The Role of Ribonuclease 1 and Ribonuclease Inhibitor 1 in Acute Kidney Injury after Open and Endovascular Thoracoabdominal Aortic Aneurysm Repair
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Approval and Design
2.2. Surgery
2.3. Serum Sampling
2.4. Human RNase Inhibitor 1 (RNH1) Enzyme-Linked Immunosorbent Assay
2.5. Human Ribonuclease (RNase) 1 Enzyme-Linked Immunosorbent Assay
2.6. Endpoints
2.7. Statistical Analysis
3. Results
3.1. Study Population
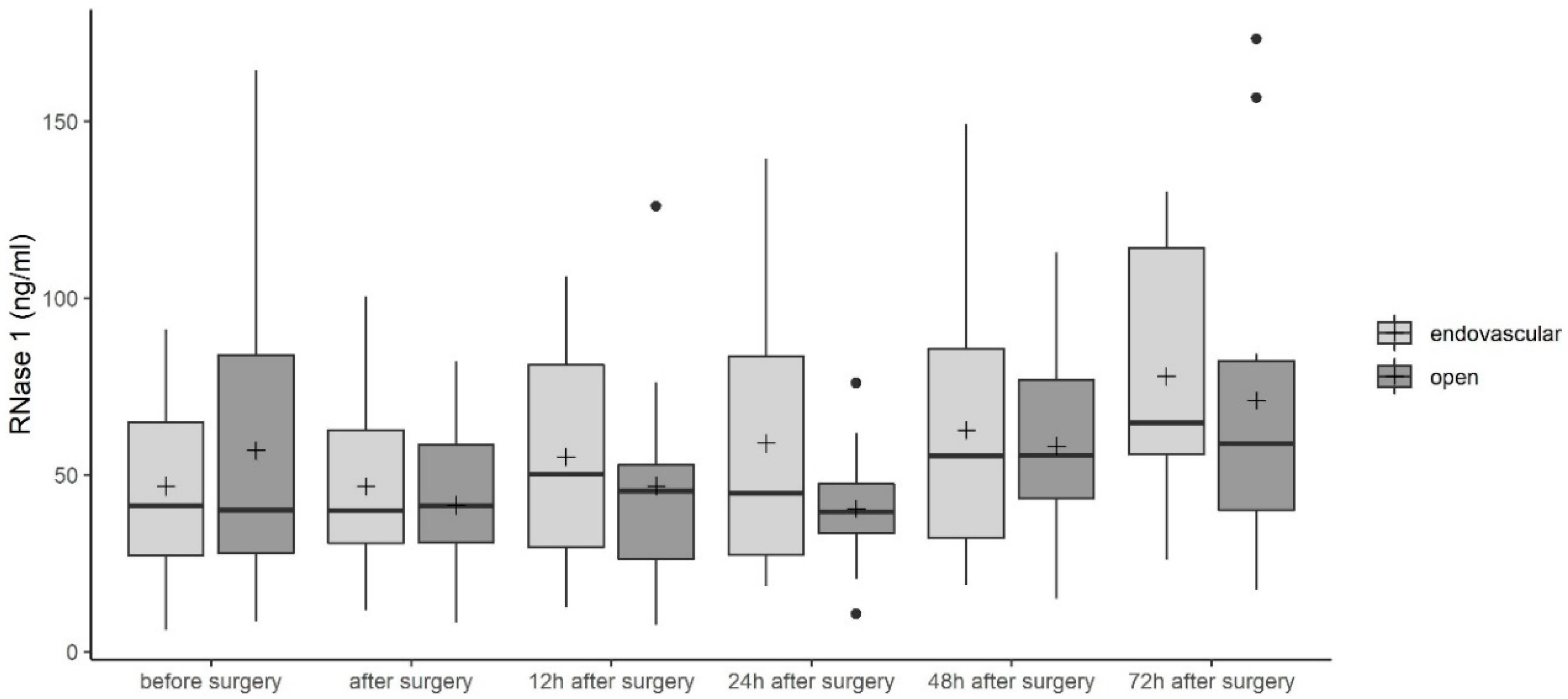
3.2. Ribonuclease (RNase) 1 Serum Levels
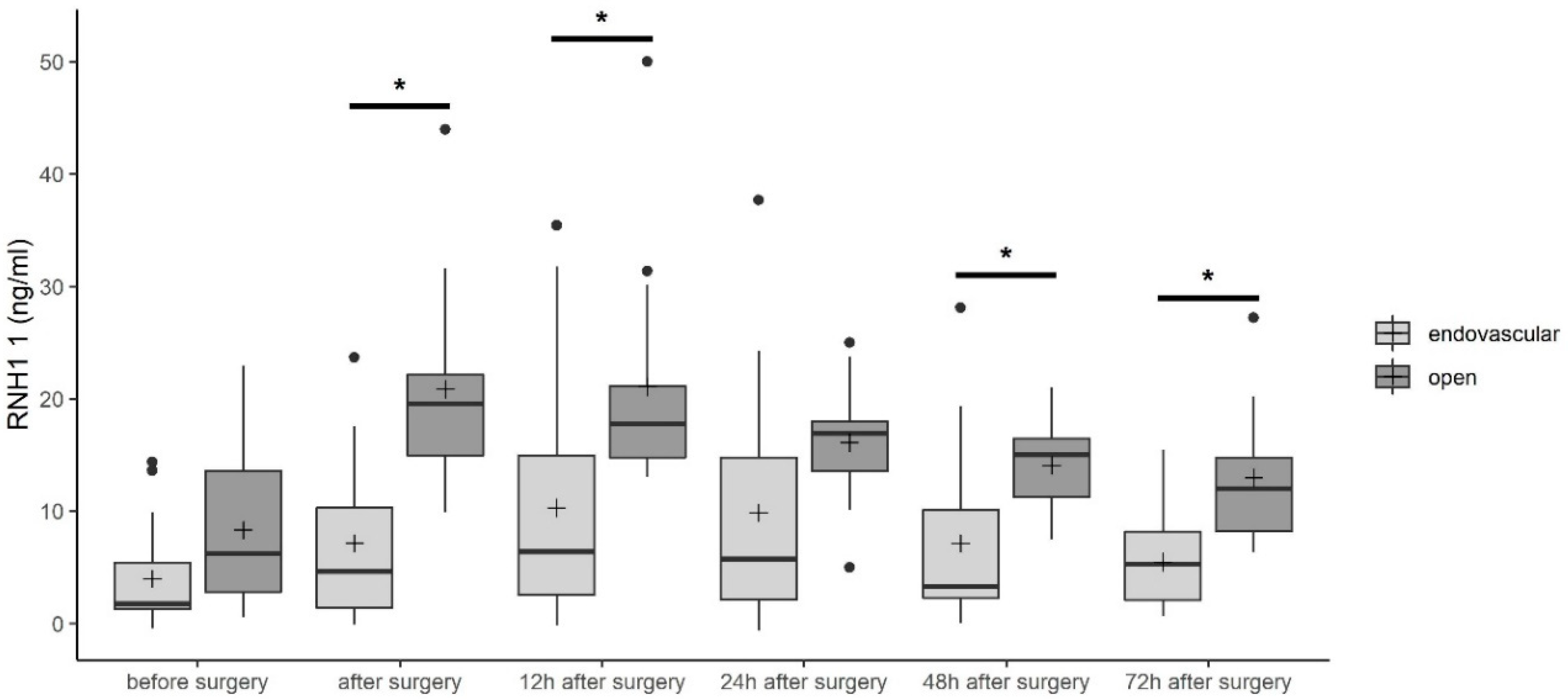
3.3. RNase Inhibitor 1 (RNH1) Serum Levels
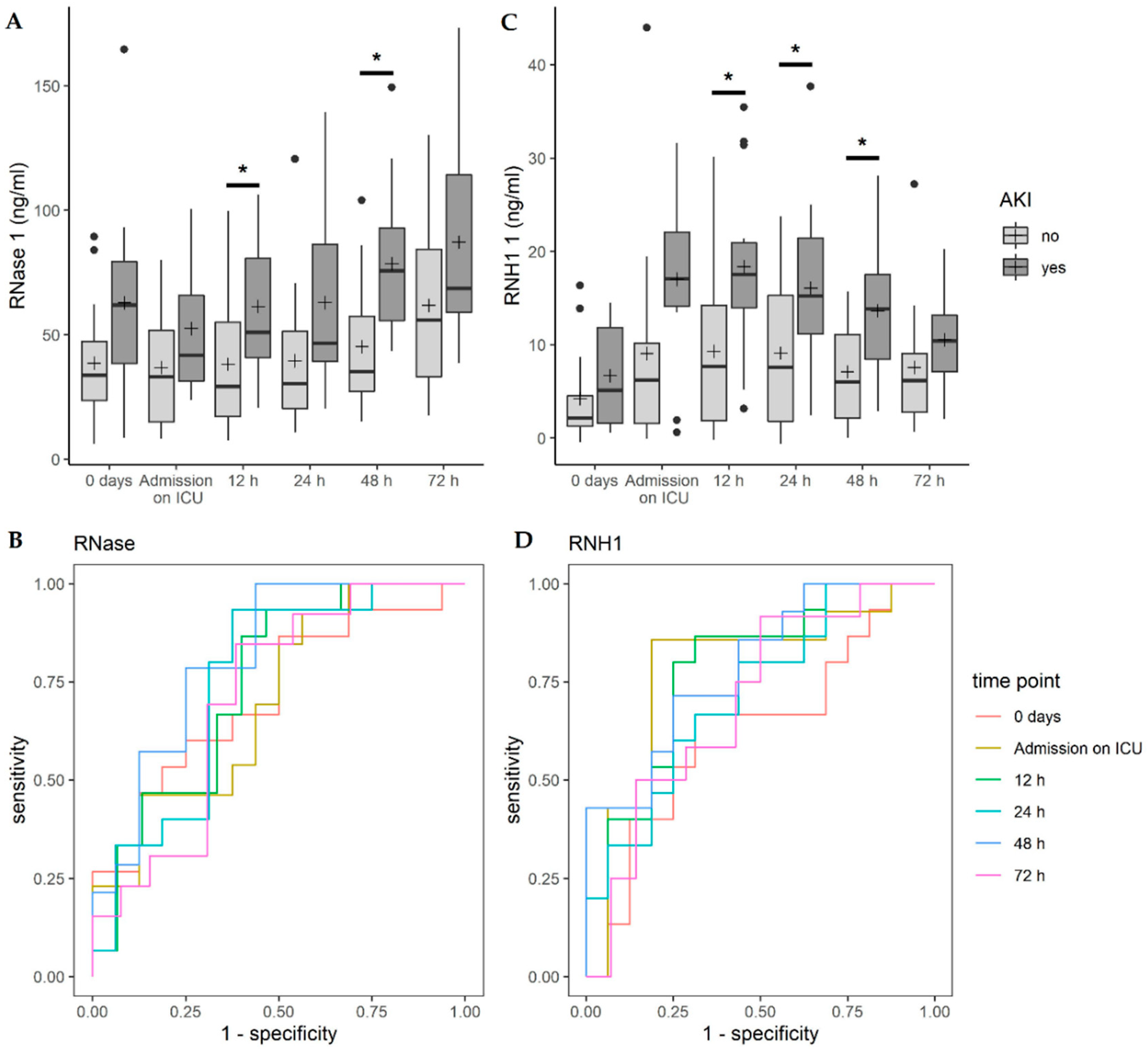
3.4. Correlation of RNase 1 and RNH1 with Acute Kidney Injury (AKI)
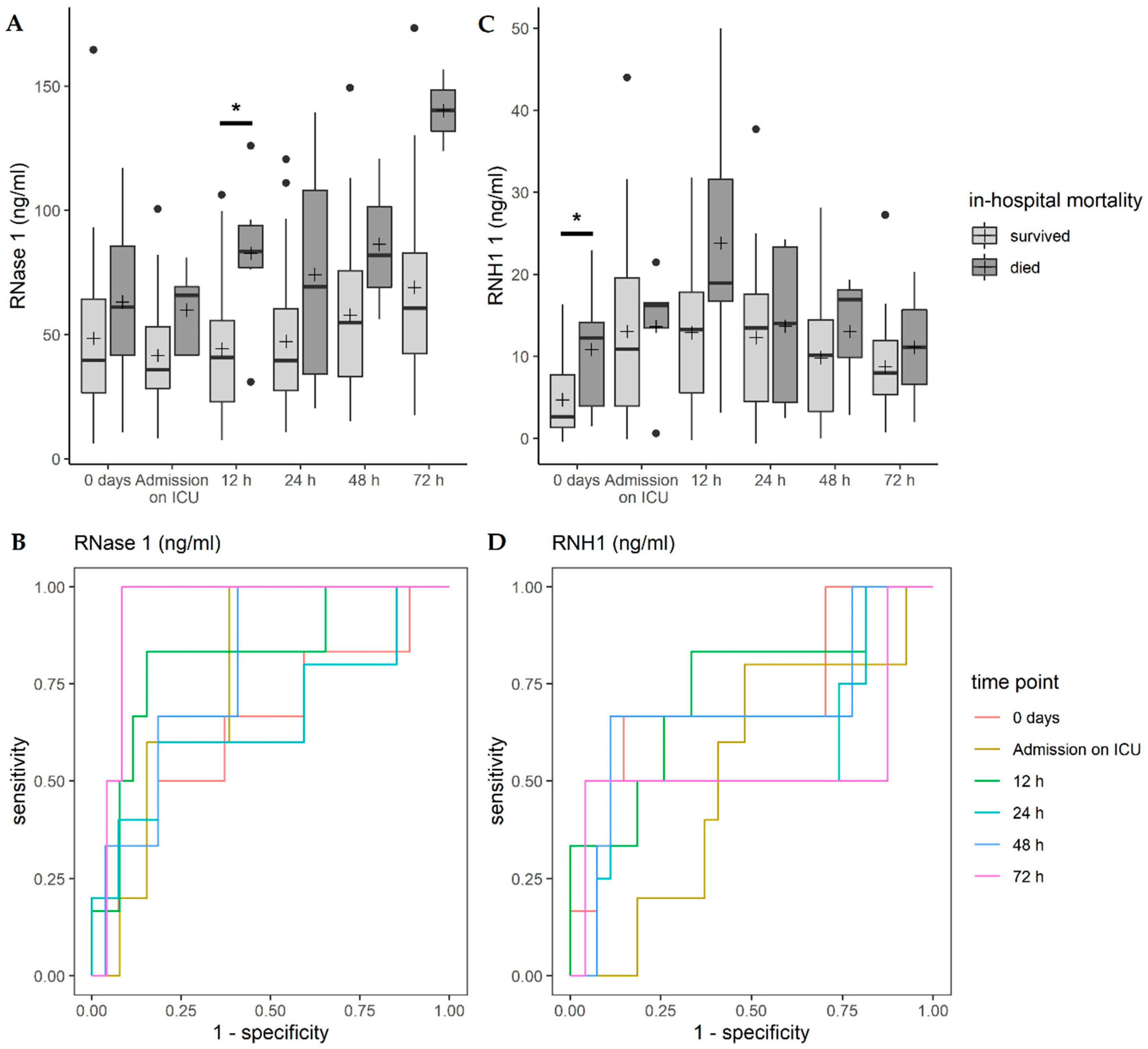
3.5. Correlation of RNase 1 and RNH1 with In-Hospital Mortality
3.6. Correlation of RNase 1 and RNH1 with Perioperative Variables
4. Discussion
5. Limitation and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Riambau, V.; Bockler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G.; Czerny, M.; Fraedrich, G.; Haulon, S.; Jacobs, M.J.; et al. Editor’s choice—Management of descending thoracic aorta diseases: Clinical practice guidelines of the european society for vascular surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, R.K.; Lu, Q.; Roselli, E.E.; Svensson, L.G.; Moon, M.C.; Hernandez, A.V.; Dowdall, J.; Cury, M.; Francis, C.; Pfaff, K.; et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: A comparison of endovascular and open techniques. Circulation 2008, 118, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.V. Perioperative acute kidney injury. Adv. Chronic Kidney Dis. 2013, 20, 67–75. [Google Scholar] [CrossRef]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef] [Green Version]
- Drews, J.D.; Patel, H.J.; Williams, D.M.; Dasika, N.L.; Deeb, G.M. The impact of acute renal failure on early and late outcomes after thoracic aortic endovascular repair. Ann. Thorac. Surg. 2014, 97, 2027–2033. [Google Scholar] [CrossRef]
- Nickolas, T.L.; Schmidt-Ott, K.M.; Canetta, P.; Forster, C.; Singer, E.; Sise, M.; Elger, A.; Maarouf, O.; Sola-Del Valle, D.A.; O’Rourke, M.; et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: A multicenter prospective cohort study. J. Am. Coll. Cardiol. 2012, 59, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, S.M.; Myers, B.D. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985, 27, 928–937. [Google Scholar] [CrossRef] [Green Version]
- Kellum, J.A.; Prowle, J.R. Paradigms of acute kidney injury in the intensive care setting. Nat. Rev. Nephrol. 2018, 14, 217–230. [Google Scholar] [CrossRef]
- Lamb, E.J.; Levey, A.S.; Stevens, P.E. The kidney disease improving global outcomes (kdigo) guideline update for chronic kidney disease: Evolution not revolution. Clin. Chem. 2013, 59, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Koczera, P.; Martin, L.; Marx, G.; Schuerholz, T. The ribonuclease a superfamily in humans: Canonical rnases as the buttress of innate immunity. Int. J. Mol. Sci. 2016, 17, 1278. [Google Scholar] [CrossRef]
- Martin, L.; Koczera, P.; Simons, N.; Zechendorf, E.; Hoeger, J.; Marx, G.; Schuerholz, T. The human host defense ribonucleases 1, 3 and 7 are elevated in patients with sepsis after major surgery—A pilot study. Int. J. Mol. Sci. 2016, 17, 294. [Google Scholar] [CrossRef] [Green Version]
- Futami, J.; Tsushima, Y.; Murato, Y.; Tada, H.; Sasaki, J.; Seno, M.; Yamada, H. Tissue-specific expression of pancreatic-type rnases and rnase inhibitor in humans. DNA Cell Biol. 1997, 16, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Dickson, K.A.; Haigis, M.C.; Raines, R.T. Ribonuclease inhibitor: Structure and function. Prog. Nucleic Acid Res. Mol. Biol. 2005, 80, 349–374. [Google Scholar] [PubMed] [Green Version]
- Zechendorf, E.; O’Riordan, C.E.; Stiehler, L.; Wischmeyer, N.; Chiazza, F.; Collotta, D.; Denecke, B.; Ernst, S.; Muller-Newen, G.; Coldewey, S.M.; et al. Ribonuclease 1 attenuates septic cardiomyopathy and cardiac apoptosis in a murine model of polymicrobial sepsis. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Averdunk, L.; Rückbeil, M.V.; Zarbock, A.; Martin, L.; Marx, G.; Jalaie, H.; Jacobs, M.J.; Stoppe, C.; Gombert, A. Slpi—A biomarker of acute kidney injury after open and endovascular thoracoabdominal aortic aneurysm (taaa) repair. Sci. Rep. 2020, 10, 3453. [Google Scholar] [CrossRef] [Green Version]
- Mommertz, G.; Sigala, F.; Langer, S.; Koeppel, T.A.; Mess, W.H.; Schurink, G.W.; Jacobs, M.J. Thoracoabdominal aortic aneurysm repair in patients with marfan syndrome. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, M.J.; Schurink, G.W. Open repair in chronic type b dissection with connective tissue disorders. Ann. Cardiothorac. Surg. 2014, 3, 325–328. [Google Scholar]
- Jacobs, M.J.; Elenbaas, T.W.; Schurink, G.W.; Mess, W.H.; Mochtar, B. Assessment of spinal cord integrity during thoracoabdominal aortic aneurysm repair. Ann. Thorac. Surg. 2002, 74, S1864–S1866. [Google Scholar] [CrossRef]
- Tshomba, Y.; Baccellieri, D.; Mascia, D.; Kahlberg, A.; Rinaldi, E.; Melissano, G.; Chiesa, R. Open treatment of extent iv thoracoabdominal aortic aneurysms. J. Cardiovasc. Surg. 2015, 56, 687–697. [Google Scholar]
- Canyigit, M.; Cetin, L.; Uguz, E.; Algin, O.; Kucuker, A.; Arslan, H.; Sener, E. Reduction of iodinated contrast load with the renal artery catheterization technique during endovascular aortic repair. Diagn. Interv. Radiol. 2013, 19, 244–250. [Google Scholar] [CrossRef]
- Thiele, R.H.; Isbell, J.M.; Rosner, M.H. Aki associated with cardiac surgery. Clin. J. Am. Soc. Nephrol. 2015, 10, 500–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Feng, Y.; Zou, L.; Wang, L.; Chen, H.H.; Cai, J.Y.; Xu, J.M.; Sosnovik, D.E.; Chao, W. Role of extracellular rna and tlr3-trif signaling in myocardial ischemia-reperfusion injury. J. Am. Heart Assoc. 2014, 3, e000683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Chen, H.; Cai, J.; Zou, L.; Yan, D.; Xu, G.; Li, D.; Chao, W. Cardiac rna induces inflammatory responses in cardiomyocytes and immune cells via toll-like receptor 7 signaling. J. Biol. Chem. 2015, 290, 26688–26698. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yang, J. Extracellular rna in renal diseases. ExRNA 2019, 1, 5. [Google Scholar] [CrossRef]
- Abtin, A.; Eckhart, L.; Mildner, M.; Ghannadan, M.; Harder, J.; Schroder, J.M.; Tschachler, E. Degradation by stratum corneum proteases prevents endogenous rnase inhibitor from blocking antimicrobial activities of rnase 5 and rnase 7. J. Investig. Dermatol. 2009, 129, 2193–2201. [Google Scholar] [CrossRef] [Green Version]
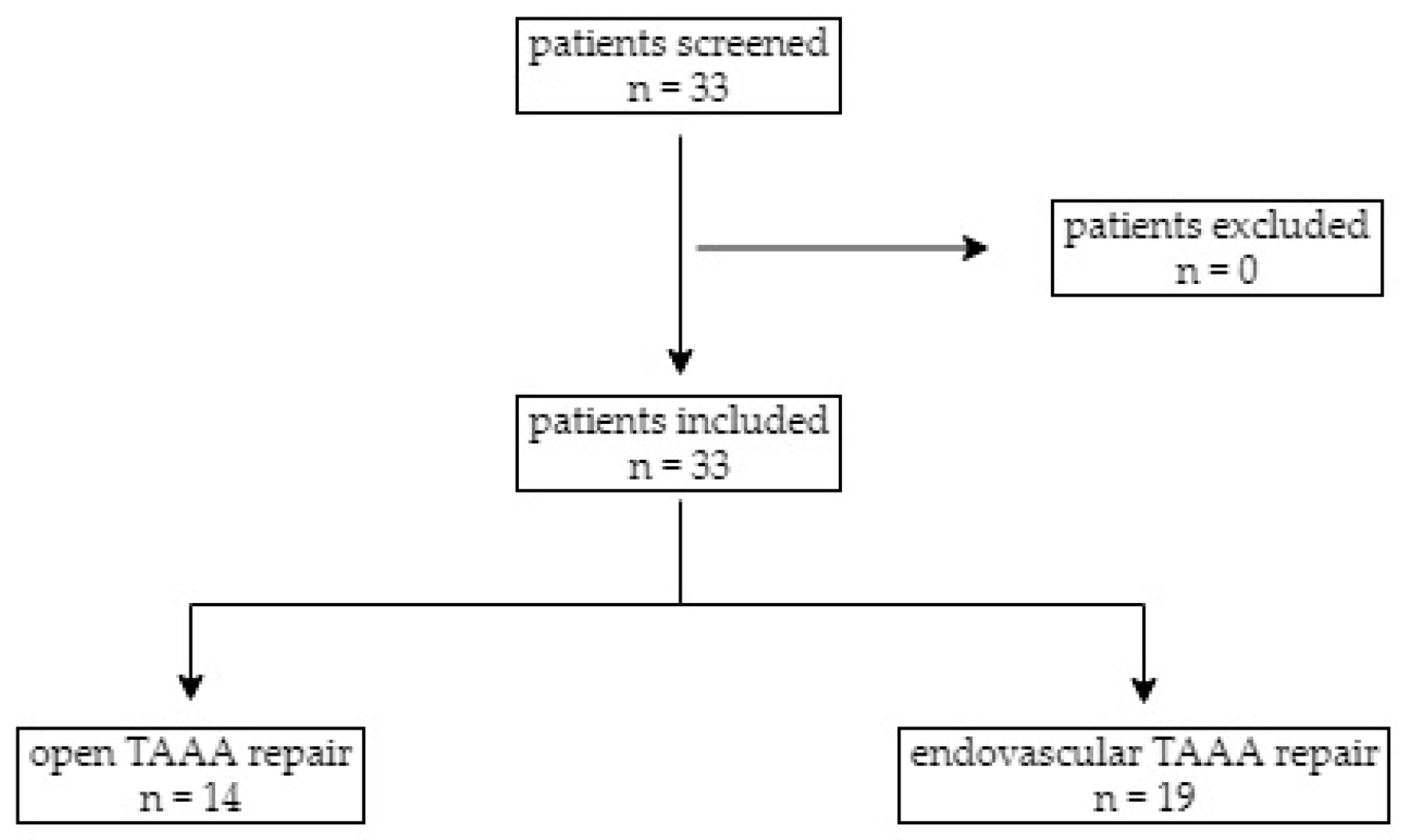
| Open TAAA Repair (n = 14) | Endovascular TAAA Repair (n = 19) | p-Value | |
|---|---|---|---|
| Age (year) (IQR) | 51 (37–65) | 74 (69–78) | <0.001 |
| Male sex (%) | 8 (57.1) | 9 (47.4) | 0.593 |
| BMI (kg/m2) (IQR) | 25.7 (20.6–30.6) | 25.4 (21.5–27.1) | 0.600 |
| Diabetes mellitus (%) | 2 (14.3) | 4 (21.1) | 0.685 |
| Smoker (%) | 4 (28.6) | 8 (42.1) | 0.440 |
| Operation time (min) (IQR) | 312.5 (295.5–474.5) | 392.0 (280.0-460.0) | 0.908 |
| LOS ICU (days) (IQR) | 5 (4–21) | 3 (6–2) | 0.075 |
| LOS in-hospital (days) (IQR) | 27 (19–38) | 15 (9–35) | 0.190 |
| In-hospital mortality (%) | 2 (14.3) | 4 (21.1) | 0.631 |
| Pre-operative renal insufficiency (%) | 3 (21.4) | 2 (10.5) | 0.4039 |
| Post-operative acute kidney injury (%) | 10 (71.4) | 7 (36.8) | 0.051 |
| Time of Measurement | Optimal Cut-Off (Youden Index) | AUC | ||||
|---|---|---|---|---|---|---|
| Cut-Off (ng/mL) | Sensitivity (%) | Specificity (%) | LR+ | LR− | ||
| 0 days n = 20 | ≥57.64 | 75.00 [19.4, 99.4] | 81.25 [54.4, 96.0] | 4.00 | 0.31 | 0.828 [0.595, 0.957] |
| Admission on ICU n = 20 | ≥31.00 | 100.00 [39.8, 100.0] | 50.00 [24.7, 75.3] | 2.00 | - | 0.672 [0.429, 0862] |
| 12 h n = 19 | ≥61.42 | 75.00 [19.4, 99.4] | 86.67 [59.5, 98.3] | 5.63 | 0.29 | 0.817 [0.575, 0.954] |
| 24 h n = 20 | ≥32.81 | 100.00 [39.8, 100.0] | 62.50 [35.4, 84.8] | 2.67 | - | 0.797 [0.560, 0.941] |
| 48 h n = 20 | ≥67.81 | 100.00 [39.8, 100.0] | 87.50 [61.7, 98.4] | 8.00 | - | 0.969 [0.779, 1.000] |
| 72 h n = 17 | ≥62.28 | 100.00 [39.8, 100.0] | 69.23 [38.6, 90.9] | 3.25 | - | 0.904 [0.663, 0.992] |
| Before Surgery | After Surgery | 12 h After Surgery | 24 h After Surgery | 48 h After Surgery | 72 h After Surgery | |
|---|---|---|---|---|---|---|
| SOFA Score | x | 0.20292 | 0.22965 | 0.25394 | 0.57451 | 0.73193 |
| (n = 6) | (n = 31) | (n = 29) | (n = 15) | (n = 8) | ||
| (p = 0.0251) | (p = 0.0390) | |||||
| Leucocytes | −0.03862 | 0.14354 | −0.07718 | −0.02492 | 0.37221 | 0.40294 |
| (n = 33) | (n = 30) | (n = 32) | (n = 28) | (n = 22) | (n = 16) | |
| PCT | −0.86603 | 0.00000 | 0.20843 | 0.24527 | 0.33636 | 0.26190 |
| (n = 3) | (n = 9) | (n = 20) | (n = 24) | (n = 11) | (n = 8) | |
| CRP | 0.22086 | 0.10000 | 0.00351 | −0.01342 | 0.01072 | 0.20000 |
| (n = 32) | (n = 9) | (n = 19) | (n = 18) | (n = 15) | (n = 15) | |
| IL-6 | −0.08571 | 0.54545 | 0.00000 | 0.54286 | ||
| (n = 1) | (n = 6) | (n = 12) | (n = 12) | (n = 6) | (n = 1) | |
| LOS ICU | 0.04169 | 0.11124 | 0.05597 | 0.22260 | 0.46103 | 0.36988 |
| (n = 33) | (n = 31) | (n = 32) | (n = 32) | (n = 30) | (n = 26) | |
| (p = 0.0103) |
| Before Surgery | After Surgery | 12 h After Surgery | 24 h After Surgery | 48 h After Surgery | 72 h After Surgery | |
|---|---|---|---|---|---|---|
| SOFA Score | x | −0.72471 | 0.31193 | 0.41576 | 0.32676 | −0.11119 |
| (n = 6) | (n = 32) | (n = 29) | (n = 15) | (n = 7) | ||
| (p = 0.0249) | ||||||
| Leukocytes | −0.03996 | −0.15045 | −0.32879 | −0.36971 | −0.04123 | 0.27857 |
| (n = 33) | (n = 31) | (n = 33) | (n = 28) | (n = 22) | (n = 15) | |
| PCT | 0.86603 | 0.69457 | 0.41986 | 0.52142 | 0.60000 | 0.60714 |
| (n = 3) | (n = 9) | (n = 20) | (n = 24) | (n = 11) | (n = 7) | |
| (p = 0.0379) | (p = 0.0090) | |||||
| CRP | −0.05668 | −0.13333 | −0.43333 | 0.23220 | 0.54334 | 0.43736 |
| (n = 32) | (n = 9) | (n = 19) | (n = 18) | (n = 15) | (n = 14) | |
| (p = 0.0363) | ||||||
| IL-6 | 0.54286 | 0.23776 | 0.75524 | 0.08571 | ||
| (n = 1) | (n = 6) | (n = 12) | (n = 12) | (n = 6) | (n = 1) | |
| (p = 0.0045) | ||||||
| LOS ICU | 0.20422 | 0.18342 | 0.20371 | 0.21947 | 0.40419 | 0.44018 |
| (n = 33) | (n = 32) | (n = 33) | (n = 31) | (n = 30) | (n = 26) | |
| (p = 0.0267) | (p = 0.0244) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zechendorf, E.; Gombert, A.; Bülow, T.; Frank, N.; Beckers, C.; Peine, A.; Kotelis, D.; Jacobs, M.J.; Marx, G.; Martin, L. The Role of Ribonuclease 1 and Ribonuclease Inhibitor 1 in Acute Kidney Injury after Open and Endovascular Thoracoabdominal Aortic Aneurysm Repair. J. Clin. Med. 2020, 9, 3292. https://doi.org/10.3390/jcm9103292
Zechendorf E, Gombert A, Bülow T, Frank N, Beckers C, Peine A, Kotelis D, Jacobs MJ, Marx G, Martin L. The Role of Ribonuclease 1 and Ribonuclease Inhibitor 1 in Acute Kidney Injury after Open and Endovascular Thoracoabdominal Aortic Aneurysm Repair. Journal of Clinical Medicine. 2020; 9(10):3292. https://doi.org/10.3390/jcm9103292
Chicago/Turabian StyleZechendorf, Elisabeth, Alexander Gombert, Tanja Bülow, Nadine Frank, Christian Beckers, Arne Peine, Drosos Kotelis, Michael J. Jacobs, Gernot Marx, and Lukas Martin. 2020. "The Role of Ribonuclease 1 and Ribonuclease Inhibitor 1 in Acute Kidney Injury after Open and Endovascular Thoracoabdominal Aortic Aneurysm Repair" Journal of Clinical Medicine 9, no. 10: 3292. https://doi.org/10.3390/jcm9103292
APA StyleZechendorf, E., Gombert, A., Bülow, T., Frank, N., Beckers, C., Peine, A., Kotelis, D., Jacobs, M. J., Marx, G., & Martin, L. (2020). The Role of Ribonuclease 1 and Ribonuclease Inhibitor 1 in Acute Kidney Injury after Open and Endovascular Thoracoabdominal Aortic Aneurysm Repair. Journal of Clinical Medicine, 9(10), 3292. https://doi.org/10.3390/jcm9103292





