Long-Term, Health-Related Quality of Life after Open and Robot-Assisted Ivor-Lewis Procedures—A Propensity Score-Matched Study
Abstract
1. Introduction
2. Material and Methods
2.1. Data Collection
2.2. Inclusion Criteria
2.3. Surgical Procedures
2.4. Outcome Measures
2.5. Statistical Analysis
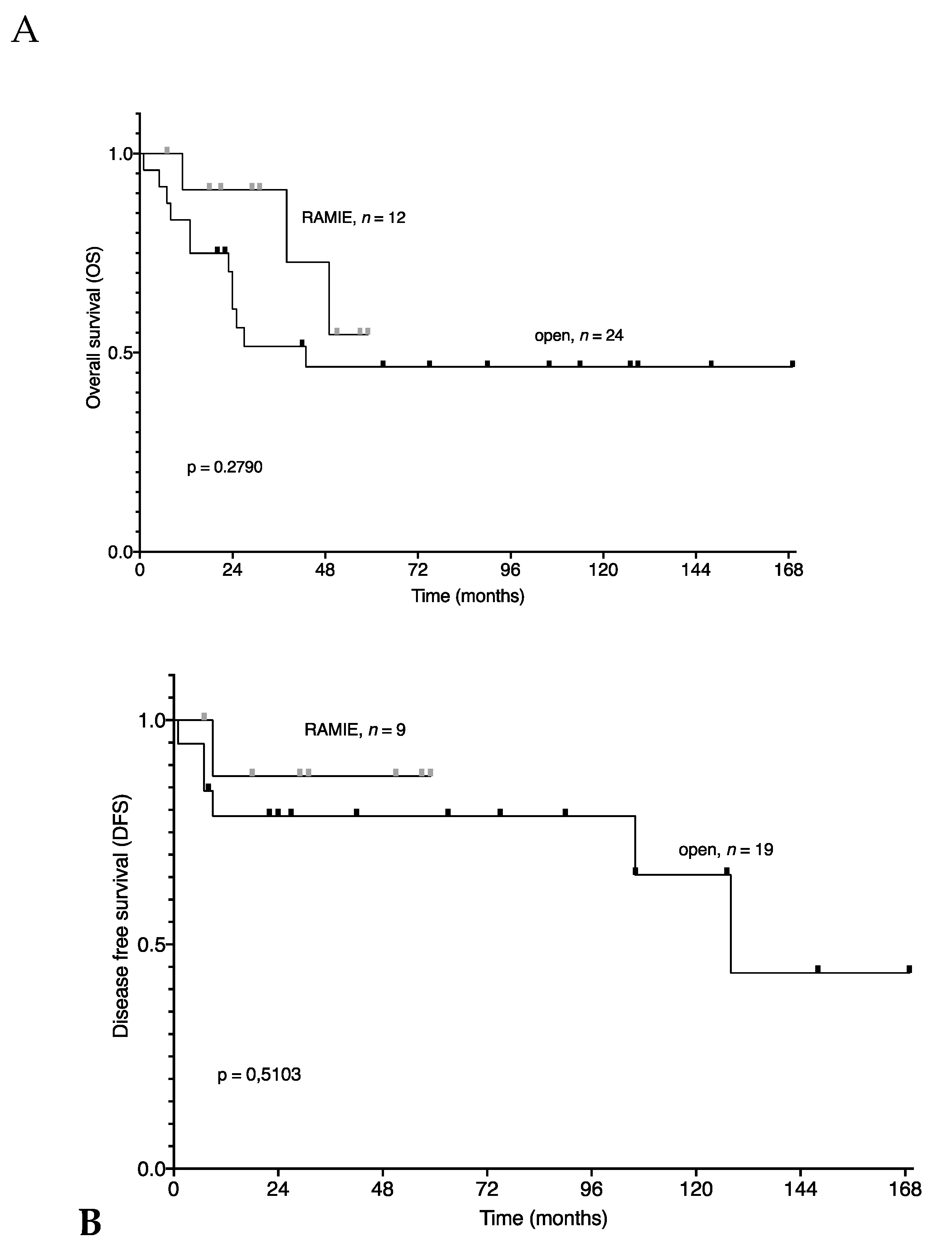
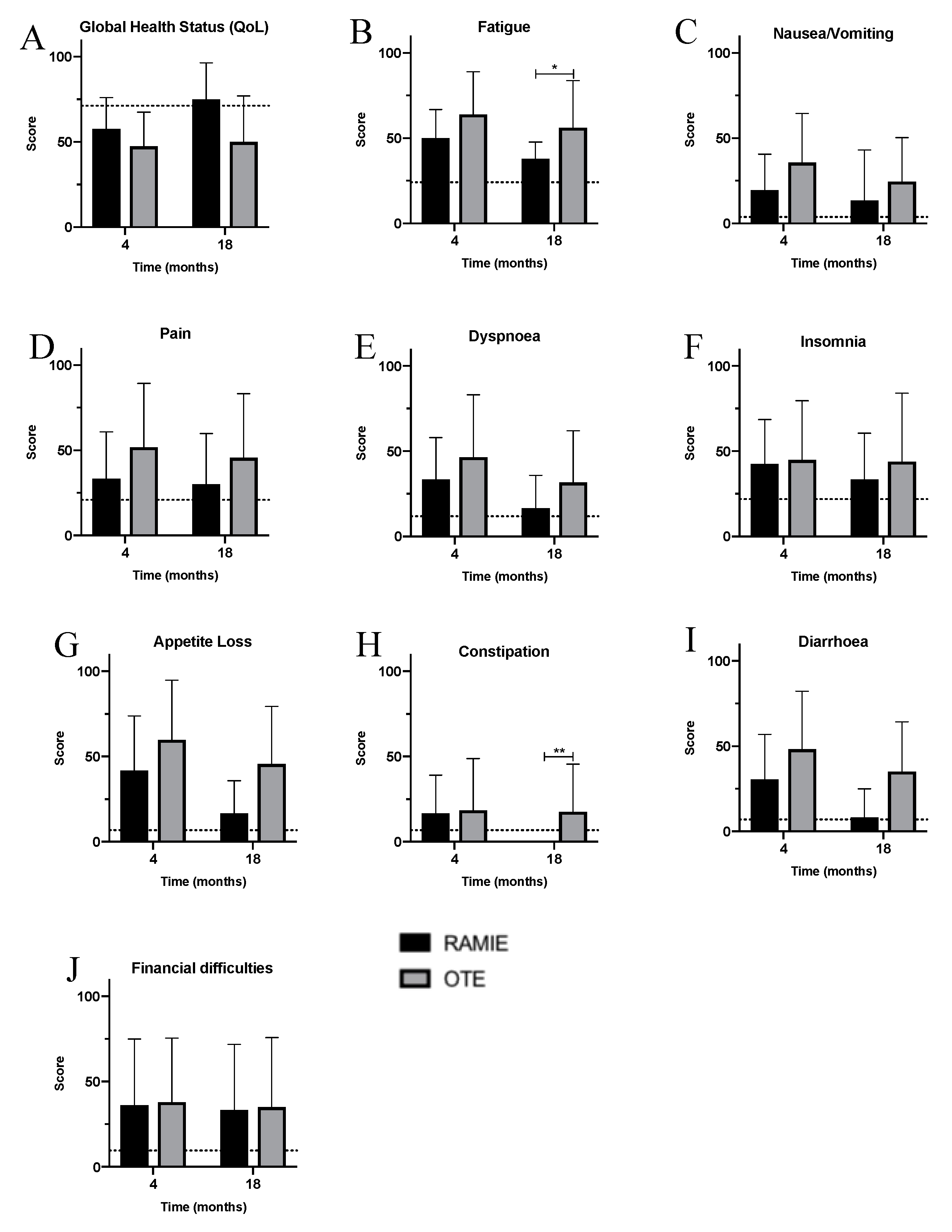
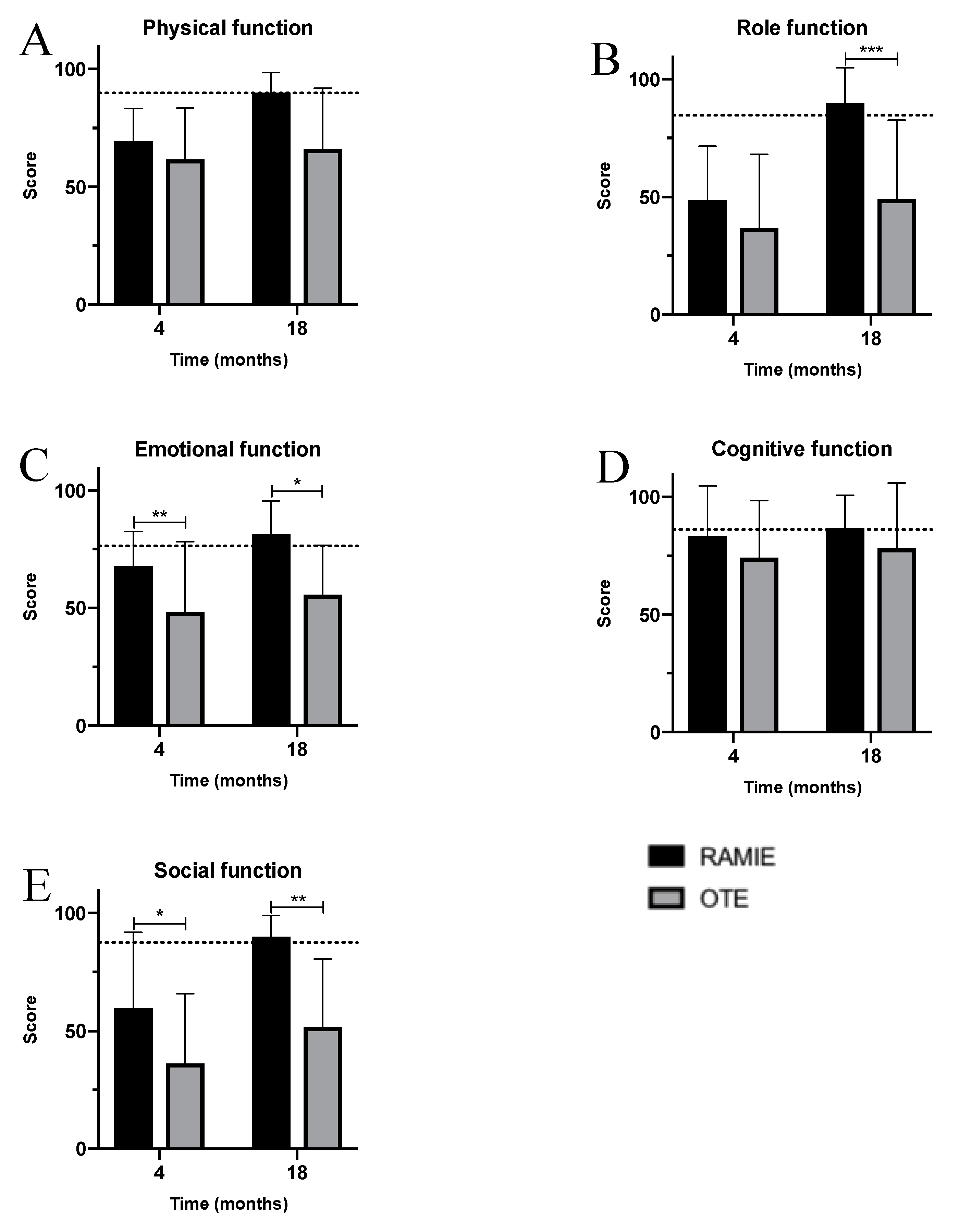
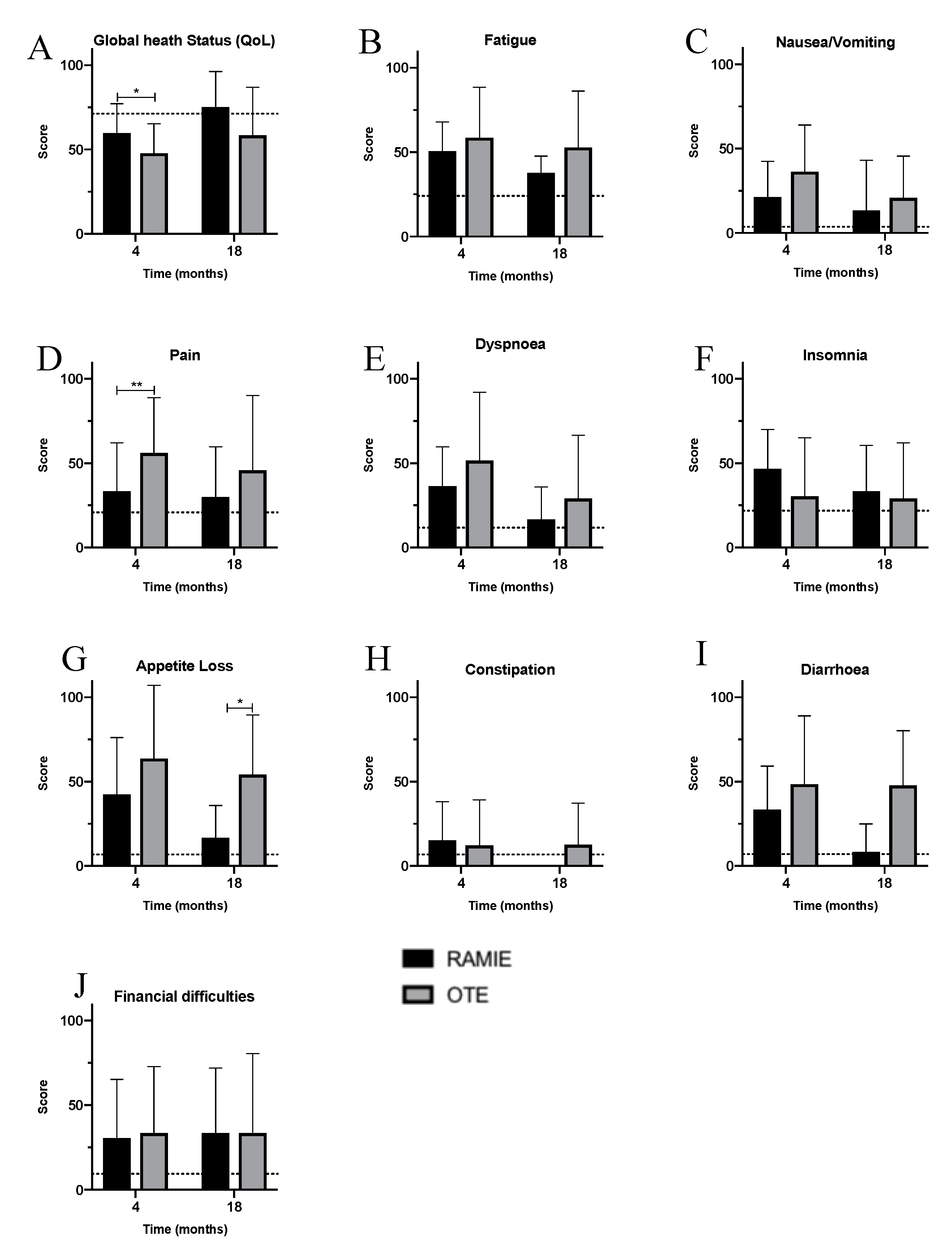
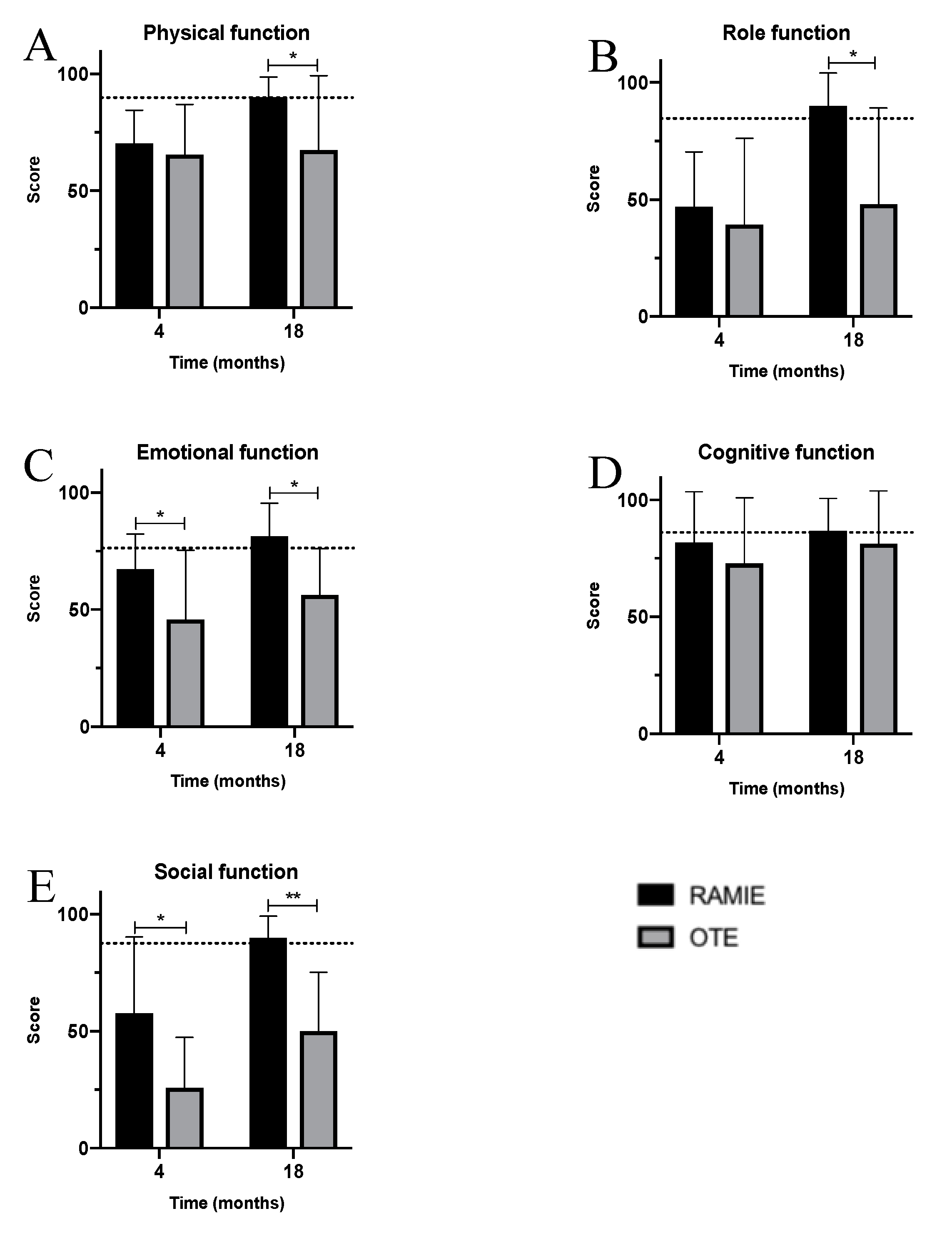
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jezerskyte, E.; Saadeh, L.M.; Hagens, E.R.C.; Sprangers, M.A.G.; Noteboom, L.; van Laarhoven, H.W.M.; Eshuis, W.J.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Long-term quality of life after total gastrectomy versus ivor lewis esophagectomy. World J. Surg. 2020, 44, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Gisbertz, S.S.; Hagens, E.R.C.; Ruurda, J.P.; Schneider, P.M.; Tan, L.J.; Domrachev, S.A.; Hoeppner, J.; van Berge Henegouwen, M.I. The evolution of surgical approach for esophageal cancer. Ann. N. Y. Acad. Sci. 2018, 1434, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Mariette, C.; Markar, S.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D‘Journo, X.B.; Brigand, C.; Perniceni, T.; Carrere, N.; et al. Health-related quality of life following hybrid minimally invasive versus open esophagectomy for patients with esophageal cancer, analysis of a multicenter, open-label, randomized phase iii controlled trial—The MIRO trial. Ann. Surg. 2019, 271, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Yamamoto, H.; Baba, H.; Miyata, H.; Watanabe, M.; Toh, Y.; Matsubara, H.; Kakeji, Y.; Seto, Y. Can minimally invasive esophagectomy replace open esopahgectomy for esophageal cancer? Latest analysis of 24,233 esophagectomies from the japanese national clinical database. Ann. Surg. 2019, 272, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Lewis, I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br. J. Surg. 1946, 34, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, J.P.; van der Horst, S.; May, A.M.; Schippers, C.; Brosens, L.A.A.; Joore, H.C.A.; Kroese, C.C.; Mohammad, N.H.; Mook, S.; Vleggaar, F.P.; et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open tranthoracic esophagectomy for resectable esophageal cancer. Ann. Surg. 2019, 269, 621–630. [Google Scholar] [CrossRef] [PubMed]
- McKeown, K.C. Total three-stage oesophagectomy for cancer of the oesophagus. Br. J. Surg. 1976, 63, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Yoshimura, S.; Yagi, K.; Nishida, M.; Aikou, S.; Yamagata, Y.; Mori, K.; Yamashita, H.; Seto, Y. Long-term health-related quality of life following robot-assisted radical transmediastinal esophagectomy. Surg. Endosc. 2019, 34, 1602–1611. [Google Scholar] [CrossRef]
- Wikman, A.; Ljung, R.; Johar, A.; Hellstadius, Y.; Lagergren, J.; Lagergren, P. Psychiatric morbidity and survival after surgery for esophageal cancer: A population-based cohort study. J. Clin. Oncol. 2015, 33, 448–453. [Google Scholar] [CrossRef]
- Mantoan, S.; Cavallin, F.; Pinto, E.; Saadeh, L.M.; Alfieri, R.; Cagol, M.; Bellissimo, M.C.; Castoro, C.; Scarpa, M. Long-term quality of life after esophagectomy with gastric pull-up. J. Surg. Oncol. 2017, 117, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Weksler, B.; Sullivan, J.L. Survival after esophagectomy: A propensity-matched study of different surgical approaches. Ann. Thorac. Surg. 2017, 104, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Briez, N.; Piessen, G.; Bonnetain, F.; Brigand, C.; Carrere, N.; Collet, D.; Doddoli, C.; Flamein, R.; Mabrut, J.Y.; Meunier, B.; et al. Open versus laparoscopically-assisted oesophagectomy for cancer: A multicentre randomised controlled phase III trial—The MIRO trial. BMC Cancer 2011, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Derogar, M.; Lagergren, P. Health-related quality of life among 5-year survivors of esophageal cancer surgery: A prospective population-based study. J. Clin. Oncol. 2012, 30, 413–418. [Google Scholar] [CrossRef]
- Taioli, E.; Schwartz, R.; Lieberman-Cribbin, W.; Moskowitz, G.; van Gerwen, M.; Flores, R. Quality of life after open or minimally invasive esophagectomy in patients with esophageal cancer—A systematic review. Semin. Thorac. Cardiovasc. Surg. 2017, 29, 377–390. [Google Scholar] [CrossRef]
- Maas, K.W.; Cuesta, M.; van Berge Henegouwen, M.I.; Roig, J.; Bonavina, L.; Rosman, C.; Gisbertz, S.S.; Biere, S.S.A.Y.; van der Peet, D.L. Quality of life and late complications after minimally invasive compared to open esophagectomy: Results of a randomized trial. World J. Surg. 2015, 39, 1986–1993. [Google Scholar] [CrossRef]
- Asti, E.; Bernardi, D.; Sozzi, M.; Bonavina, L. Minimally invasive esophagectomy for Barrett’s adenocarcinoma. Transl. Gastroenterol. Hepatol. 2018, 3, 1–7. [Google Scholar] [CrossRef]
- Na, K.J.; Park, S.; Park, I.K.; Kim, Y.T.; Kang, C.H. Outcomes after total robotic esophagectomy for esophageal cancer: A propensity-matched comparison with hybrid robotic esophagectomy. J. Thorac. Dis. 2019, 11, 5310–5320. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Li, B.; Li, Z.; Sun, Y.; Mao, T.; Hua, R.; Yang, Y.; Guo, X.; He, Y.; et al. Robot-assisted esophagectomy (RAE) versus conventional minimally invasive esophagectomy (MIE) for resectable esophageal squamous cell carcinoma: Protocol for a multicenter prospective randomized controlled trial (RAMIE trial, robot-assisted minimally invasive Esophagectomy). BMC Cancer 2019, 19, 1–8. [Google Scholar]
- Kernstine, K.H.; Dearmond, D.; Karimi, M.; Van Natta, T.L.; Campos, J.C.; Yoder, M.R.; Everett, J.E. The robotic, 2-stage, 3-field esophagolymphadenectomy. J. Thorac. Cardiovasc. Surg. 2004, 127, 1847. [Google Scholar] [CrossRef]
- Egberts, J.H.; Schniewind, B.; Bestmann, B.; Schafmayer, C.; Egberts, F.; Faendrich, F.; Kuechler, T.; Tepel, J. Impact of the site of anastomosis after oncologic esophagectomy on quality of life—A prospective, longitudinal outcome study. J. Surg. Oncol. 2008, 15, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Egberts, J.H.; Stein, H.; Aselmann, H.; Hendricks, H.; Becker, T. Fully robotic da Vinci Ivor–Lewis esophagectomy in four-arm technique—Problems and solutions. Dis. Esophagus 2017, 30, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Egberts, J.H.; Biebl, M.; Perez, D.R.; Mees, S.T.; Grimminger, O.O.; Müller-Stich, B.P.; Stein, H.; Fuchs, H.; Bruns, C.J.; Hackert, T.; et al. Robot-assisted oesophagectomy: Recommendations towards a standardised ivor lewis procedure. J. Gastroenterol. Surg. 2019, 23, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Kröll, D.; Borbély, Y.M.; Dislich, B.; Haltmeier, T.; Malinka, T.; Biebl, M.; Langer, R.; Candinas, D.; Seiler, C. Favourable long-term survival of patients with esophageal cancer treated with extended transhiatal esophagectomy combined with en bloc lymphadenectomy: Results from a retrospective observational cohort study. BMC Cancer 2020, 20, 1–10. [Google Scholar]
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’Journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking complications associated with esophagectomy. Ann. Surg. 2019, 269, 291–298. [Google Scholar] [CrossRef]
- Linder, G.; Jestin, C.; Sundbom, M.; Hedberg, J. Safe introduction of minimally invasive esophagectomy at a medium volume center. SJS 2020, 109, 121–126. [Google Scholar] [CrossRef]
- Carroll, P.; Jacob, N.; Yeung, J.C.; Darling, G.E. Using benchmarking standards to evaluate trainsition to minimally invasive esophagectomy. Ann. Thorac. Surg. 2020, 109, 383–388. [Google Scholar] [CrossRef]
- Schmidt, H.M.; Gisbertz, S.S.; Moons, J.; Rouvelas, I.; Kauppi, J.; Brown, A.; Asti, E.; Luyer, M.; Lagarde, S.M.; Berlth, F.; et al. Defining benchmarks for transthoracic esophagectomy: A multicenter analysis of total minimally invasive esophagectomy in low risk patients. Ann. Surg. 2017, 266, 814–821. [Google Scholar] [CrossRef]
- Mariette, C.; Markar, S.R.; Dabakuyo-Yonli, T.S.; Pharm, D.; Meunier, B.; Pezet, D.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N. Engl. J. Med. 2019, 380, 152–162. [Google Scholar] [CrossRef]
- Biere, S.S.; van Berge Henegouwen, M.I.; Maas, K.W.; Bonavina, L.; Rosman, C.; Garcia, J.R.; Gisbertz, S.S.; Klinkenbijl, J.H.; Hollmann, M.W.; de Lange, E.S.; et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A mul-ticentre, open-label, randomised controlled trial. Lancet 2012, 379, 1887–1922. [Google Scholar] [CrossRef]
- Sarkaria, I.S.; Rizk, N.P.; Goldman, D.A.; Sima, C.; Tan, K.S.; Bains, M.S.; Adusumilli, P.S.; Molena, D.; Bott, M.; Atkinson, T.; et al. Early quality of life outcomes after robotic-assisted minimally invasive and open esophagectomy. Ann. Thorac. Surg. 2019, 108, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Na, K.J.; Chang, H.K. Current issues in minimally invasive esophagectomy. Korean J. Thorac. Cardiovasc. Surg. 2020, 53, 152–159. [Google Scholar] [CrossRef]
- Schröder, W.; Raptis, D.A.; Schmidt, H.M.; Gisbertz, S.S.; Moons, J.; Asti, E.; Luyer, M.; Hölscher, A.H.; Schneider, P.M.; van Berge Henegouwen, M.I.; et al. Anastomotic Techniques and Associated Morbidity in Total Minimally Invasive Transthoracic Esophagectomy Results From the EsoBenchmark Database. Ann. Surg. 2019, 270, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Derogar, M.; Orsini, N.; Sadr-Azodi, O.; Lagergren, P. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J. Clin. Oncol. 2012, 30, 1615–1619. [Google Scholar] [CrossRef] [PubMed]
- Noordman, B.J.; der Bekker-Grob, E.W.; Coene, P.P.L.O.; van der Harst, E.; Lagarde, S.M.; Shapiro, J.; Wijnhoven, B.P.L.; van Lanschot, J.J.B. Patients’ preferences for treatment after neoadjuvant chemoradiotherapy for oesophageal cancer. BJS 2018, 105, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Bulamu, B.N.; Chen, G.; Ratcliff, J.; Schloite, A.; Brigth, T.; Watson, D.I. Health-related quality of life associated with barrett’s esophagus and cancer. World J. Surg. 2019, 43, 1554–1562. [Google Scholar] [CrossRef]
- Sunde, B.; Klevebro, F.; Johar, A.; Johnsen, G.; Jacobsen, A.B.; Glenjen, N.I.; Friesland, S.; Lindblad, M.; Ajengui, A.; Lundell, L.; et al. Health-related quality of life in a randomized trial of neoadjuvant chemotherapy or chemoradiotherapy plus surgery in patients with oesophageal cancer (NeoRes trial). Br. J. Surg. 2019, 106, 1452–1463. [Google Scholar] [CrossRef]
- Gaspar, L.E.; Winter, K.; Kocha, W.I.; Pinover, W.H.; Herskovic Graham, M.; Gunderson, L. Swallowing function and weight change observed in a phase I/II study of external-beam radiation, brachytherapy and concurrent chemotherapy in localized cancer of the esophagus (RTOG 9207). Cancer J. 2001, 7, 388–394. [Google Scholar]
- Lai, A.; Lipka, A.; Kumar, A.; Sethi, S.; Bromberg, D.; Li, N.; Shen, H.; Stefaniwsky, L.; Brady, P. Role of esophageal metal stents placement and combination therapy in inoperable esophageal carcinoma: A systematic review and meta-analysis. Dig. Dis. Sci. 2018, 63, 1025–1034. [Google Scholar] [CrossRef]
- Noordman, B.J.; Verdam, M.; Onstenk, B.; Heisterkamp, J.; Jansen, W.J.B.; Martijnse, I.S.; Lagarde, S.M.; Wijnhoven, B.P.L.; Acosta, C.M.M.; van der Gaast, A.; et al. Quality of life during and after completion of neoadjuvant chemoradiotherapy for esophageal and junctional cancer. Ann. Surg. Oncol. 2019, 26, 4765–4772. [Google Scholar] [CrossRef]
- Noordman, B.J.; Wijnhoven, B.; Lagarde, S.M.; Biermann, K.; van der Gaast, A.; Spaander, M.C.W.; Valkema, F.; van Lanschot, J.J.B. Active surveillance in clinically complete responders after neoadjuvant chemoradiotherapy for esophageal or junctional cancer. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schandl, A.; Lagergren, J.; Johar, A.; Lagergren, P. Health-related quality of life 10 years after oesophageal cancer surgery. Eur. J. Cancer 2016, 69, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Brierley, R.C.; Gaunt, D.; Metcalfe, C.; Blazeby, J.M.; Blencowe, N.S.; Jepson, M.; Berrisford, R.G.; Avery, K.N.L.; Hollingworth, W.; Rice, C.T.; et al. Laparoscopically assisted versus open oesophagectomy for patients with oesophageal cancer—The Randomised Oesophagectomy: Minimally Invasive or Open (ROMIO) study: Protocol for a randomised controlled trial (RCT). BMJ Open 2019, 1–8. [Google Scholar] [CrossRef]
- Hauser, C.; Patett, C.; von Schoenfels, W.; Heits, N.; Schafmayer, C.; Malchow, B.; Hampe, J.; Schniewind, B.; Becker, T.; Egberts, J.H. Does neoadjuvant treatment before oncologic esophagectomy affect the postoperative quality of life? A prospective, longitudinal outcome study. Dis. Esophagus 2015, 28, 625–659. [Google Scholar] [CrossRef]
- Schwarz, R.; Hinz, A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur. J. Cancer 2001, 37, 1345–1351. [Google Scholar] [CrossRef]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. EORTC Quality of Life Group. In The EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2001; pp. 365–376. [Google Scholar]
- Franke, F.; Moeller, T.; Mehdorn, A.S.; Beckmann, J.H.; Becker, T.; Egberts, J.H. Ivor-Lewis esophagectomy: A standardized operative technique in 11 steps. Int. J. Med Robot. Comput. Assist. Surg. 2020. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Heits, N.; Bernsmeier, A.; Reichert, B.; Hauser, C.; Hendricks, A.; Seifert, D.; Richter, F.; Schafmayer, C.; Ellrichmann, M.; Schniewind, B.; et al. Long-term quality of life after endovac-therapy in anastomotic leakages after esophagectomy. J. Thorac. Dis. 2018, 10, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Elshiekh, M.A.F.; Lo, T.T.H.; Shipolini, A.R.; McCormack, D.J. Does muscle-sparing thoracotomy as opposed to posterolateral thoracotomy result in better recovery? Interact. Cardiovasc. Thorac. Surg. 2013, 16, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Tran, T.; Mayo, N.E.; Carli, F.; Feldman, L.S. What does it really mean to ‘‘recover’’ from an operation? Surgery 2014, 155, 211–216. [Google Scholar] [CrossRef]
- Anandavadivelan, P.; Wikman, A.; Johar, A.; Lagergren, P. Impact of weight loss and eating difficulties on health-related quality of life up to 10 years after oesophagectomy for cancer. Br. J. Surg. 2018, 105, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, Z.; Zhang, T.; Fang, S.; Zhang, S.; Shen, G.; Wu, M. Long-term outcomes of 530 esophageal squamous cell carcinoma patients with minimally invasive Ivor Lewis esophagectomy. J. Surg. Oncol. 2018, 117, 1–13. [Google Scholar]
- Klevebro, F.; Kauppila, J.H.; Markar, S.; Johar, A.; Lagergren, P. Health-related quality of life following total minimally invasive, hybrid minimally invasive or open oesophagectomy: A population-based cohort study. BJS 2020. [Google Scholar] [CrossRef]
- Scarpa, M.; Saadeh, L.; Fasolo, A.; Alfieri, R.; Cagol, M.; Cavallin, F.; Pinto, E.; Zaninotto, G.; Ancona, E.; Castoro, C. Health-related quality of life in patients with oesophageal cancer: Analysis at different steps of the treatment pathway. J. Gastrointest. Surg. 2013, 17, 421–433. [Google Scholar] [CrossRef]
- Rutegard, M.; Lagergren, J.; Rouvelas, I.; Lindblad, M.; Blazeby, J.M.; Lagergren, P. Population-based study of surgical factors in relation to health-related quality of life after oesophageal cancer resection. BJS 2008, 95, 592–601. [Google Scholar] [CrossRef]
- Kauppila, J.H.; Johar, A.; Lagegreen, P. Postoperative complications and health-related quality of life 10 years after esophageal cancer surgery. Ann. Surg. 2020, 271, 311–317. [Google Scholar] [CrossRef]
- Kauppila, J.H.; Johar, A.; Lagegreen, P. Medical and surgical complications and health-related quality of life after esophageal cancer surgery. Ann. Surg. 2020, 271, 502–509. [Google Scholar] [CrossRef]

| RAMIE (n = 12) | OTE (n = 29) | р-Value | |
|---|---|---|---|
| Age (years), mean ± SD (range) | 64.5 ± 9.1 (50–75) | 61.5 ± 8.2 (51–81) | 0.332 a |
| Gender, % male | 83.3 | 86.2 | 0.813 b |
| BMI (kg/m2), median ± SD (range) | 26.5 ± 4.6 (21.6–34.0) | 28.3 ± 4.5 (19.8–44.2) | 0.264 a |
| Comorbidities, % | |||
| Arterial hypertension | 25.0 | 55.2 | 0.078 b |
| Coronary heart disease | 16.7 | 6.9 | 0.337 b |
| Myocardial infarction | 0 | 0 | |
| Heart failure | 8.3 | 6.9 | 0.872 b |
| Diabetes | 0 | 17.2 | 0.965 b |
| COPD | 16.7 | 10.3 | 0.574 b |
| Alcohol consumption | 8.3 | 6.9 | 0.872 b |
| Smoking | 41.7 | 24.1 | 0.262 b |
| ASA-classification, % | 0.136 b | ||
| I | 0 | 0 | |
| II | 25.0 | 50.0 | |
| III | 66.7 | 50.0 | |
| IV | 8.3 | 0 | |
| Additional treatment, % | |||
| Neoadjuvant chemotherapy | 75.0 | 62.1 | 0.472 b |
| Neoadjuvant radiotherapy | 16.7 | 10.3 | 0.574 b |
| Adjuvant chemotherapy | 58.3 | 40.0 | 0.295 b |
| Adjuvant radiotherapy | 8.3 | 7.1 | 0.896 b |
| RAMIE (n = 12) | OTE (n = 29) | p-Value | |
|---|---|---|---|
| Total time of surgery (min), mean ± SD (range) | 357.8 ± 86.7 (232–524) | 394.5 ± 101.1 (245–589) | 0.278 a |
| Type of anastomosis, % | 0.014 b | ||
| End-to-end | 8.3 | 7.1 | |
| End-to-side | 33.3 | 78.6 | |
| Hand-sewn | 58.3 | 14.3 | |
| Postoperative complications, % | |||
| 0 | 58.3 | 62.1 | |
| I | 0 | 27.6 | |
| II | 41.7 | 10.3 | |
| Histopathological results, % | 0.283 (T) 0.317 (N) 0.125 (M) 0.355 (R) TNM 0.430 | ||
| pT0 | 25.0 | 10.3 | |
| pT1 | 0 | 20.7 | |
| pT2 | 25.0 | 20.7 | |
| pT3 | 50.0 | 48.3 | |
| pN0 | 50.0 | 48.3 | |
| pN1 | 25.0 | 37.9 | |
| pN2 | 25.0 | 6.9 | |
| pN3 | 0 | 6.9 | |
| pM0 | 100.0 | 82.8 | |
| pM1 | 0 | 17.2 | |
| pR0 | 91.66 | 93.1 | |
| pR1 | 8.33 | 6.9 | |
| Postoperative tumor stage (UICC), % | |||
| 0 | 25.0 | 10.3 | |
| IA | 0 | 17.2 | |
| IB | 0 | 0 | |
| IIA | 16.7 | 6.9 | |
| IIB | 0 | 3.4 | |
| IIIA | 25 | 17.2 | |
| IIIB | 33.3 | 27.6 | |
| IVA | 0 | 3.4 | |
| IVB | 0 | 13.8 | |
| Size of tumor (mm), mean ± SD (min-max) | 31.9 ± 11.7 (17–46) | 20.6 ± 20.9 (2.5–65) | 0.164 a |
| Lymph nodes harvested, n | 31.0 ± 10.0 (20–46) | 18.7 ± 12.1 (7–47) | 0.004 a |
| Positive lymph nodes harvested, n | 1.4 ± 1.9 (1–5) | 1.9 ± 2.7 (1–10) | 0.609 a |
| Length of stay in hospital (days), mean ± SD (min-max) | 18.9 ± 8.6 (12–42) | 15.3 ± 3.5 (9–24) | 0.180 a |
| RAMIE (n = 11) | OTE (n = 11) | p-Value | |
|---|---|---|---|
| Age (years), mean ± SD (range) | 64.4 ± 9.5 (48–75) | 63.2 ± 6.0 (51–73) | 0.732 a |
| Gender, % male | 81.8 | 72.7 | 0.611 b |
| BMI (kg/m2), median ± SD (range) | 27.0 ± 4.5 (19.8–29.3) | 27.8 ± 4.3 (23.1–30.9) | 0.651 a |
| Comorbidities (yes), % | |||
| Arterial hypertension | 27.3 | 63.6 | 0.087 b |
| Coronary heart disease | 18.2 | 0 | 0.138 b |
| Heart failure | 0 | 9.1 | 0.306 b |
| Myocardial infarction | 0 | 0 | |
| Diabetes mellitus | 18.2 | 27.3 | 0.611 b |
| COPD | 18.2 | 0 | 0.138 b |
| Alcohol consumption | 9.1 | 9.1 | 1.000 b |
| Smoking | 36.4 | 18.2 | 0.338 b |
| ASA-classification, % | 0.647 b | ||
| I | 0 | 0 | |
| II | 27.3 | 36.4 | |
| III | 72.7 | 63.6 | |
| IV | 0 | 0 | |
| Additional treatment, % | |||
| Neoadjuvant chemotherapy | 72.7 | 72.7 | 1.000 b |
| Neoadjuvant radiotherapy | 18.2 | 9.1 | 0.534 b |
| Adjuvant chemotherapy | 54.5 | 25.0 | 0.198 b |
| Adjuvant radiotherapy | 9.1 | 0 | 0.329 b |
| RAMIE (n = 11) | OTE (n = 11) | p-Value | |
|---|---|---|---|
| Total time of surgery (min), mean ± SD (range) | 357.7 ± 91.0 (232–524) | 369.4 ± 83.1 (205–460) | 0.757 a |
| Type of anastomosis, % | 0.190 b | ||
| End-to-end | 9.1 | 10.0 | |
| End-to-side | 36.4 | 90.0 | |
| Hand-sewn | 54.5 | 0 | |
| Type of stapler, % | |||
| Circular stapler | 45.5 | 100 | 0.004b |
| Stapler | 54.5 | ||
| Postoperative complications, % | |||
| 0 | 54.5 | 54.5 | |
| I | 0 | 27.3 | |
| II | 45.5 | 18.3 | |
| Postoperative tumor stage (UICC), % | 0.881 (T) 0.330 (N) 0 (M) 0.384 (R) TNM 0.952 | ||
| 0 | 27.3 | 27.3 | |
| I A | 0 | 0 | |
| I B | 0 | 0 | |
| II A | 18.2 | 18.2 | |
| II B | 0 | 0 | |
| III A | 27.3 | 36.4 | |
| III B | 27.3 | 18.2 | |
| IV A | 0 | 0 | |
| IV B | 0 | 0 | |
| Size of tumor (mm), mean ± SD (min-max) | 31.8 ± 11.7 (7–48) | 16.0 ± 22.24 (3–60) | 0.156 a |
| Lymph nodes harvested, n | 29.9 ± 9.8 (19–46) | 18.1 ± 13.8 (4–40) | 0.031 a |
| Tumor-positive lymph nodes harvested, n | 1.3 ± 2.0 (1–10) | 1.8 ± 3.3 (2–5) | 0.640 a |
| Length of stay in hospital (days), mean ± SD (min-max) | 19.3 ± 8.9 (12–42) | 14.2 ± 2.4 (9–17) | 0.082 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdorn, A.-S.; Möller, T.; Franke, F.; Richter, F.; Kersebaum, J.-N.; Becker, T.; Egberts, J.-H. Long-Term, Health-Related Quality of Life after Open and Robot-Assisted Ivor-Lewis Procedures—A Propensity Score-Matched Study. J. Clin. Med. 2020, 9, 3513. https://doi.org/10.3390/jcm9113513
Mehdorn A-S, Möller T, Franke F, Richter F, Kersebaum J-N, Becker T, Egberts J-H. Long-Term, Health-Related Quality of Life after Open and Robot-Assisted Ivor-Lewis Procedures—A Propensity Score-Matched Study. Journal of Clinical Medicine. 2020; 9(11):3513. https://doi.org/10.3390/jcm9113513
Chicago/Turabian StyleMehdorn, Anne-Sophie, Thorben Möller, Frederike Franke, Florian Richter, Jan-Niclas Kersebaum, Thomas Becker, and Jan-Hendrik Egberts. 2020. "Long-Term, Health-Related Quality of Life after Open and Robot-Assisted Ivor-Lewis Procedures—A Propensity Score-Matched Study" Journal of Clinical Medicine 9, no. 11: 3513. https://doi.org/10.3390/jcm9113513
APA StyleMehdorn, A.-S., Möller, T., Franke, F., Richter, F., Kersebaum, J.-N., Becker, T., & Egberts, J.-H. (2020). Long-Term, Health-Related Quality of Life after Open and Robot-Assisted Ivor-Lewis Procedures—A Propensity Score-Matched Study. Journal of Clinical Medicine, 9(11), 3513. https://doi.org/10.3390/jcm9113513





