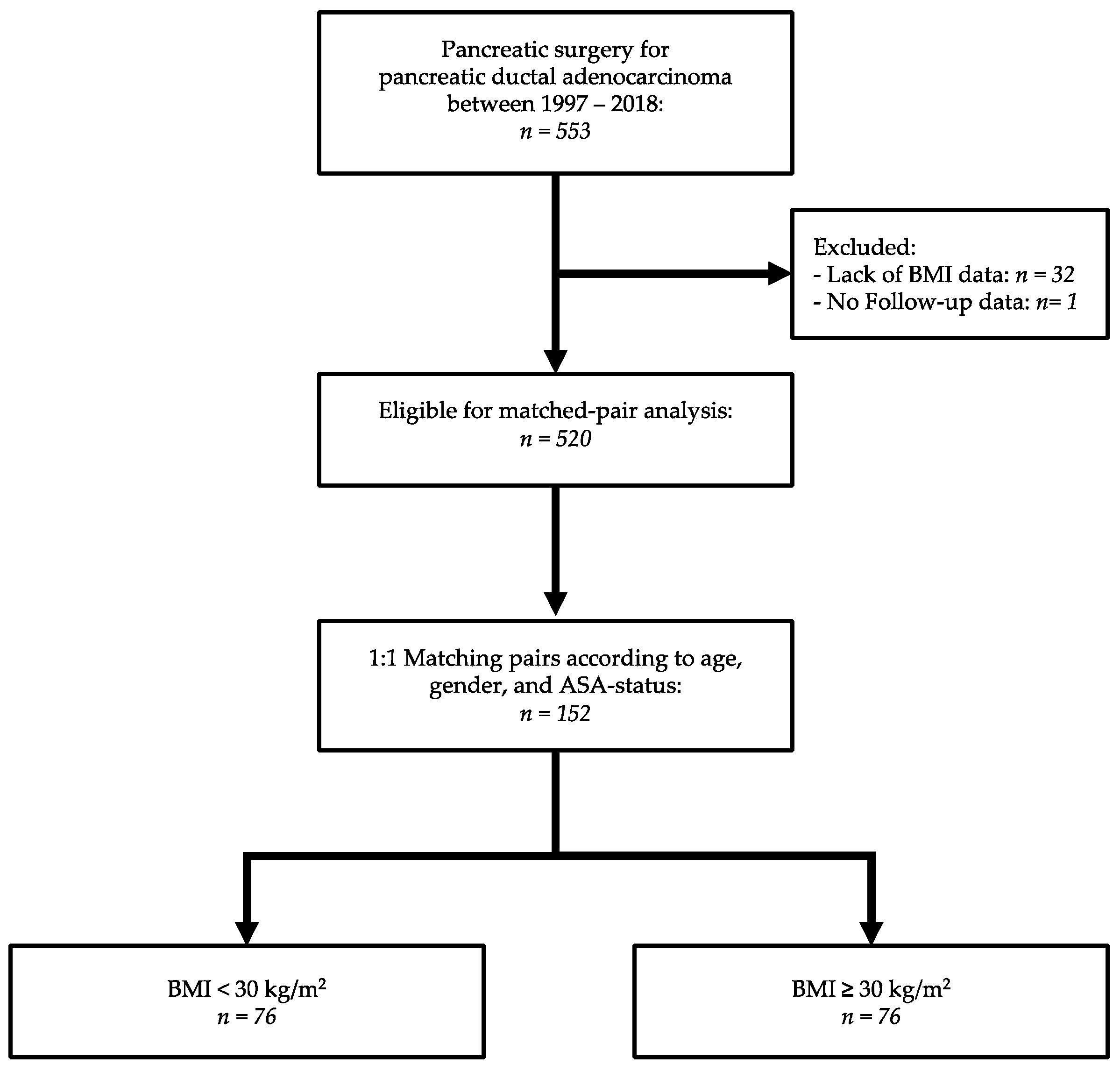
2. Materials and Methods
2.1. Study Design
2.2. Definitions and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Intraoperative Outcome and Pathological Characteristics
3.3. Postoperative Morbidity
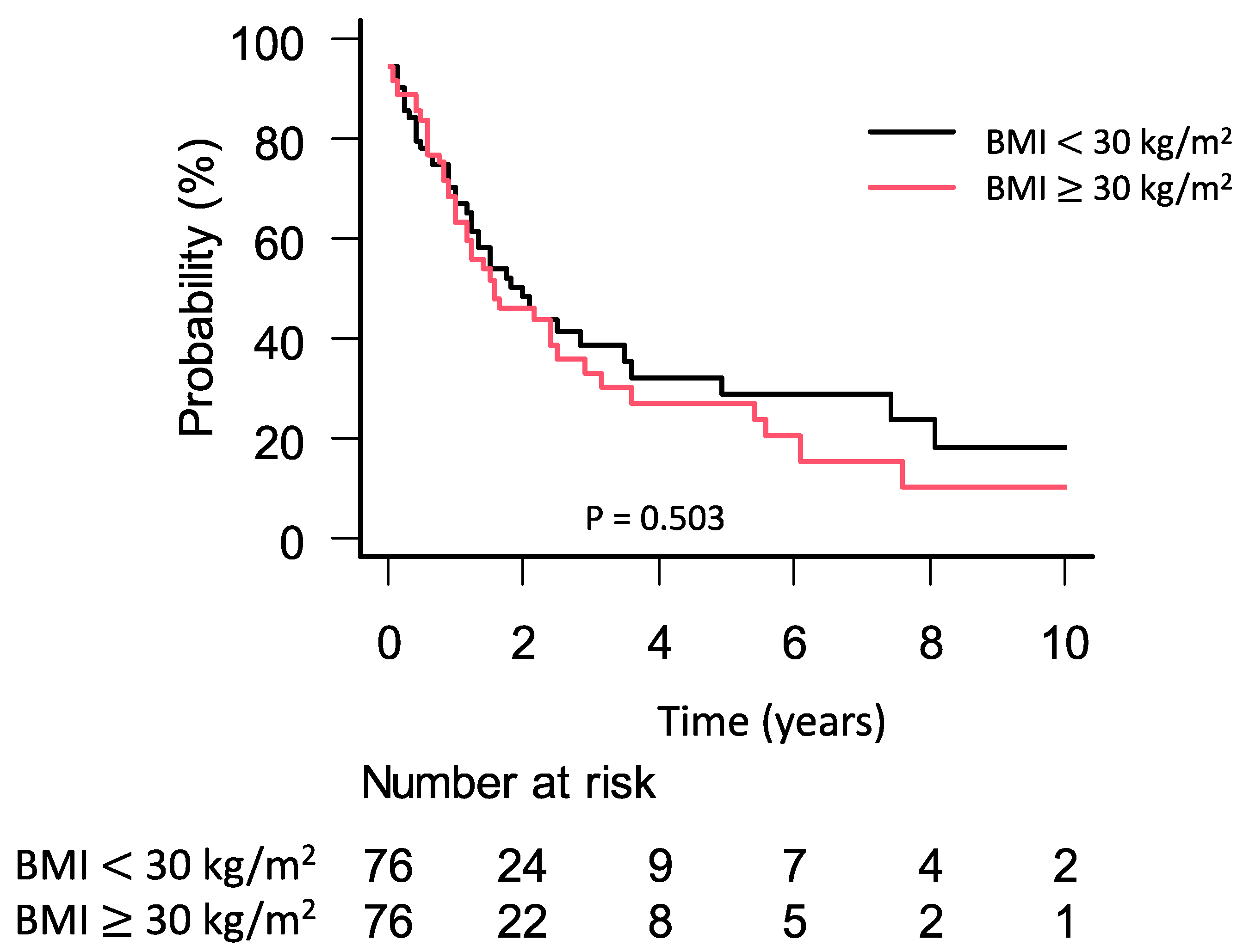
3.4. Survival Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Popkin, B.M. Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 2006, 84, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Slining, M.M. New dynamics in global obesity facing low- and middle-income countries. Obes. Rev. 2013, 14, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.R.; Li, L.; Pan, Y.H.; Tian, G.D.; Lin, W.L.; Li, Z.; Chen, Z.Y.; Gong, Y.L.; Kikano, G.E.; Stange, K.C.; et al. Prevalence of metabolic syndrome among urban community residents in China. BMC Pub. Health 2013, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC) State-specific prevalence of obesity among adults-United States, 2005. MMWR Morb. Mortal. Wkly. Rep. 2006, 55, 985–988.
- Berger, N.A. Obesity and cancer pathogenesis. Ann. N. Y. Acad. Sci. 2014, 1311, 57–76. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. International Agency for Research on Cancer Handbook Working Group Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Téoule, P.; Bartel, F.; Birgin, E.; Rückert, F.; Wilhelm, T.J. The Clavien-Dindo Classification in Pancreatic Surgery: A Clinical and Economic Validation. J. Invest. Surg. 2019, 32, 314–320. [Google Scholar] [CrossRef]
- Nimptsch, U.; Krautz, C.; Weber, G.F.; Mansky, T.; Grützmann, R. Nationwide In-hospital Mortality Following Pancreatic Surgery in Germany is Higher than Anticipated. Ann. Surg. 2016, 264, 1082–1090. [Google Scholar] [CrossRef]
- Adams, K.F.; Schatzkin, A.; Harris, T.B.; Kipnis, V.; Mouw, T.; Ballard-Barbash, R.; Hollenbeck, A.; Leitzmann, M.F. Overweight, Obesity, and Mortality in a Large Prospective Cohort of Persons 50 to 71 Years Old. N. Engl. J. Med. 2006, 355, 763–778. [Google Scholar] [CrossRef]
- Benns, M.; Woodall, C.; Scoggins, C.; McMasters, K.; Martin, R. The impact of obesity on outcomes following pancreatectomy for malignancy. Ann. Surg. Oncol. 2009, 16, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.K.; Rosato, E.L.; Kennedy, E.P.; Chojnacki, K.A.; Andrel, J.; Hyslop, T.; Doria, C.; Sauter, P.K.; Bloom, J.; Yeo, C.J.; et al. Impact of obesity on perioperative morbidity and mortality after pancreaticoduodenectomy. J. Am. Coll. Surg. 2009, 208, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Koga, R.; Saiura, A.; Natori, T.; Yamaguchi, T.; Yamamoto, J. Factors influencing infectious complications after pancreatoduodenectomy. J. Hepatobil. Pancreat. Sci 2010, 17, 174–179. [Google Scholar] [CrossRef] [PubMed]
- House, M.G.; Fong, Y.; Arnaoutakis, D.J.; Sharma, R.; Winston, C.B.; Protic, M.; Gonen, M.; Olson, S.H.; Kurtz, R.C.; Brennan, M.F.; et al. Preoperative predictors for complications after pancreaticoduodenectomy: Impact of BMI and body fat distribution. J. Gastrointest. Surg. 2008, 12, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Balentine, C.J.; Enriquez, J.; Cruz, G.; Hodges, S.; Bansal, V.; Jo, E.; Ahern, C.; Sansgiry, S.; Petersen, N.; Silberfein, E.; et al. Obesity does not increase complications following pancreatic surgery. J. Surg. Res. 2011, 170, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Del Chiaro, M.; Rangelova, E.; Ansorge, C.; Blomberg, J.; Segersvärd, R. Impact of body mass index for patients undergoing pancreaticoduodenectomy. World J. Gastroint. Pathophysiol. 2013, 4, 37–42. [Google Scholar] [CrossRef]
- Tsai, S.; Choti, M.A.; Assumpcao, L.; Cameron, J.L.; Gleisner, A.L.; Herman, J.M.; Eckhauser, F.; Edil, B.H.; Schulick, R.D.; Wolfgang, C.L.; et al. Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: A large single-institution study. J. Gastrointest. Surg. 2010, 14, 1143–1150. [Google Scholar] [CrossRef]
- Fleming, J.B.; Gonzalez, R.J.; Petzel, M.Q.B.; Lin, E.; Morris, J.S.; Gomez, H.; Lee, J.E.; Crane, C.H.; Pisters, P.W.T.; Evans, D.B. Influence of obesity on cancer-related outcomes after pancreatectomy to treat pancreatic adenocarcinoma. Arch. Surg. 2009, 144, 216–221. [Google Scholar] [CrossRef]
- Gaujoux, S.; Cortes, A.; Couvelard, A.; Noullet, S.; Clavel, L.; Rebours, V.; Lévy, P.; Sauvanet, A.; Ruszniewski, P.; Belghiti, J. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery 2010, 148, 15–23. [Google Scholar] [CrossRef]
- Li, D.; Morris, J.S.; Liu, J.; Hassan, M.M.; Day, R.S.; Bondy, M.L.; Abbruzzese, J.L. Body Mass Index and Risk, Age of Onset, and Survival in Patients With Pancreatic Cancer. JAMA 2009, 301, 2553–2562. [Google Scholar] [CrossRef]
- McWilliams, R.R.; Matsumoto, M.E.; Burch, P.A.; Kim, G.P.; Halfdanarson, T.R.; de Andrade, M.; Reid-Lombardo, K.; Bamlet, W.R. Obesity Adversely Affects Survival in Pancreatic Cancer Patients. Cancer 2010, 116, 5054–5062. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Bao, Y.; Wu, C.; Kraft, P.; Ogino, S.; Ng, K.; Qian, Z.R.; Rubinson, D.A.; Stampfer, M.J.; Giovannucci, E.L.; et al. Prediagnostic Body Mass Index and Pancreatic Cancer Survival. J. Clin. Oncol. 2013, 31, 4229–4234. [Google Scholar] [CrossRef] [PubMed]
- Kasenda, B.; Bass, A.; Koeberle, D.; Pestalozzi, B.; Borner, M.; Herrmann, R.; Jost, L.; Lohri, A.; Hess, V. Survival in overweight patients with advanced pancreatic carcinoma: A multicentre cohort study. BMC Cancer 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.H.; Chou, J.F.; Ludwig, E.; O’Reilly, E.; Allen, P.J.; Jarnagin, W.R.; Bayuga, S.; Simon, J.; Gonen, M.; Reisacher, W.R.; et al. Allergies, obesity, other risk factors and survival from pancreatic cancer. Int. J. Cancer 2010, 127, 2412–2419. [Google Scholar] [CrossRef]
- Pelucchi, C.; Galeone, C.; Polesel, J.; Manzari, M.; Zucchetto, A.; Talamini, R.; Franceschi, S.; Negri, E.; La Vecchia, C. Smoking and body mass index and survival in pancreatic cancer patients. Pancreas 2014, 43, 47–52. [Google Scholar] [CrossRef]
- Dandona, M.; Linehan, D.; Hawkins, W.; Strasberg, S.; Gao, F.; Wang-Gillam, A. Influence of obesity and other risk factors on survival outcomes in patients undergoing pancreaticoduodenectomy for pancreatic cancer. Pancreas 2011, 40, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Birgin, E.; Hablawetz, P.; Téoule, P.; Rückert, F.; Wilhelm, T.J. Chronic pancreatitis and resectable synchronous pancreatic carcinoma: A survival analysis. Pancreatology 2018, 18, 394–398. [Google Scholar] [CrossRef]
- Birgin, E.; Reeg, A.; Téoule, P.; Rahbari, N.N.; Post, S.; Reissfelder, C.; Rückert, F. Early postoperative pancreatitis following pancreaticoduodenectomy: What is clinically relevant postoperative pancreatitis? HPB (Oxford) 2019, 21, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, W.; Gluth, A.; Hinz, U.; Koliogiannis, D.; Strobel, O.; Hackert, T.; Werner, J.; Büchler, M.W. Outcomes after extended pancreatectomy in patients with borderline resectable and locally advanced pancreatic cancer. Br. J. Surg. 2016, 103, 1683–1694. [Google Scholar] [CrossRef]
- Albrecht, R.; Haase, D.; Zippel, R.; Koch, H.; Settmacher, U. Robot-assisted surgery—Progress or expensive toy?: Matched-pair comparative analysis of robot-assisted cholecystectomy vs. laparoscopic cholecystectomy. Chirurg 2017, 88, 1040–1045. [Google Scholar] [CrossRef]
- Deichmann, S.; Bolm, L.R.; Honselmann, K.C.; Wellner, U.F.; Lapshyn, H.; Keck, T.; Bausch, D. Perioperative and Long-term Oncological Results of Minimally Invasive Pancreatoduodenectomy as Hybrid Technique—A Matched Pair Analysis of 120 Cases. Zentralbl. Chir. 2018, 143, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Téoule, P.; Kunz, B.; Schwarzbach, M.; Birgin, E.; Rückert, F.; Wilhelm, T.J.; Niedergethmann, M.; Post, S.; Rahbari, N.N.; Reißfelder, C.; et al. Influence of Clinical pathways on treatment and outcome quality for patients undergoing pancreatoduodenectomy?—A retrospective outcome cohort study. Asian. J. Surg. 2019. [Google Scholar] [CrossRef]
- Téoule, P.; Römling, L.; Schwarzbach, M.; Birgin, E.; Rückert, F.; Wilhelm, T.J.; Niedergethmann, M.; Post, S.; Rahbari, N.N.; Reißfelder, C.; et al. Clinical Pathways For Pancreatic Surgery: Are They A Suitable Instrument For Process Standardization To Improve Process And Outcome Quality Of Patients Undergoing Distal And Total Pancreatectomy?—A Retrospective Cohort Study. Ther. Clin. Risk. Manag. 2019, 15, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Saklad, M. Grading of Patients for Surgical Procedures. Anesthesiology 1941, 2, 281–284. [Google Scholar] [CrossRef]
- WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [CrossRef]
- Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253.
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [Google Scholar] [CrossRef]
- Bassi, C.; Dervenis, C.; Butturini, G.; Fingerhut, A.; Yeo, C.; Izbicki, J.; Neoptolemos, J.; Sarr, M.; Traverso, W.; Buchler, M.; et al. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery 2005, 138, 8–13. [Google Scholar] [CrossRef]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy hemorrhage (PPH): An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Bertuccio, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2014. Ann. Oncol. 2014, 25, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.E.; Chien, M.W.; Earle, C.C. Prognostic factors following curative resection for pancreatic adenocarcinoma: A population-based, linked database analysis of 396 patients. Ann. Surg. 2003, 237, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Sohn, T.A.; Yeo, C.J.; Cameron, J.L.; Koniaris, L.; Kaushal, S.; Abrams, R.A.; Sauter, P.K.; Coleman, J.; Hruban, R.H.; Lillemoe, K.D. Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. J. Gastrointest. Surg. 2000, 4, 567–579. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Gleisner, A.L.; Cameron, J.L.; Winter, J.M.; Assumpcao, L.; Lillemoe, K.D.; Wolfgang, C.; Hruban, R.H.; Schulick, R.D.; Yeo, C.J.; et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007, 141, 610–618. [Google Scholar] [CrossRef]
- Riediger, H.; Keck, T.; Wellner, U.; zur Hausen, A.; Adam, U.; Hopt, U.T.; Makowiec, F. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J. Gastrointest. Surg. 2009, 13, 1337–1344. [Google Scholar] [CrossRef]
- Stark, A.P.; Sacks, G.D.; Rochefort, M.M.; Donahue, T.R.; Reber, H.A.; Tomlinson, J.S.; Dawson, D.W.; Eibl, G.; Hines, O.J. Long-term Survival in Patients with Pancreatic Ductal Adenocarcinoma. Surgery 2016, 159, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Greenwood, D.C.; Chan, D.S.M.; Vieira, R.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Cade, J.E.; Burley, V.J.; Norat, T. Body mass index, abdominal fatness and pancreatic cancer risk: A systematic review and non-linear dose-response meta-analysis of prospective studies. Ann. Oncol. 2012, 23, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Muller, M.K.; Weber, M.; Clavien, P.-A. Obesity in general elective surgery. Lancet 2003, 361, 2032–2035. [Google Scholar] [CrossRef]
- Lunevicius, R.; Nakanishi, H.; Ito, S.; Kozaki, K.; Kato, T.; Tatematsu, M.; Yasui, K. Clinicopathological significance of fibrotic capsule formation around liver metastasis from colorectal cancer. J. Cancer Res. Clin. Oncol. 2001, 127, 193–199. [Google Scholar] [CrossRef]
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016, 6, 852–869. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Harvie, M.; Cutress, R.I.; Leitzmann, M.; Pischon, T.; Howell, S.; Howell, A. How to Manage the Obese Patient With Cancer. JCO 2016, 34, 4284–4294. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-H.; Eibl, G. Obesity-Induced Adipose Tissue Inflammation as a Strong Promotional Factor for Pancreatic Ductal Adenocarcinoma. Cells 2019, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Nazzani, S.; Preisser, F.; Mazzone, E.; Tian, Z.; Mistretta, F.A.; Shariat, S.F.; Saad, F.; Graefen, M.; Tilki, D.; Montanari, E.; et al. In-hospital length of stay after major surgical oncological procedures. Eur. J. Surg. Oncol. 2018, 44, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.W.; Friess, H.; Kleeff, J.; Dahmen, R.; Wagner, M.; Hinz, U.; Breisch-Girbig, D.; Ceyhan, G.O.; Büchler, M.W. Is there still a role for total pancreatectomy? Ann. Surg. 2007, 246, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Fisher, W.E.; Hodges, S.E.; Wu, M.-F.; Hilsenbeck, S.G.; Brunicardi, F.C. Assessment of the learning curve for pancreaticoduodenectomy. Am. J. Surg. 2012, 203, 684–690. [Google Scholar] [CrossRef]
- Richter, A.; Niedergethmann, M.; Sturm, J.W.; Lorenz, D.; Post, S.; Trede, M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J. Surg. 2003, 27, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, I.; Lion, E.; Hetjens, S.; Vassilev, G.; Galata, C.; Reissfelder, C.; Otto, M. Trocar Site HERnias after Bariatric Laparoscopic Surgery (HERBALS): A Prospective Cohort Study. Obes. Surg. 2020, 30, 1820–1826. [Google Scholar] [CrossRef]
| Variables | BMI < 30 kg/m2 n = 76 (%) | BMI ≥ 30 kg/m2 n = 76 (%) | p-Value |
|---|---|---|---|
| Age, years * | 68 (9) | 68 (9) | ≥0.99 |
| Sex ratio (M:F) | 38:38 | 38:38 | ≥0.99 |
| BMI * | 25 (3) | 33 (4) | 0.001 |
| BMI 30–35 kg/m2 | - | 64 (84) | |
| BMI 35–40 kg/m2 | - | 8 (10) | |
| BMI ≤ 40 kg/m2 | - | 4 (6) | |
| ASA | ≥0.99 | ||
| I | 25 (33) | 25 (33) | |
| II | 18 (24) | 18 (24) | |
| III | 33 (43) | 33 (43) | |
| Cardiac comorbidity | 29 (38) | 25 (33) | 0.611 |
| Pulmonary comorbidity | 10 (13) | 17 (22) | 0.202 |
| Arterial hypertension | 36 (47) | 56 (74) | 0.002 |
| Diabetes mellitus | 28 (37) | 37 (49) | 0.189 |
| History of chronic pancreatitis | 9 (12) | 10 (13) | ≥0.99 |
| Preoperative stenting | 4 (6) | 4 (6) | ≥0.99 |
| Preoperative blood values * | |||
| Albumin (g/L) | 26 (14) | 27 (13) | 0.776 |
| Bilirubin (mg/dL) | 5.3 (5.9) | 5.6 (6.2) | 0.758 |
| Creatinine (mg/dL) | 0.9 (0.3) | 0.9 (0.2) | 0.313 |
| C-reactive protein (mg/dL) | 16 (23) | 22 (40) | 0.304 |
| Hemoglobin (g/dL) | 12.8 (1.6) | 12.7 (1.4) | 0.583 |
| Platelets (10E9/L) | 275 (76) | 255 (107) | 0.407 |
| International normalized ratio | 1.0 (0.1) | 1.0 (0.1) | 0.869 |
| Adjuvant Chemotherapy | 44 (58) | 46 (61) | 0.330 |
| Chemotherapeutic regimen | 0.232 | ||
| Single agent | 37 (49) | 36 (47) | |
| Double agents | 5 (7) | 2 (3) | |
| Triple agents | 1 (1) | 4 (5) | |
| X | 1 (1) | 4 (5) | |
| Additional targeted therapy | 4 (5) | 2 (3) | 0.431 |
| Radiation Therapy | 1 (1) | 1 (1) | 0.360 |
| Radiochemotherapy | 3 (4) | 1 (1) | ≥0.99 |
| Variables | BMI < 30 kg/m2 n = 76 (%) | BMI ≥ 30 kg/m2 n = 76 (%) | p-Value |
|---|---|---|---|
| Operating time (min) * | 333 (100) | 360 (104) | 0.108 |
| Blood loss (mL) * | 809 (607) | 1050 (760) | 0.039 |
| Type of surgery | ≥0.99 | ||
| Whipple | 8 (11) | 9 (12) | |
| PPPD | 48 (63) | 48 (63) | |
| Total pancreatectomy | 4 (5) | 6 (8) | |
| Distal pancreatectomy | 8 (11) | 8 (11) | |
| Palliative Bypass | 6 (8) | 5 (7) | |
| Vascular resection | 38 (50) | 37 (52) | ≥0.99 |
| Perioperative blood transfusion | 17 (22) | 23 (30) | 0.357 |
| T classification | 0.705 | ||
| 1 | 1 (1) | 3 (4) | |
| 2 | 6 (8) | 9 (12) | |
| 3 | 59 (78) | 57 (75) | |
| 4 | 5 (7) | 4 (5) | |
| X | 5 (7) | 2 (3) | |
| Nodal status | 0.150 | ||
| 0 | 24 (32) | 22 (29) | |
| 1 | 44 (58) | 49 (64) | |
| 2 | 2 (3) | 0 (0) | |
| X | 6 (8) | 5 (7) | |
| M status | 0.882 | ||
| 0 | 71 (93) | 71 (93) | |
| 1 | 4 (5) | 5 (7) | |
| X | 1 (1) | 0 (0) | |
| Grading | 0.748 | ||
| 1 | 1 (1) | 2 (3) | |
| 2 | 41 (54) | 37 (49) | |
| 3 | 25 (33) | 31 (41) | |
| 4 | 1 (1) | 0 (0) | |
| X | 8 (11) | 6 (8) | |
| Resection status | 0.344 | ||
| 0 | 51 (67) | 59 (78) | |
| 1 | 15 (20) | 9 (12) | |
| 2 | 1 (1) | 3 (4) | |
| X | 10 (13) | 5 (7) | |
| Perineural invasion | 0.033 | ||
| 0 | 21 (28) | 9 (12) | |
| 1 | 32 (42) | 40 (53) | |
| x | 23 (30) | 27 (36) | |
| Lymphatic invasion | 0.570 | ||
| 0 | 22 (29) | 17 (22) | |
| 1 | 32 (42) | 38 (50) | |
| X | 22 (29) | 21(28) | |
| Vascular invasion | ≥0.99 | ||
| 0 | 39 (51) | 40 (53) | |
| 1 | 30 (39) | 31(41) | |
| X | 6 (8) | 5 (7) |
| Variables | BMI < 30 kg/m2 n = 76 (%) | BMI ≥ 30 kg/m2 n = 76 (%) | p-Value |
|---|---|---|---|
| Clavien–Dindo Classification | 0.645 | ||
| Grade I | 14 (18) | 10 (13) | |
| Grade II | 15 (20) | 12 (16) | |
| Grade III | 6 (8) | 13 (17) | |
| Grade IV | 2 (3) | 2 (3) | |
| Grade V (death) | 4 (5) | 5 (7) | |
| 30-day mortality rate | 4 (5) | 4 (5) | ≥0.99 |
| Clinically-relevant complications | 12 (16) | 20 (26) | 0.163 |
| Surgical site infection | 7 (9) | 5 (7) | 0.765 |
| Burst abdomen | 1 (1) | 2 (3) | 0.559 |
| Invasive interventions | 4 (5) | 8 (11) | 0.368 |
| Endoscopic intervention | 2 (3) | 5 (7) | 0.442 |
| Radiologic intervention | 2 (3) | 4 (5) | 0.681 |
| DGE | 0.558 | ||
| Grade B/C | 5 (7) | 8 (11) | |
| POPF | 0.582 | ||
| Grade B/C | 6 (8) | 8 (11) | |
| PPH | 0.360 | ||
| Grade B/C | 3 (4) | 1 (1) | |
| Length of hospital stay (d) * | 15 (13–20) | 16 (13–25) | 0.201 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Téoule, P.; Rasbach, E.; Oweira, H.; Otto, M.; Rahbari, N.N.; Reissfelder, C.; Rückert, F.; Birgin, E. Obesity and Pancreatic Cancer: A Matched-Pair Survival Analysis. J. Clin. Med. 2020, 9, 3526. https://doi.org/10.3390/jcm9113526
Téoule P, Rasbach E, Oweira H, Otto M, Rahbari NN, Reissfelder C, Rückert F, Birgin E. Obesity and Pancreatic Cancer: A Matched-Pair Survival Analysis. Journal of Clinical Medicine. 2020; 9(11):3526. https://doi.org/10.3390/jcm9113526
Chicago/Turabian StyleTéoule, Patrick, Erik Rasbach, Hani Oweira, Mirko Otto, Nuh N. Rahbari, Christoph Reissfelder, Felix Rückert, and Emrullah Birgin. 2020. "Obesity and Pancreatic Cancer: A Matched-Pair Survival Analysis" Journal of Clinical Medicine 9, no. 11: 3526. https://doi.org/10.3390/jcm9113526
APA StyleTéoule, P., Rasbach, E., Oweira, H., Otto, M., Rahbari, N. N., Reissfelder, C., Rückert, F., & Birgin, E. (2020). Obesity and Pancreatic Cancer: A Matched-Pair Survival Analysis. Journal of Clinical Medicine, 9(11), 3526. https://doi.org/10.3390/jcm9113526






