Efficacy and Safety of High-Dose Immunoglobulin-Based Regimen in Statin-Associated Autoimmune Myopathy: A Multi-Center and Multi-Disciplinary Retrospective Study
Abstract
1. Introduction
2. Experimental Section
2.1. Data Collection
2.2. Eligibility Criteria
- -
- Clinical and laboratory evidence of muscle damage, including objective decrease in muscle strength by using MMT, elevation in serum CK levels, myopathy pattern in EMG and/or muscle biopsy);
- -
- Positive anti-HMGCR antibody (cut-off value >20 UA/mL) measured by chemiluminescence assay (BioFlash, Inova, CA, USA).
2.3. Statistical Analysis
2.4. Compliance with Ethical Standards
3. Results
3.1. Clinical Features
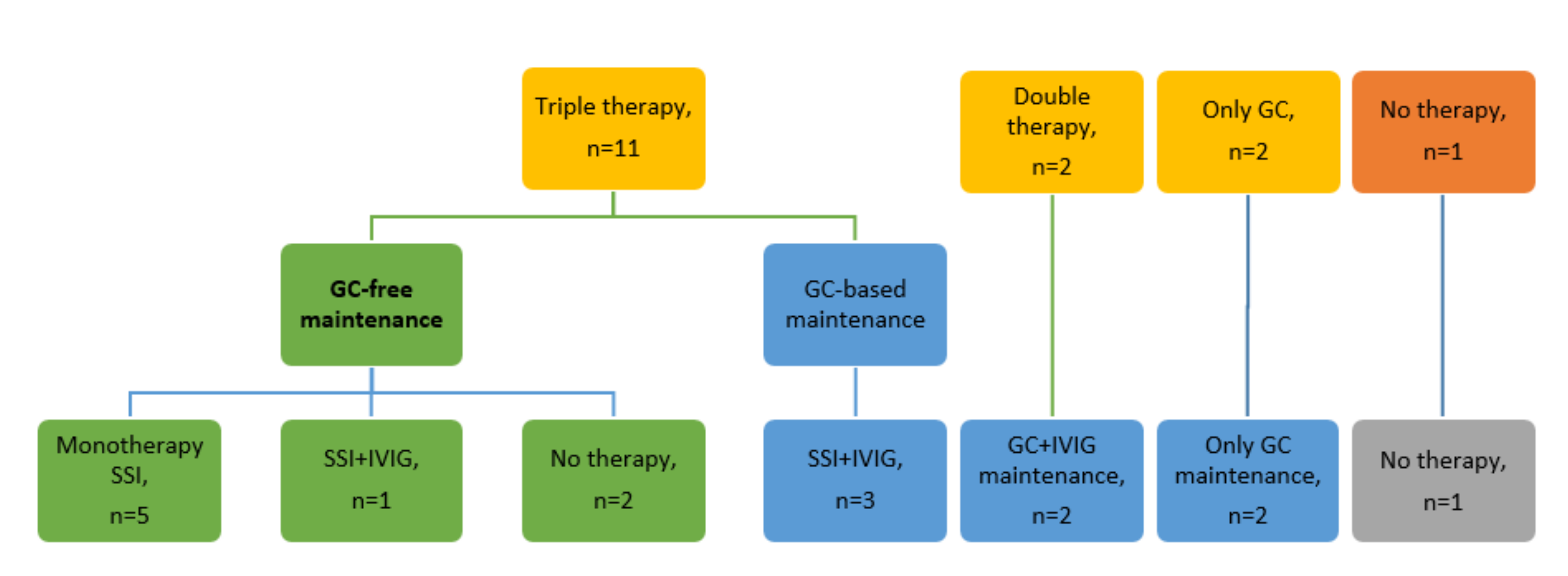
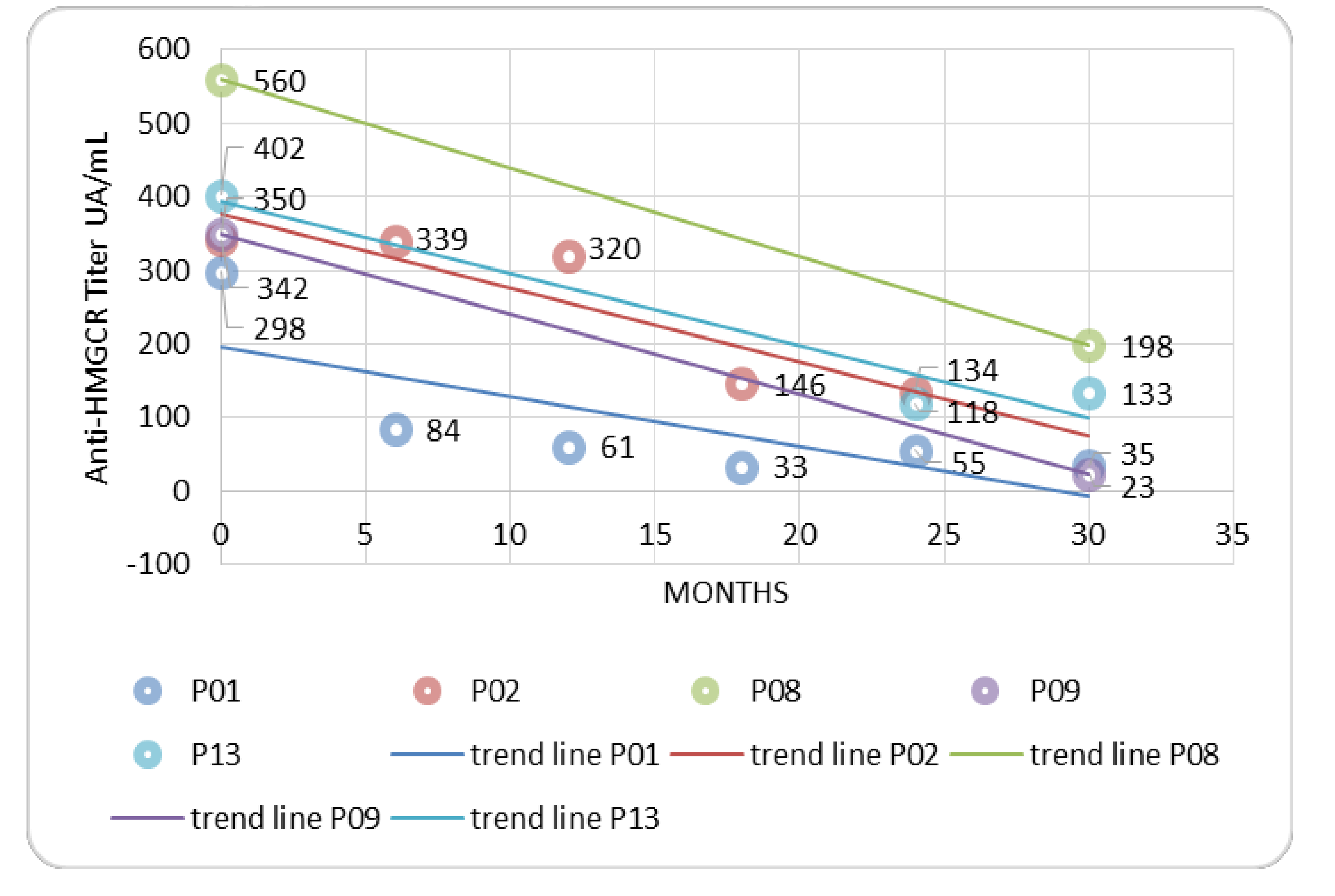
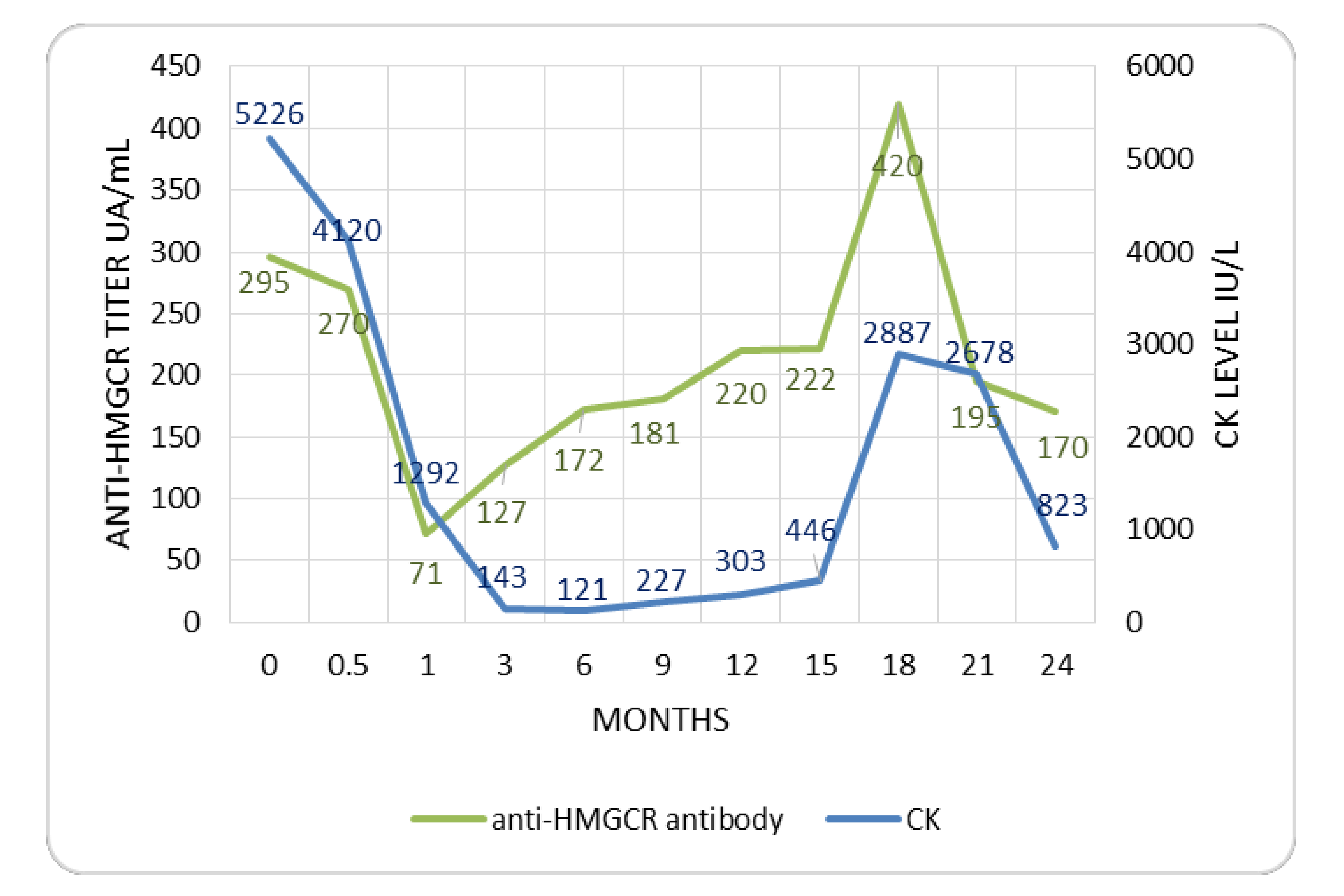
3.2. Treatment and Outcome
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christopher-Stine, L.; Casciola-Rosen, L.A.; Hong, G.; Chung, T.; Corse, A.M.; Mammen, A.L. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010, 62, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Mammen, A.L.; Chung, T.; Christopher-Stine, L.; Rosen, P.; Rosen, A.; Doering, K.R.; Casciola-Rosen, L.A. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011, 63, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Mohassel, P.; Mammen, A.L. Anti-HMGCR Myopathy. J. Neuromuscul. Dis. 2018, 5, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Basharat, P.; Lahouti, A.H.; Paik, J.J.; Albayda, J.; Pinal-Fernandez, I.; Bichile, T.; Lloyd, T.E.; Danoff, S.K.; Casciola-Rosen, L.; Mammen, A.L.; et al. Statin-Induced Anti-HMGCR-Associated Myopathy. J. Am. Coll. Cardiol. 2016, 68, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Hilton-Jones, D. Statin-related myopathies. Pract. Neurol. 2018, 18, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Musset, L.; Allenbach, Y.; Benveniste, O.; Boyer, O.; Bossuyt, X.; Bentow, C.; Phillips, J.; Mammen, A.; Van Damme, P.; Westhovens, R.; et al. Anti-HMGCR antibodies as a biomarker for immune-mediated necrotizing myopathies: A history of statins and experience from a large international multi-center study. Autoimmun. Rev. 2016, 15, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Mammen, A.L. Statin-Associated Autoimmune Myopathy. N. Engl. J. Med. 2016, 374, 664–669. [Google Scholar] [CrossRef]
- Tiniakou, E.; Christopher-Stine, L. Immune-mediated necrotizing myopathy associated with statins: History and recent developments. Curr. Opin. Rheumatol. 2017, 29, 604–611. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Miller, F.W.; Tjärnlund, A.; Bottai, M. Diagnosis and classification of Idiopathic Inflammatory Myopathies. J. Intern. Med. 2016, 280, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Allenbach, Y.; Mammen, A.L.; Benveniste, O.; Stenzel, W. Immune-Mediated Necrotizing Myopathies Working Group 224th ENMC International Workshop: Clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul. Disord. NMD 2018, 28, 87–99. [Google Scholar] [CrossRef]
- Bergua, C.; Chiavelli, H.; Allenbach, Y.; Arouche-Delaperche, L.; Arnoult, C.; Bourdenet, G.; Jean, L.; Zoubairi, R.; Guerout, N.; Mahler, M.; et al. In vivo pathogenicity of IgG from patients with anti-SRP or anti-HMGCR autoantibodies in immune-mediated necrotising myopathy. Ann. Rheum. Dis. 2019, 78, 131–139. [Google Scholar] [CrossRef]
- Mammen, A.L.; Gaudet, D.; Brisson, D.; Christopher-Stine, L.; Lloyd, T.E.; Leffell, M.S.; Zachary, A.A. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res. 2012, 64, 1233–1237. [Google Scholar] [CrossRef]
- Meyer, A.; Troyanov, Y.; Drouin, J.; Oligny-Longpré, G.; Landon-Cardinal, O.; Hoa, S.; Hervier, B.; Bourré-Tessier, J.; Mansour, A.-M.; Hussein, S.; et al. Statin-induced anti-HMGCR myopathy: Successful therapeutic strategies for corticosteroid-free remission in 55 patients. Arthritis Res. Ther. 2020, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Tiniakou, E.; Pinal-Fernandez, I.; Lloyd, T.E.; Albayda, J.; Paik, J.; Werner, J.L.; Parks, C.A.; Casciola-Rosen, L.; Christopher-Stine, L.; Mammen, A.L. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatol. Oxf. Engl. 2017, 56, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Vencovský, J.; Alexanderson, H.; Lundberg, I.E. Idiopathic Inflammatory Myopathies. Rheum. Dis. Clin. N. Am. 2019, 45, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Dyck, P.J.; Boes, C.J.; Mulder, D.; Millikan, C.; Windebank, A.J.; Dyck, P.J.B.; Espinosa, R. History of standard scoring, notation, and summation of neuromuscular signs. A current survey and recommendation. J. Peripher. Nerv. Syst. JPNS 2005, 10, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Lohani, S.; Tachamo, N.; Poudel, D.; Donato, A. Statin-Associated Autoimmune Myopathy: A Systematic Review of 100 Cases. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2017, 23, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Selva-O’Callaghan, A.; Alvarado-Cardenas, M.; Pinal-Fernández, I.; Trallero-Araguás, E.; Milisenda, J.C.; Martínez, M.Á.; Marín, A.; Labrador-Horrillo, M.; Juárez, C.; Grau-Junyent, J.M. Statin-induced myalgia and myositis: An update on pathogenesis and clinical recommendations. Expert Rev. Clin. Immunol. 2018, 14, 215–224. [Google Scholar] [CrossRef]
- Kishi, T.; Rider, L.G.; Pak, K.; Barillas-Arias, L.; Henrickson, M.; McCarthy, P.L.; Shaham, B.; Weiss, P.F.; Horkayne-Szakaly, I.; Targoff, I.N.; et al. Association of Anti-3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Autoantibodies With DRB1*07:01 and Severe Myositis in Juvenile Myositis Patients. Arthritis Care Res. 2017, 69, 1088–1094. [Google Scholar] [CrossRef]
- Farkouh, A.; Baumgärtel, C. Mini-review: Medication safety of red yeast rice products. Int. J. Gen. Med. 2019, 12, 167–171. [Google Scholar] [CrossRef]
- Mammen, A.L.; Pak, K.; Williams, E.K.; Brisson, D.; Coresh, J.; Selvin, E.; Gaudet, D. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res. 2012, 64, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Floyd, J.S.; Brody, J.A.; Tiniakou, E.; Psaty, B.M.; Mammen, A. Absence of anti-HMG-CoA reductase autoantibodies in severe self-limited statin-related myopathy. Muscle Nerve 2016, 54, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C. Necrotising autoimmune myopathy (NAM): Antibodies seem to be specific markers in aiding diagnosis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1037. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.J.; Limaye, V. Clinico-serologic features of statin-induced necrotising autoimmune myopathy in a single-centre cohort. Clin. Rheumatol. 2018, 37, 543–547. [Google Scholar] [CrossRef]
- Oddis, C.V. Update on the pharmacological treatment of adult myositis. J. Intern. Med. 2016, 280, 63–74. [Google Scholar] [CrossRef]
- Arouche-Delaperche, L.; Allenbach, Y.; Amelin, D.; Preusse, C.; Mouly, V.; Mauhin, W.; Tchoupou, G.D.; Drouot, L.; Boyer, O.; Stenzel, W.; et al. Pathogenic role of anti-signal recognition protein and anti-3-Hydroxy-3-methylglutaryl-CoA reductase antibodies in necrotizing myopathies: Myofiber atrophy and impairment of muscle regeneration in necrotizing autoimmune myopathies. Ann. Neurol. 2017, 81, 538–548. [Google Scholar] [CrossRef]
- Mammen, A.L.; Casciola-Rosen, L.; Christopher-Stine, L.; Lloyd, T.E.; Wagner, K.R. Myositis-specific autoantibodies are specific for myositis compared to genetic muscle disease. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e172. [Google Scholar] [CrossRef]
- Hoogendijk, J.E.; Amato, A.A.; Lecky, B.R.; Choy, E.H.; Lundberg, I.E.; Rose, M.R.; Vencovsky, J.; de Visser, M.; Hughes, R.A. 119th ENMC international workshop: Trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul. Disord. NMD 2004, 14, 337–345. [Google Scholar] [CrossRef]
| Pts | Age at Onset/ Gender/ Ethnicity | Statin Exposure and Drug | Type of Muscle Weakness (MRC) | CK (IU/L) | Anti HMGCR Antibody | Biopsy Findings | Induction Therapy | Time to Achieve Clinical Remission | Time to Reach Low-Doses of GCs (<15mg/day Prednisone-Equivalent) | Outcome (Maintenance Therapy at Last Follow Up) | Relapse |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P01 | 76/F/ Caucasian | Yes, atorvastatin | Upper and lower limbs (3/5), myalgia, skin involvement | 13171 IU/L | Positive, 289 UA/mL | Lymphocytic infiltrates and polyfragmentation of elastic fibers | Steroid, IVIG, MTX | 6 months | 2 months | Remission, (MTX) | No |
| P02 | 78/M/ Caucasian | Yes, atorvastatin | Upper and lower limbs (3/5), myalgia, dysphagia | 6181 IU/L | Positive, 320 UA/mL | Necrosis, atrophic fibres, secondary mitochondrial alterations, absent inflammatory infiltrate | Steroid, IVIG, MTX | 22 months | 3 months | Remission (MTX) | No |
| P03 | 74/M/ Caucasian | Yes, atorvastatin | Upper and lower limbs (3/5), myalgia | 5226 IU/L | Positive, 295 UA/mL | Biopsy not performed | Steroid, IVIG, MTX | 3 months | 1 months | Remission (MTX, IVIG), one relapse | Yes |
| P04 | 72/M/ Caucasian | Yes, atorvastatin | Lower limbs (3/5), myalgia | 5649 IU/L | Positive, 192 UA/mL | Biopsy not performed | Steroid, IVIG, MTX | 6 months | 2 months | Remission (MTX) | |
| P05 | 80/F/ Caucasian | Yes, atorvastatin | Upper and lower limbs (3/5), myalgia, dysphagia, skin involvement | 3585 IU/L | Positive, NA | Minimum focal inflammation, CD3+/CD8+, CD68+ cells | Steroid, IVIG, MTX | 3 months | 3 months | Remission (MTX, IVIG, GCs) | No |
| P06 | 66/M/ Caucasian | Yes, atorvastatin | Upper and lower limbs (1/5), dyspnea with oxygen therapy, dysphagia with parenteral nutrition | 11393 IU/L | Positive, NA | Necrosis | Steroid, IVIG, MTX | 3 months | 3 months | Remission(IVIG), one relapse | Yes |
| P07 | 80/F/ Caucasian | Yes, atorvastatin | Upper and lower limbs (2/5) | 3540 IU/L | Positive, NA | Muscle fiber phagocytosis | Steroid, IVIG | 2 months | 4 months | Remission (IVIG, GCs) | No |
| P08 | 49/F/ Caucasian | No | Upper and lower limbs (3/5), dysphagia | 359 IU/L | Positive, NA | Necrosis, myopathic alteration without inflammatory deposits | Steroid, IVIG, MTX | 6 months | 6 months | Remission (AZA, IVIG, GCs) | No |
| P09 | 76/M/ Caucasian | Yes, atorvastatin | Upper and lower limbs (2/5), myalgia, skin involvement | 5733 IU/L | Positive, 350 UA/mL | Necrosis, inflammation | Steroid, IVIG, MTX | 12 months | 3 months | Remission (no therapy) | No |
| P10 | 86/M/ Caucasian | NA | Upper and lower limbs (3/5), myalgia | 2100 IU/L | Positive, 62 UA/mL | Biopsy not performed | Steroid | 9 months | 6 months | Remission (GCs) | No |
| P11 | 82/M/ Caucasian | Yes, atorvastatin | Upper and lower limbs (3/5), dysphagia | 9600 IU/L | Positive, NA | Biopsy not performed | Steroid, IVIG, MTX | 9 months | 5 months | Remission (MTX) | No |
| P12 | 55/F/ Caucasian | Yes, red rice | Lower limbs (4/5), Myalgia | 1000 IU/L | Positive, NA | Biopsy not performed | No therapy, suspension red rice | 3 months | Non applicable | Remission (no therapy) | No |
| P13 | 68/F/ Caucasian | Yes, atorvastatin | Upper and lower limbs (1/5), myalgia, dysphagia | 10345 IU/L | Positive, NA | Biopsy not performed | Steroid, IVIG, MTX | 6 months | 3 months | Remission (RTX), one relapse | Yes |
| P14 | 81/M/ Caucasian | NA | Upper and lower limbs (3/5), dyspnea without oxygen therapy, acute renal failure | 1377 IU/L | Positive, NA | Necrosis | Steroid | 18 months | 2 months | Remission (GCs) | No |
| P15 | 60/M/ Caucasian | Yes, atorvastatin | Upper and lower limbs (4/5), myalgia | 8900 IU/L | Positive, 161 UA/mL | Necrosis | Steroid, IVIG, MTX | 9 months | 6 months | Remission (IVIG, MTX, GCs) | No |
| P16 | 75/F/ Caucasian | Yes, atorvastatin | Upper and lower limbs (4/5) | 7000 IU/L | Positive, 126 UA/mL | Necrosis | Steroid, IVIG | 6 months | 5 months | Remission (IVIG, GCs) | No |
| Demography | N (%); mean ± SD or Median (Range) |
| Age, years | 72.4 ± 10.3 |
| Female gender | 7 (43.8) |
| Prior statin use | 13 (81.3) |
| Atorvastatin | 12/13 (92.3) |
| Type 2 diabetes | 7 (43.8) |
| Cardiovascular disease | 8 (50) |
| Arterial hypertension | 11 (68.8) |
| Cancer during follow-up | 0 (0) |
| Baseline Muscle Evaluation | |
| Proximal muscle weakness | 15 (93.8) |
| MRC score | 3 (1–4) |
| Myalgia | 10 (62.5) |
| Oropharyngeal dysphagia | 7 (43.8) |
| Serum CK level at onset (IU/L) | 5691 (359–13171) |
| Muscle biopsy availability | 10 (62.5) |
| Necrosis | 9/10 (90) |
| Myopathic EMG | 14/14 (87.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treppo, E.; Infantino, M.; Benucci, M.; Ravagnani, V.; Palterer, B.; Fabris, M.; Tomietto, P.; Manfredi, M.; Giudizi, M.G.; Ligobbi, F.; et al. Efficacy and Safety of High-Dose Immunoglobulin-Based Regimen in Statin-Associated Autoimmune Myopathy: A Multi-Center and Multi-Disciplinary Retrospective Study. J. Clin. Med. 2020, 9, 3454. https://doi.org/10.3390/jcm9113454
Treppo E, Infantino M, Benucci M, Ravagnani V, Palterer B, Fabris M, Tomietto P, Manfredi M, Giudizi MG, Ligobbi F, et al. Efficacy and Safety of High-Dose Immunoglobulin-Based Regimen in Statin-Associated Autoimmune Myopathy: A Multi-Center and Multi-Disciplinary Retrospective Study. Journal of Clinical Medicine. 2020; 9(11):3454. https://doi.org/10.3390/jcm9113454
Chicago/Turabian StyleTreppo, Elena, Maria Infantino, Maurizio Benucci, Viviana Ravagnani, Boaz Palterer, Martina Fabris, Paola Tomietto, Mariangela Manfredi, Maria Grazia Giudizi, Francesca Ligobbi, and et al. 2020. "Efficacy and Safety of High-Dose Immunoglobulin-Based Regimen in Statin-Associated Autoimmune Myopathy: A Multi-Center and Multi-Disciplinary Retrospective Study" Journal of Clinical Medicine 9, no. 11: 3454. https://doi.org/10.3390/jcm9113454
APA StyleTreppo, E., Infantino, M., Benucci, M., Ravagnani, V., Palterer, B., Fabris, M., Tomietto, P., Manfredi, M., Giudizi, M. G., Ligobbi, F., Cammelli, D., Grandis, M., Parronchi, P., De Vita, S., & Quartuccio, L. (2020). Efficacy and Safety of High-Dose Immunoglobulin-Based Regimen in Statin-Associated Autoimmune Myopathy: A Multi-Center and Multi-Disciplinary Retrospective Study. Journal of Clinical Medicine, 9(11), 3454. https://doi.org/10.3390/jcm9113454






