Photo-Optical Transcutaneous Oxygen Tension Measurement Is of Added Value to Predict Diabetic Foot Ulcer Healing: An Observational Study
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Study Procedure
2.3. Outcome Parameters
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morbach, S.; Furchert, H.; Groblinghoff, U.; Hoffmeier, H.; Kersten, K.; Klauke, G.T.; Klemp, U.; Roden, T.; Icks, A.; Haastert, B.; et al. Long-term prognosis of diabetic foot patients and their limbs: Amputation and death over the course of a decade. Diabetes Care 2012, 35, 2021–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, A.I.; Erqou, S.; Lima, T.A.; Robinson, A.H. Association between glycated haemoglobin and the risk of lower extremity amputation in patients with diabetes mellitus-review and meta-analysis. Diabetologia 2010, 53, 840–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrepnek, G.H.; Armstrong, D.G.; Mills, J.L. Open bypass and endovascular procedures among diabetic foot ulcer cases in the United States from 2001 to 2010. J. Vasc. Surg. 2014, 60, 1255–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmonds, M. Modern treatment of infection and ischaemia to reduce major amputation in the diabetic foot. Curr. Pharm. Des. 2013, 19, 5008–5015. [Google Scholar] [CrossRef]
- Siersma, V.; Thorsen, H.; Holstein, P.E.; Kars, M.; Apelqvist, J.; Jude, E.B.; Piaggesi, A.; Bakker, K.; Edmonds, M.; Jirkovska, A.; et al. Importance of factors determining the low health-related quality of life in people presenting with a diabetic foot ulcer: The Eurodiale study. Diabet. Med. 2013, 30, 1382–1387. [Google Scholar] [CrossRef]
- Brownrigg, J.R.; Schaper, N.C.; Hinchliffe, R.J. Diagnosis and assessment of peripheral arterial disease in the diabetic foot. Diabet. Med. 2015, 32, 738–747. [Google Scholar] [CrossRef]
- Wang, Z.; Hasan, R.; Firwana, B.; Elraiyah, T.; Tsapas, A.; Prokop, L.; Mills, J.L.; Murad, M.H. A systematic review and meta-analysis of tests to predict wound healing in diabetic foot. J. Vasc. Surg. 2016, 63, 29S–36S.e2. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Vanhoutte, P.M. Macro- and microvascular endothelial dysfunction in diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef] [Green Version]
- Vesterager, P. Transcutaneous Po2 electrode. Scand. J. Clin. Lab. Investig. Suppl. 1977, 146, 27–30. [Google Scholar] [CrossRef]
- Urban, M.; Fouasson-Chailloux, A.; Signolet, I.; Colas Ribas, C.; Feuilloy, M.; Abraham, P. Comparison of two devices for measuring exercise transcutaneous oxygen pressures in patients with claudication. Vasa 2015, 44, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Rumsey, W.L.; Vanderkooi, J.M.; Wilson, D.F. Imaging of phosphorescence: A novel method for measuring oxygen distribution in perfused tissue. Science 1988, 241, 1649–1651. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L.; Conte, M.S.; Armstrong, D.G.; Pomposelli, F.B.; Schanzer, A.; Sidawy, A.N.; Andros, G.; Society for Vascular Surgery Lower Extremity Guidelines Writing Group. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on wound, ischemia, and foot infection (WIfI). J. Vasc. Surg. 2014, 59, 220–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathioudakis, N.; Hicks, C.W.; Canner, J.K.; Sherman, R.L.; Hines, K.F.; Lum, Y.W.; Perler, B.A.; Abularrage, C.J. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system predicts wound healing but not major amputation in patients with diabetic foot ulcers treated in a multidisciplinary setting. J. Vasc. Surg. 2017, 65, 1698–1705.e1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicks, C.W.; Canner, J.K.; Mathioudakis, N.; Sherman, R.; Malas, M.B.; Black, J.H., 3rd; Abularrage, C.J. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification independently predicts wound healing in diabetic foot ulcers. J. Vasc. Surg. 2018, 68, 1096–1103. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763. [Google Scholar] [CrossRef] [Green Version]
- Leenstra, B. Photo-optical transcutaneous oxygen tension measurement in patients with peripheral arterial disease. J. Cardiovasc. Surg. 2019. Submitted. [Google Scholar]
- Ubbink, D.T.; Jacobs, M.J.; Tangelder, G.J.; Slaaf, D.W.; Reneman, R.S. The usefulness of capillary microscopy, transcutaneous oximetry and laser Doppler fluxmetry in the assessment of the severity of lower limb ischaemia. Int. J. Microcirc. Clin. Exp. 1994, 14, 34–44. [Google Scholar] [CrossRef]
- Kalani, M.; Brismar, K.; Fagrell, B.; Ostergren, J.; Jorneskog, G. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care 1999, 22, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Ballard, J.L.; Eke, C.C.; Bunt, T.J.; Killeen, J.D. A prospective evaluation of transcutaneous oxygen measurements in the management of diabetic foot problems. J. Vasc. Surg. 1995, 22, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; Group, T.I.W. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [Green Version]
- Faglia, E.; Clerici, G.; Caminiti, M.; Quarantiello, A.; Curci, V.; Morabito, A. Predictive values of transcutaneous oxygen tension for above-the-ankle amputation in diabetic patients with critical limb ischemia. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 731–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladurner, R.; Kuper, M.; Konigsrainer, I.; Lob, S.; Wichmann, D.; Konigsrainer, A.; Coerper, S.; Beckert, S. Predictive value of routine transcutaneous tissue oxygen tension (tcpO2) measurement for the risk of non-healing and amputation in diabetic foot ulcer patients with non-palpable pedal pulses. Med. Sci. Monit. 2010, 16, CR273–CR277. [Google Scholar] [PubMed]
- Dhatariya, K.K.; Li Ping Wah-Pun Sin, E.; Cheng, J.O.S.; Li, F.Y.N.; Yue, A.W.Y.; Gooday, C.; Nunney, I. The impact of glycaemic variability on wound healing in the diabetic foot-A retrospective study of new ulcers presenting to a specialist multidisciplinary foot clinic. Diabetes Res. Clin. Pract. 2018, 135, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J. Pediatr. 1994, 125, 177–188. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 infectious diseases society of america clinical practice guideline for the diagnosis and treatment of diabetic foot infections. J. Am. Podiatr. Med. Assoc. 2013, 103, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Manu, C.; Lacopi, E.; Bouillet, B.; Vouillarmet, J.; Ahluwalia, R.; Ludemann, C.; Garcia-Klepzig, J.L.; Meloni, M.; De Buruaga, V.R.; Sanchez-Rios, J.P.; et al. Delayed referral of patients with diabetic foot ulcers across Europe: Patterns between primary care and specialised units. J. Wound Care 2018, 27, 186–192. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

| Baseline Characteristics | Healed (n = 68) | Non-Healed (n = 35) | p |
|---|---|---|---|
| Sex (male) (percentage) | 44 (64.7) | 28 (80.0) | 0.109 |
| Age (σ | 71 (12.3) | 69 (12.8) | 0.701 |
| BMI (n, %) | 0.196 | ||
| <25 | 37 (54.4) | 21 (60.0) | |
| 25–30 | 26 (3.2) | 8 (22.9) | |
| 30> | 3 (4.4) | 5 (14.3) | |
| n/a | 2 (2.9) | 1 (2.9) | |
| Smoking history (n, %) | 0.641 | ||
| Active | 12 (21.8) | 9 (31.0) | |
| Former smoker | 22 (40.0) | 12 (41.4) | |
| Non-smoker | 18 (32.7) | 6 (20.7) | |
| n/a | 3 (5.5) | 2 (6.9) | |
| Hypertension (%) | 32 (47.1) | 21 (60.0) | 0.213 |
| Dyslipidemia (%) | 35 (51.5) | 20 (58.8) | 0.482 |
| HBA1c (sd) | 63.0 (15.8) | 62.9 (17.5) | 0.866 |
| Wound Healing Outcome | (Patients, n) |
|---|---|
| Healed | 68 |
| Non-healed | 35 |
| Amputation | 29 |
| Deterioration without amputation | 6 |
| Wound Classification | All (n = 103) | Healed (n = 68) | Non-Healed (n = 35) | p |
|---|---|---|---|---|
| Wound location (n, %) | 0.271 | |||
| Toe | 60 (58.3) | 37 (54.5) | 23 (65.7) | |
| Foot | 43 (41.7) | 31 (45.6) | 12 (34.3) | |
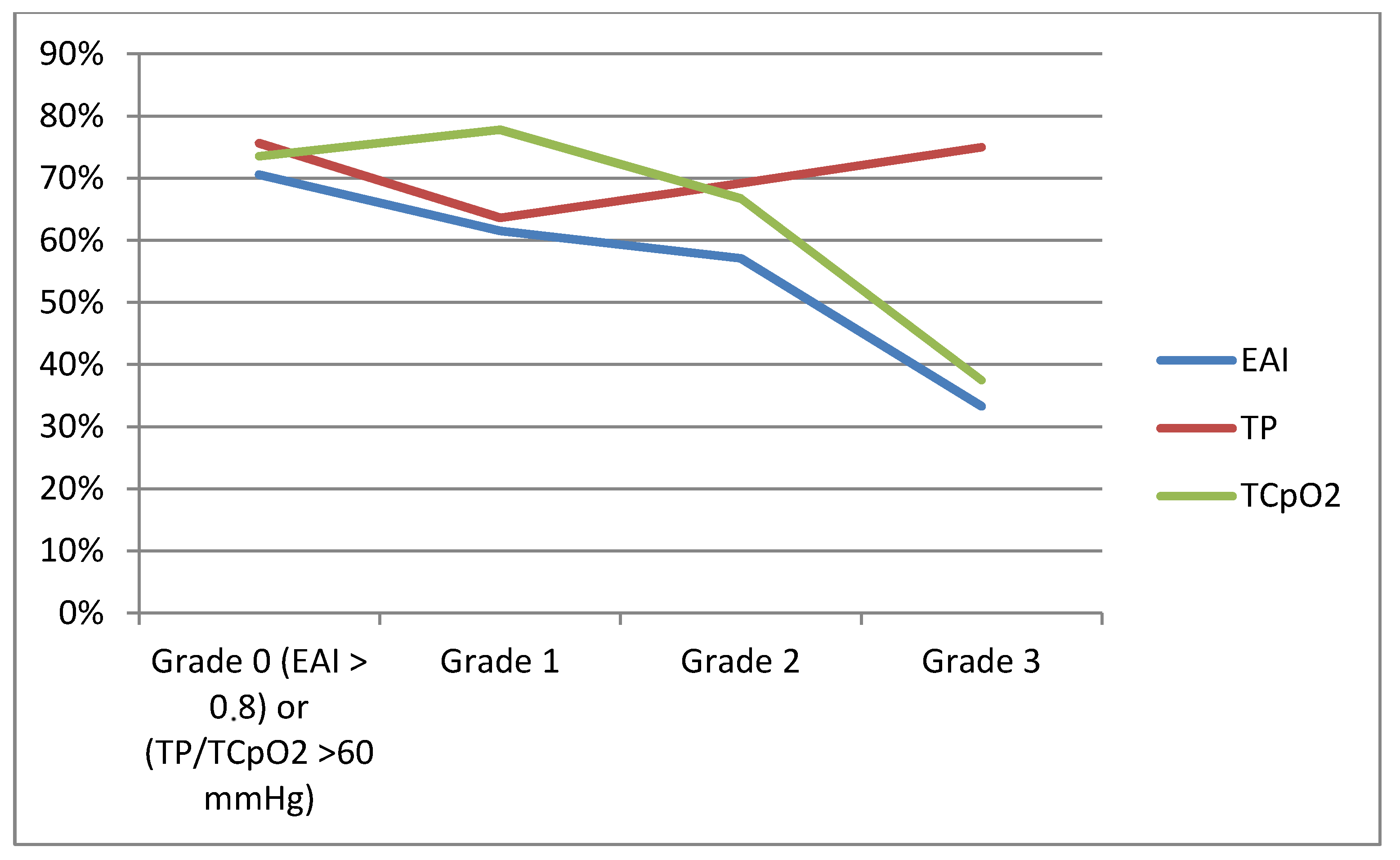
| WIfI classification | ||||
| Wound (n, %) | 0.003 | |||
| 1 | 81 (78.6) | 60 (88.2) | 21 (60.0) | |
| 2 | 21 (20.4) | 8 (11.8) | 13 (37.1) | |
| 3 | 1 (1.0) | 0 (0) | 1 (2.9) | |
| Ischemia (n, %) | 0.231 | |||
| 0 | 43 (41.7) | 32 (47.1) | 11 (31.4) | |
| 1 | 37 (35.9) | 25 (36.8) | 12 (34.3) | |
| 2 | 8 (7.8) | 4 (5.9) | 4 (11.4) | |
| 3 | 6 (5.8) | 2 (2.9) | 4 (11.4) | |
| Not measurable (n, %) | 9 (8.7) | 5 (7.4) | 4 (11.4) | |
| Foot Infection (n, %) | 0.194 | |||
| 0 | 72 (69.9) | 50 (73.5) | 22 (62.9) | |
| 1 | 14 (13.6) | 10 (14.7) | 4 (11.4) | |
| 2 | 17 (16.5) | 8 (11.8) | 9 (25.7) |
| Measurements | All (n = 103) | Healed (n = 68) | Non-Healed (n = 35) | p |
|---|---|---|---|---|
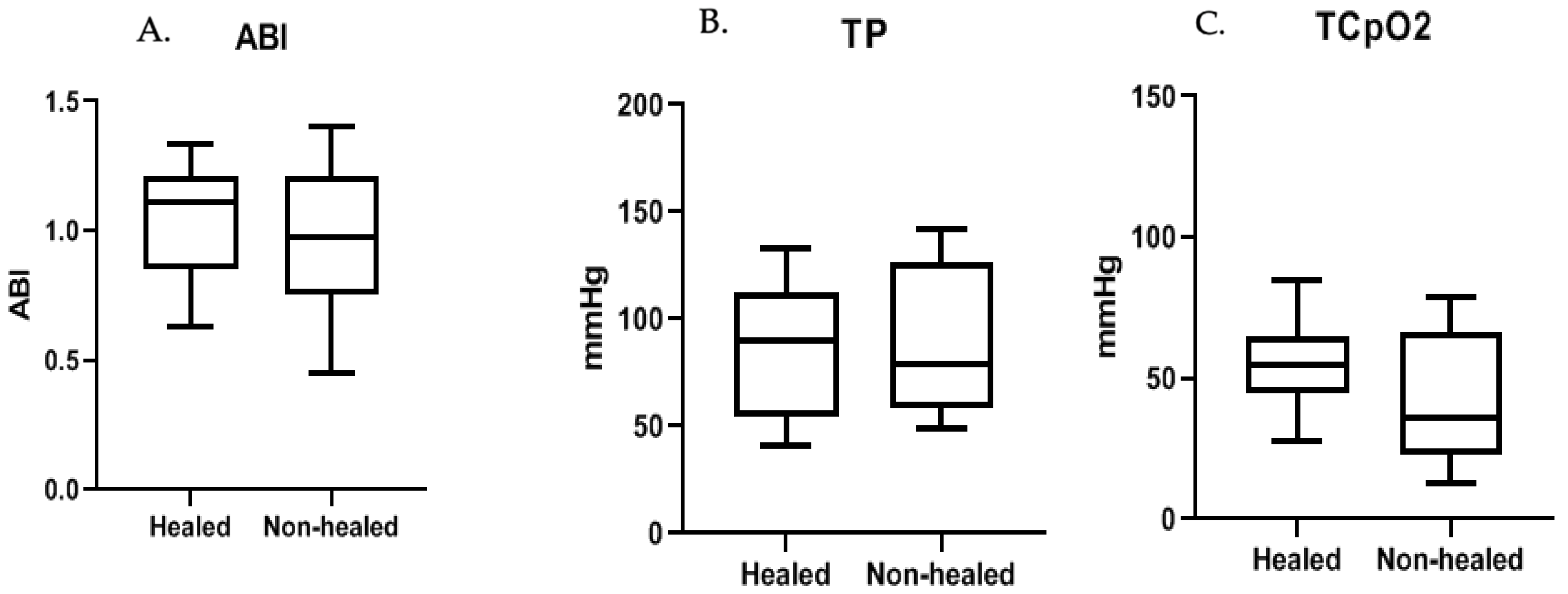
| Brachial pressure (mmHg, σ) | 152 | 149 (24) | 157 (28) | 0.128 |
| Ankle pressure (mmHg, σ) | 150 | 152 (45) | 145 (54) | 0.531 |
| Toe-pressure (mmHg, σ) | 89 | 88 (36) | 91 (38) | 0.799 |
| TCpO2 reference probe (mmHg, σ) | 72 | 75 (29) | 68 (22) | 0.177 |
| TCpO2 forefoot (mmHg, σ) | 51 | 55 (21) | 41 (24) | 0.005 |
| Ankle-brachial index (σ) | 1.00 | 1.03 (0.28) | 0.96 (0.37) | 0.386 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leenstra, B.; de Kleijn, R.; Kuppens, G.; Verhoeven, B.A.N.; Hinnen, J.W.; de Borst, G.J. Photo-Optical Transcutaneous Oxygen Tension Measurement Is of Added Value to Predict Diabetic Foot Ulcer Healing: An Observational Study. J. Clin. Med. 2020, 9, 3291. https://doi.org/10.3390/jcm9103291
Leenstra B, de Kleijn R, Kuppens G, Verhoeven BAN, Hinnen JW, de Borst GJ. Photo-Optical Transcutaneous Oxygen Tension Measurement Is of Added Value to Predict Diabetic Foot Ulcer Healing: An Observational Study. Journal of Clinical Medicine. 2020; 9(10):3291. https://doi.org/10.3390/jcm9103291
Chicago/Turabian StyleLeenstra, Bernard, Robert de Kleijn, Geoffrey Kuppens, Bart Arnoldus Nicolaas Verhoeven, Jan Willem Hinnen, and Gert J. de Borst. 2020. "Photo-Optical Transcutaneous Oxygen Tension Measurement Is of Added Value to Predict Diabetic Foot Ulcer Healing: An Observational Study" Journal of Clinical Medicine 9, no. 10: 3291. https://doi.org/10.3390/jcm9103291
APA StyleLeenstra, B., de Kleijn, R., Kuppens, G., Verhoeven, B. A. N., Hinnen, J. W., & de Borst, G. J. (2020). Photo-Optical Transcutaneous Oxygen Tension Measurement Is of Added Value to Predict Diabetic Foot Ulcer Healing: An Observational Study. Journal of Clinical Medicine, 9(10), 3291. https://doi.org/10.3390/jcm9103291






