Helicobacter pylori Related Diseases and Osteoporotic Fractures (Narrative Review)
Abstract
1. Introduction
2. Brief Overview of Helicobacter pylori Infection
3. HPI and Bone Status
4. HPI-Induced Upper Gut Diseases and Osteoporotic Fractures
4.1. Peptic Ulcer Disease
4.2. Chronic/Atrophic Gastritis
4.3. Gastric Cancer
4.4. Gastroesophageal Reflux Disease (GERD)
4.5. Effects of Acid-Suppressive Drugs
5. HPI-Associated Chronic Extra-Gastroduodenal Diseases, Medication Use and Osteoporotic Fractures
5.1. Chronic Extra-Gastroduodenal Diseases
5.2. Falls
5.3. Medications
6. Potential Pathophysiological Mechanisms
7. Clinical Implications and Recommendations
8. Limitations
9. Conclusions
10. Key Points
- H.p. colonizes about half of the world population. HPI as a multi-system condition confined not only to gastroduodenal morbidity but also many chronic extra-digestive diseases (CVD, neurodegenerative, endocrine, CLD, CKD, etc.) might directly and/or indirectly affect bone status, predispose to falls and, consequently, to OFs.
- The relationship between HPI and OP/OFs, two common, multifactorial and heterogeneous conditions, depends on complex interactions of multiple factors, including microbe’s virulence, host genetic predisposition, local gastroduodenal and systemic responses (biochemical, metabolic, hormonal, immunologic and inflammatory) and environmental influences. Therefore, microbe’s contribution to development and progression of OP/OF and the risk profile in colonized individuals could vary significantly. When studying the role of HPI in OP/OF, correction for the mentioned factors, is essential.
- The data on associations between HPI and OP/OFs in the literature are inconsistent, but there is growing evidence that HPI (especially in persons infected with virulent strains, e.g., cagA+) increases risk of OP/OF approximately 1.5–2-fold.
- Given the widespread prevalence of HPI in the population, the practical implication for these data is that comprehensive assessment for OP/OF risks should include evaluation for HPI-related diseases and disorders and vice versa (assessment for HPI in subjects with established OP, falls and low energy fractures); such approach would assist in individualized prevention and treatment of OP/OFs and should be considered at health care policy level.
- The usefulness and applicability of a practical strategy addressing HPI, an easy identifiable and treatable condition, as a potential pathophysiological co-factor of OP/OF, are worth further investigation in controlled, long-term studies with simultaneous assessment of H.p., host’s and environmental characteristics; a better understanding of the mechanisms underlying HPI–OP/OF relationship and individual outcomes should be achieved.
Addendum
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMD | bone mineral density |
| bALP | bone-specific alkaline phosphatase |
| β-CTX | β-collagen I carboxy terminal telopeptide |
| BO | Barrett’s esophagus |
| Ca | calcium |
| CAD | coronary artery disease |
| cagA | cytotoxin-associated gene A |
| CagA | cytotoxin associated antigen A |
| CHF | congestive heart failure |
| CKD | chronic kidney disease |
| COPD | chronic obstructive pulmonary disease |
| CVD | cardiovascular disease |
| DC | dendritic cell |
| DM | diabetes mellitus |
| GERD | gastroesophageal reflux disease |
| HRT | hormone replacement therapy |
| H.p. | Helicobacter pylori |
| HPI | Helicobacter pylori infection |
| H2RA | histamine-2 receptor antagonist |
| HR | hazard ratio |
| IL | interleukin |
| ITT | idiopathic thrombocytopenic purpura |
| Mg | magnesium |
| MALT | mucosa-associated lymphoid tissue B-cell lymphoma |
| MAFLD | metabolic associated fatty liver disease |
| OC | osteocalcin |
| OF | osteoporotic fracture |
| OP | osteoporosis |
| OPG | osteoprotegerin |
| RF | risk factor |
| P1NP | N-terminal cross-links of human procollagen type I |
| PO4 | phosphate |
| PPI | proton pump inhibitor |
| PTH | parathyroid hormone |
| PUD | peptic ulcer disease |
| RA | rheumatoid arthritis |
| RANK | receptor activator of nuclear factor κB |
| RANKL | receptor activator of nuclear factor-kappa B (NF-kB) ligand |
| TNF-α | tumor necrosis factor alpha |
References
- Karsenty, G.; Ferron, M. The contribution of bone to whole-organism physiology. Nat. Cell Biol. 2012, 481, 314–320. [Google Scholar] [CrossRef]
- Kitay, A.M.; Geibel, J.P. Stomach and Bone. Adv. Exp. Med. Biol. 2017, 1033, 97–131. [Google Scholar]
- Suchacki, K.J.; Roberts, F.; Lovdel, A.; Farquharson, C.; Morton, N.M.; Macrae, V.E.; Cawthorn, W.P.; Morton, N.M.; Cawthorn, W. Skeletal energy homeostasis: A paradigm of endocrine discovery. J. Endocrinol. 2017, 234, R67–R79. [Google Scholar] [CrossRef]
- Ramsey, W.; Isales, C.M. Intestinal Incretins and the Regulation of Bone Physiology. Adv. Exp. Med. Biol. 2017, 1033, 13–33. [Google Scholar]
- Schiellerup, S.P.; Skov-Jeppesen, K.; Windeløv, J.A.; Svane, M.S.; Holst, J.J.; Hartmann, B.; Rosenkilde, M. Gut Hormones and Their Effect on Bone Metabolism. Potential Drug Therapies in Future Osteoporosis Treatment. Front. Endocrinol. 2019, 10, 75. [Google Scholar] [CrossRef]
- Guntur, A.R.; Rosen, C.J. Bone as an Endocrine Organ. Endocr. Pract. 2012, 18, 758–762. [Google Scholar] [CrossRef]
- Wong, I.P.; Baldock, P.A.; Herzog, H. Gastrointestinal peptides and bone health. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 44–50. [Google Scholar] [CrossRef]
- Blaser, M.J.; Atherton, J.C. Helicobacter pylori persistence: Biology and disease. J. Clin. Investig. 2004, 113, 321–333. [Google Scholar] [CrossRef]
- Atherton, J.C.; Blaser, M.J. Coadaptation of Helicobacter pylori and humans: Ancient history, modern implications. J. Clin. Investig. 2009, 119, 2475–2487. [Google Scholar] [CrossRef]
- Hooi, J.K.; Lai, W.Y.; Ng, W.K.; Suen, M.M.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.; Wu, J.C.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Franceschi, F.; Tortora, A.; Gasbarrini, G.; Gasbarrini, A. Helicobacter pyloriand Extragastric Diseases. Helicobacter 2014, 19, 52–58. [Google Scholar] [CrossRef]
- Upala, S.; Sanguankeo, A.; Wijarnpreecha, K.; Jaruvongvanich, V. Association between Helicobacter pylori infection and osteoporosis: A systematic review and meta-analysis. J. Bone Miner. Metab. 2015, 34, 482–483. [Google Scholar] [CrossRef]
- Papamichael, K.; Papaioannou, G.; Cheifetz, M.A.; Cheifetz, A.S. Bone of Contention: Helicobacter pylori and Osteoporosis-Is There an Association? Dig. Dis. Sci. 2019, 64, 2736–2739. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Zhang, Q.; Ge, B.; Zhang, J.; Yu, L.; Cai, T.; Zhang, Y.; Xiong, H. Relationship betweenHelicobacter pyloriinfection and osteoporosis: A systematic review and meta-analysis. BMJ Open 2019, 9, e027356. [Google Scholar] [CrossRef]
- Kodaman, N.; Pazos, A.; Schneider, B.G.; Piazuelo, M.B.; Mera, R.; Sobota, R.S.; Sicinschi, L.A.; Shaffer, C.L.; Romero-Gallo, J.; De Sablet, T.; et al. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proc. Natl. Acad. Sci. USA 2014, 111, 1455–1460. [Google Scholar] [CrossRef]
- Warren, J.R. Unidentified Curved Bacilli on Gastric Epithelium in Active Chronic Gastritis. Lancet 1983, 321, 1273–1275. [Google Scholar]
- Marshall, B.; Warren, J. Unidentified Curved Bacilli in The Stomach of Patients with Gastritis And Peptic Ulceration. Lancet 1984, 323, 1311–1315. [Google Scholar] [CrossRef]
- Medina, M.L.; Medina, M.G.; Merino, L.A. Correlation between virulence markers of Helicobacter pylori in the oral cavity and gastric biopsies. Arq. Gastroenterol. 2017, 54, 217–221. [Google Scholar] [CrossRef]
- Lu, L.-J.; Hao, N.-B.; Liu, J.-J.; Li, X.; Wang, R.-L. Correlation between Helicobacter pylori Infection and Metabolic Abnormality in General Population: A Cross-Sectional Study. Gastroenterol. Res. Pract. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Roesler, B.M.; Rabelo-Gonçalves, E.M.; Zeitune, J.M. Virulence Factors of Helicobacter pylori: A Review. Clin. Med. Insights: Gastroenterol. 2014, 7, 9–17. [Google Scholar]
- Waldum, H.L.; Kleveland, P.M.; Sørdal, Ø.F. Helicobacter pyloriand gastric acid: An intimate and reciprocal relationship. Ther. Adv. Gastroenterol. 2016, 9, 836–844. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Survival of Helicobacter pylori in gastric acidic territory. Helicobacter 2017, 22, e12386. [Google Scholar] [CrossRef]
- Smolka, A.J.; Schubert, M.L. Helicobacter pylori-Induced Changes in Gastric Acid Secretion and Upper Gastrointestinal Disease. Future HIV 1 Ther. 2017, 400, 227–252. [Google Scholar]
- Marcus, E.A.; Tokhtaeva, E.; Jimenez, J.L.; Wen, Y.; Naini, B.V.; Heard, A.N.; Kim, S.; Capri, J.; Cohn, W.; Whitelegge, J.P.; et al. Helicobacter pylori infection impairs chaperone-assisted maturation of Na-K-ATPase in gastric epithelium. Am. J. Physiol. Liver Physiol. 2020, 318, G931–G945. [Google Scholar] [CrossRef]
- Navabi, N.; Johansson, M.E.V.; Raghavan, S.; Linden, S.K. Helicobacter pylori Infection Impairs the Mucin Production Rate and Turnover in the Murine Gastric Mucosa. Infect. Immun. 2012, 81, 829–837. [Google Scholar] [CrossRef]
- Fosslien, E. Mitochondrial medicine--molecular pathology of defective oxidative phosphorylation. Ann. Clin. Lab. Sci. 2001, 31, 25–67. [Google Scholar]
- Sachs, G.; Weeks, D.L.; Melchers, K.; Scott, D.R. The Gastric Biology of Helicobacter pylori. Annu. Rev. Physiol. 2003, 65, 349–369. [Google Scholar] [CrossRef]
- Kemmerly, T.; Kaunitz, J.D. Gastroduodenal mucosal defense. Curr. Opin. Gastroenterol. 2013, 29, 642–649. [Google Scholar] [CrossRef]
- De Brito, B.B.; Da Silva, F.A.F.; Soares, A.S.; Pereira, V.A.; Santos, M.L.C.; Sampaio, M.M.; Neves, P.H.M.; Melo, F. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol. 2019, 25, 5578–5589. [Google Scholar] [CrossRef]
- Zhang, X.; Arnold, I.C.; Müller, A. Mechanisms of persistence, innate immune activation and immunomodulation by the gastric pathogen Helicobacter pylori. Curr. Opin. Microbiol. 2020, 54, 1–10. [Google Scholar] [CrossRef]
- Šterbenc, A.; Jarc, E.; Poljak, M.; Homan, M. Helicobacter pylori virulence genes. World J. Gastroenterol. 2019, 25, 4870–4884. [Google Scholar] [CrossRef]
- Marcus, E.A.; Sachs, G.; Scott, D.R. Acid-regulated gene expression of Helicobacter pylori: Insight into acid protection and gastric colonization. Helicobacter 2018, 23, e12490. [Google Scholar] [CrossRef]
- Mayerle, J.; Hoed, C.M.D.; Schurmann, C.; Stolk, L.; Homuth, G.; Peters, M.J.; Capelle, L.G.; Zimmermann, K.; Rivadeneira, F.; Gruska, S.; et al. Identification of Genetic Loci Associated with Helicobacter pylori Serologic Status. JAMA 2013, 309, 1912–1920. [Google Scholar] [CrossRef]
- Hunt, R.H.; Camilleri, M.; Crowe, S.; El-Omar, E.M.; Fox, J.; Kuipers, E.; Malfertheiner, P.; McColl, K.; Pritchard, D.; Rugge, M.; et al. The stomach in health and disease. Gut 2015, 64, 1650–1668. [Google Scholar] [CrossRef]
- Bartels, L.E.; Dahlerup, J.F. Association of Helicobacter pylori and Crohn’s Disease Incidence: An Inversion Reaction? Dig. Dis. Sci. 2017, 62, 2217–2219. [Google Scholar] [CrossRef]
- Ishida, Y. Significant association between Helicobacter pylori infection and serum C-reactive protein. Int. J. Med. Sci. 2008, 224. [Google Scholar] [CrossRef]
- Jackson, L.; Britton, J.; Lewis, S.A.; McKeever, T.M.; Atherton, J.; Fullerton, D.; Fogarty, A.W. A Population-Based Epidemiologic Study of Helicobacter Pylori Infection and its Association with Systemic Inflammation. Helicobacter 2009, 14, 460–465. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Mirzaee, V.; Ahmad-Beygi, H.; Nemati, M.; Rezayati, M.T. Association of the CagA status of Helicobacter pyloriand serum levels of interleukin (IL)-17 and IL-23 in duodenal ulcer patients. J. Dig. Dis. 2009, 10, 107–112. [Google Scholar] [CrossRef]
- Akada, J.; Okuda, M.; Hiramoto, N.; Kitagawa, T.; Zhang, X.; Kamei, S.; Ito, A.; Nakamura, M.; Uchida, T.; Hiwatani, T.; et al. Proteomic Characterization of Helicobacter pylori CagA Antigen Recognized by Child Serum Antibodies and Its Epitope Mapping by Peptide Array. PLoS ONE 2014, 9, e104611. [Google Scholar] [CrossRef]
- Shimoda, A.; Ueda, K.; Nishiumi, S.; Murata-Kamiya, N.; Mukai, S.-A.; Sawada, S.-I.; Azuma, T.; Hatakeyama, M.; Akiyoshi, K. Exosomes as nanocarriers for systemic delivery of the Helicobacter pylori virulence factor CagA. Sci. Rep. 2016, 6, 18346. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc. Jpn. Acad. Ser. B 2017, 93, 196–219. [Google Scholar] [CrossRef] [PubMed]
- Chmiela, M.; Gonciarz, W. Molecular mimicry inHelicobacter pyloriinfections. World J. Gastroenterol. 2017, 23, 3964–3977. [Google Scholar] [CrossRef]
- Franceschi, F.; Sepulveda, A.R.; Gasbarrini, A.; Pola, P.; Silveri, N.G.; Gasbarrini, G.; Graham, D.Y.; Genta, R.M. Cross-Reactivity of Anti-CagA Antibodies with Vascular Wall Antigens. Circulation 2002, 106, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Rožanković, P.B.; Huzjan, A.L.; Cupic, H.; Benčić, I.J.; Bašić, S.; Demarin, V. Influence of CagA-positive Helicobacter pylori strains on atherosclerotic carotid disease. J. Neurol. 2010, 258, 753–761. [Google Scholar] [CrossRef]
- Tohidpour, A. CagA-mediated pathogenesis of Helicobacter pylori. Microb. Pathog. 2016, 93, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and its Pathogenicity. Toxins 2019, 11, 677. [Google Scholar] [CrossRef]
- Knorr, J.; Ricci, V.; Hatakeyama, M.; Backert, S. Classification of Helicobacter pylori Virulence Factors: Is CagA a Toxin or Not? Trends Microbiol. 2019, 27, 731–738. [Google Scholar] [CrossRef]
- Higashi, H.; Tsutsumi, R.; Fujita, A.; Yamazaki, S.; Asaka, M.; Azuma, T.; Hatakeyama, M. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc. Natl. Acad. Sci. USA 2002, 99, 14428–14433. [Google Scholar] [CrossRef]
- Higashi, H.; Tsutsumi, R.; Muto, S.; Sugiyama, T.; Azuma, T.; Asaka, M.; Hatakeyama, M. SHP-2 Tyrosine Phosphatase as an Intracellular Target of Helicobacter pylori CagA Protein. Science 2001, 295, 683–686. [Google Scholar] [CrossRef]
- Higashi, H.; Yokoyama, K.; Fujii, Y.; Ren, S.; Yuasa, H.; IMurata-Kamiya, S.N.; Azuma, T.; Hatakeyama, M. EPIYA motif is a membrane-targeting signal of Helicobacter pylori virulence factor CagA in mammalian cells. J. Biol. Chem. 2005, 280, 23130–23137. [Google Scholar] [CrossRef]
- Backert, S.; Haas, R.; Gerhard, M.; Naumann, M. The Helicobacter pylori Type IV Secretion System Encoded by the cag Pathogenicity Island: Architecture, Function, and Signaling. Future HIV 1 Ther. 2017, 413, 187–220. [Google Scholar]
- Graham, D.Y.; Opekun, A.R.; Belson, G.; El-Zimaity, H.M.T.; Carlson, M.R. Novel bismuth-metronidazole-tetracycline triple-layer tablet for treatment of Helicobacter pylori. Aliment. Pharm. 2005, 21, 165–168. [Google Scholar] [CrossRef]
- Brandt, S.; Kwok, T.; Hartig, R.; Konig, W.; Backert, S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 2005, 102, 9300–9305. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M. Linking epithelial polarity and carcinogenesis by multitasking Helicobacter pylori virulence factor CagA. Oncogene 2008, 27, 7047–7054. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kodama, T.; Gutierrez, O.; Kim, J.G.; Kashima, K.; Graham, D.Y. Relationship between Helicobacter pylori iceA, cagA, and vacA Status and Clinical Outcome: Studies in Four Different Countries. J. Clin. Microbiol. 1999, 37, 2274–2279. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Orito, E.; Mizokami, M.; Gutierrez, O.; Saitou, N.; Kodama, T.; Osato, M.S.; Kim, J.G.; Ramirez, F.C.; Mahachai, V.; et al. Helicobacter pyloriin North and South America before Columbus. FEBS Lett. 2002, 517, 180–184. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kato, M.; Asaka, M. Geographic Differences in Gastric Cancer Incidence Can be Explained by Differences between Helicobacter pylori Strains. Intern. Med. 2008, 47, 1077–1083. [Google Scholar] [CrossRef]
- Yamaoka, Y. Pathogenesis of Helicobacter pylori-Related Gastroduodenal Diseases from Molecular Epidemiological Studies. Gastroenterol. Res. Pract. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Suzuki, H.; Mori, H. Different Pathophysiology of Gastritis between East and West? An Asian Perspective. Inflamm. Intest. Dis. 2016, 1, 123–128. [Google Scholar] [CrossRef]
- Wirth, H.-P.; Yang, M. Different Pathophysiology of Gastritis in East and West? A Western Perspective. Inflamm. Intest. Dis. 2016, 1, 113–122. [Google Scholar] [CrossRef]
- Nakajima, N.; Kuwayama, H.; Ito, Y.; Iwasaki, A.; Arakawa, Y. Helicobacter pylori, Neutrophils, Interleukins, and Gastric Epithelial Proliferation. J. Clin. Gastroenterol. 1997, 25, S198–S202. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Reddy, R.; Graham, D.Y. Helicobacter pylori Virulence Factor Genotypes in Children in the United States: Clues about Genotype and Outcome Relationships. J. Clin. Microbiol. 2010, 48, 2550–2551. [Google Scholar] [CrossRef]
- Selgrad, M.; Tammer, I.; Langner, C.; Bornschein, J.; Meißle, J.; Kandulski, A.; Varbanova, M.; Wex, T.; Schlüter, D.; Malfertheiner, P. Different antibiotic susceptibility between antrum and corpus of the stomach, a possible reason for treatment failure of Helicobacter pyloriinfection. World J. Gastroenterol. 2014, 20, 16245–16251. [Google Scholar] [CrossRef]
- den Hollander, W.J.; Sostres, C.; Kuipers, E.J.; Lanas, A. Helicobacter pyloriand Nonmalignant Diseases. Helicobacter 2013, 18, 24–27. [Google Scholar] [CrossRef]
- Link, A.; Langner, C.; Schirrmeister, W.; Habendorf, W.; Weigt, J.; Venerito, M.; Tammer, I.; Schlüter, D.; Schlaermann, P.; Meyer, T.F.; et al. Helicobacter pylorivacA genotype is a predominant determinant of immune response toHelicobacter pyloriCagA. World J. Gastroenterol. 2017, 23, 4712–4723. [Google Scholar] [CrossRef]
- Stein, M.; Ruggiero, P.; Rappuoli, R.; Bagnoli, F. Helicobacter pylori CagA: From Pathogenic Mechanisms to Its Use as an Anti-Cancer Vaccine. Front. Immunol. 2013, 4, 328. [Google Scholar] [CrossRef]
- Chang, W.-L.; Yeh, Y.-C.; Sheu, B.-S. The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. J. Biomed. Sci. 2018, 25, 68. [Google Scholar] [CrossRef]
- Meng, W.-P.; Wang, Z.-Q.; Deng, J.-Q.; Liu, Y.; Deng, M.-M.; Lü, M.-H. The Role of H. pyloriCagA in Regulating Hormones of Functional Dyspepsia Patients. Gastroenterol. Res. Pract. 2016, 2016, 7150959. [Google Scholar] [CrossRef]
- Van Doorn, L.J.; Figueiredo, C.; Sanna, R.; Blaser, M.J.; Quint, W.G. Distinct variants of Helicobacter pylori cagA are associated with vacA subtypes. J. Clin. Microbiol. 1999, 37, 2306–2311. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Yamaoka, Y. Helicobacter pylorivirulence genes and host genetic polymorphisms as risk factors for peptic ulcer disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1535–1547. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Liu, Y.; Han, J.; Ma, X.; Luo, Y.; Liang, Y.; Zhang, L.; Hu, Y. Association between HLA-Ⅱgene polymorphism and Helicobacter pylori infection in Asian and European population: A meta-analysis. Microb. Pathog. 2015, 82, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, D.; Hu, X.; Li, J.; Cao, M.; Dong, W. Associations between cytokine gene polymorphisms and susceptibility to Helicobacter pylori infection and Helicobacter pylori related gastric cancer, peptic ulcer disease: A meta-analysis. PLoS ONE 2017, 12, e0176463. [Google Scholar] [CrossRef] [PubMed]
- Dooley, C.P.; Cohen, H.; Fitzgibbons, P.L.; Bauer, M.; Appleman, M.D.; Perez, G.I.P.; Blaser, M.J. Prevalence of Helicobacter pyloriInfection and Histologic Gastritis in Asymptomatic Persons. N. Engl. J. Med. 1989, 321, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Blaser, M.J. Bacterial populations as perfect gases: Genomic integrity and diversification tensions in Helicobacter pylori. Nat. Rev. Genet. 2006, 4, 826–836. [Google Scholar] [CrossRef]
- Cover, T.L.; Blaser, M.J. Helicobacter pylori in Health and Disease. Gastroenterology 2009, 136, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Sachs, G.; Scott, D.R. Helicobacter pylori: Eradication or Preservation. F1000 Med. Rep. 2012, 4, 7. [Google Scholar] [PubMed]
- Kayali, S.; Manfredi, M.; Gaiani, F.; Bianchi, L.; Bizzarri, B.; Leandro, G.; di Mario, F.; de Ángelis Gian, L. Helicobacter pylori, transmission routes and recurrence of infection: State of the art. Acta Biomed. 2018, 89, 72–76. [Google Scholar]
- Arnold, I.C.; Hitzler, I.; Müller, A. The Immunomodulatory Properties of Helicobacter pylori Confer Protection Against Allergic and Chronic Inflammatory Disorders. Front. Cell. Infect. Microbiol. 2012, 2, 10. [Google Scholar] [CrossRef]
- Kyburz, A.; Müller, A. Helicobacter pylori and Extragastric Diseases. Future HIV 1 Ther. 2017, 400, 325–347. [Google Scholar]
- Malfertheiner, P. The Intriguing Relationship of Helicobacter pylori Infection and Acid Secretion in Peptic Ulcer Disease and Gastric Cancer. Dig. Dis. 2011, 29, 459–464. [Google Scholar] [CrossRef]
- Walker, M.M.; Talley, N.J. Review article: Bacteria and pathogenesis of disease in the upper gastrointestinal tract-beyond the era of Helicobacter pylori. Aliment. Pharm. 2014, 39, 767–779. [Google Scholar] [CrossRef]
- Paoluzi, O.A.; Blanco, D.V.G.; Caruso, R.; Monteleone, I.; Monteleone, G.; Pallone, F. Impairment of ghrelin synthesis inHelicobacter pylori-colonized stomach: New clues for the pathogenesis of H. pylori-related gastric inflammation. World J. Gastroenterol. 2014, 20, 639–646. [Google Scholar] [CrossRef]
- Schubert, M.L. Physiologic, pathophysiologic, and pharmacologic regulation of gastric acid secretion. Curr. Opin. Gastroenterol. 2017, 33, 430–438. [Google Scholar] [CrossRef]
- Burclaff, J.; Osaki, L.H.; Liu, D.; Goldenring, J.R.; Mills, J.C. Targeted Apoptosis of Parietal Cells Is Insufficient to Induce Metaplasia in Stomach. Gastroenterology 2017, 152, 762–766.e7. [Google Scholar] [CrossRef]
- Testerman, T.L. Beyond the stomach: An updated view of Helicobacter pyloripathogenesis, diagnosis, and treatment. World J. Gastroenterol. 2014, 20, 12781–12808. [Google Scholar] [CrossRef]
- Zavos, C.; Kountouras, J.; Sakkias, G.; Venizelos, I.; Deretzi, G.; Arapoglou, S. Histological Presence of Helicobacter pylori Bacteria in the Trabeculum and Iris of Patients with Primary Open-Angle Glaucoma. Ophthalmic Res. 2012, 47, 150–156. [Google Scholar] [CrossRef]
- Al Sayed, A.; Anand, P.S.; Kamath, K.P.; Patil, S.; Preethanath, R.S.; Anil, S. Oral Cavity as an Extragastric Reservoir of Helicobacter pylori. ISRN Gastroenterol. 2014, 2014, 261369. [Google Scholar] [CrossRef]
- Payão, S.L.M.; Rasmussen, L.T. Helicobacter pyloriand its reservoirs: A correlation with the gastric infection. World J. Gastrointest. Pharm. 2016, 7, 126–132. [Google Scholar] [CrossRef]
- Yee, J.K.C. Are the view of Helicobacter pylori colonized in the oral cavity an illusion? Exp. Mol. Med. 2017, 49, e397. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Bielanski, W.; Guzik, T.J.; Loster, B.; Konturek, S.J. Helicobacter pylori in the oral cavity and its implications for gastric infection, periodontal health, immunology and dyspepsia. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2005, 77–89. [Google Scholar]
- Veiga, N.; Pereira, C.; Resende, C.; Amaral, O.; Ferreira, M.; Nelas, P.; Chaves, C.; Duarte, J.; Cirnes, L.; Machado, J.C.; et al. Oral and Gastric Helicobacter Pylori: Effects and Associations. PLoS ONE 2015, 10, e0126923. [Google Scholar] [CrossRef]
- Malfertheiner, M.V.; Kandulski, A.; Schreiber, J.; Malfertheiner, P. Helicobacter pylori Infection and the Respiratory System: A Systematic Review of the Literature. Digestion 2011, 84, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Jahromy, S.H.; Siavoshi, F.; Malekzadeh, R.; Sattari, T.N.; Latifi-Navid, S. Reciprocal impact of host factors andHelicobacter pylorigenotypes on gastric diseases. World J. Gastroenterol. 2015, 21, 9317–9327. [Google Scholar] [CrossRef] [PubMed]
- Zabaleta, J. MicroRNA: A Bridge from H. pylori Infection to Gastritis and Gastric Cancer Development. Front. Genet. 2012, 3, 294. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-M.; Hsu, T.-Y.; Chen, C.-Y.; Lin, C.-L.; Kao, C.-H.; Chen, C.-H.; Yang, T.-Y.; Chen, W.-K. Analysis of Patients with Helicobacter pylori Infection and the Subsequent Risk of Developing Osteoporosis after Eradication Therapy: A Nationwide Population-Based Cohort Study. PLoS ONE 2016, 11, e0162645. [Google Scholar] [CrossRef] [PubMed]
- Kalantarhormozi, M.R.; Assadi, M.; Vahdat, K.; Asadipooya, K.; Ostovar, A.; Raissi, K.; Darabi, H.; Farrokhi, S.; Dobaradaran, S.; Farrokhnia, M.; et al. Chlamydia pneumoniae and Helicobacter pylori IgG seropositivities are not predictors of osteoporosis-associated bone loss: A prospective cohort study. J. Bone Miner. Metab. 2015, 34, 422–428. [Google Scholar] [CrossRef]
- Chung, Y.H.; Gwak, J.S.; Hong, S.W.; Hyeon, J.H.; Lee, C.M.; Oh, S.W.; Kwon, H. Helicobacter pylori: A Possible Risk Factor for Bone Health. Korean J. Fam. Med. 2015, 36, 239–244. [Google Scholar] [CrossRef][Green Version]
- Figura, N.; Gennari, L.; Merlotti, D.; Lenzi, C.; Campagna, M.S.; Franci, M.B.; Lucani, B.; Trabalzini, L.; Bianciardi, L.; Gonnelli, C.; et al. Prevalence of Helicobacter pylori Infection in Male Patients with Osteoporosis and Controls. Dig. Dis. Sci. 2005, 50, 847–852. [Google Scholar] [CrossRef]
- Figura, N.; Gennai, L.; Merlotti, D.; Campagna, M.; Franci, B.; Avanzati, A.; Lucani, B.; Calabro, A.; Lardiello, S.; Nuti, R. H. pylori (HP) infection and osteoporosis: A population based study. In Proceedings of the European Helicobacter Study 23nd International Workshop on Helicobacter Related Bacteria in Chronic Digestive Inflammation and Gastric Cancer, Rotterdam, The Netherlands, 16–18 September 2010; Blackwell Publishing Ltd., Helicobacter: Rotterdam, The Netherlands, 2010; pp. 334–335. [Google Scholar]
- Cooper, C.; Cole, Z.A.; Holroyd, C.R.; Earl, S.C.; Harvey, N.C.; Dennison, E.M.; Melton, L.J.; Cummings, S.R.; Kanis, J.A.; The IOF CSA Working Group on Fracture Epidemiology. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos. Int. 2011, 22, 1277–1288. [Google Scholar] [CrossRef]
- Ballane, G.; Cauley, A.J.; Luckey, M.M.; Fuleihan, G.E.-H. Secular Trends in Hip Fractures Worldwide: Opposing Trends East Versus West. J. Bone Miner. Res. 2014, 29, 1745–1755. [Google Scholar] [CrossRef]
- Ballane, G.; Cauley, J.A.; Luckey, M.M.; Fuleihan, G.E.-H. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos. Int. 2017, 28, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.-L.; Bin Ang, S.; Chadha, M.; Chow, E.S.-L.; Chung, Y.-S.; Hew, F.L.; Jaisamrarn, U.; Ng, H.; Takeuchi, Y.; Wu, C.-H.; et al. An updated hip fracture projection in Asia: The Asian Federation of Osteoporosis Societies study. Osteoporos. Sarcopenia 2018, 4, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Thambiah, S.C.; Yeap, S.S. Osteoporosis in South-East Asian Countries. Clin. Biochem. Rev. 2020, 41, 29–40. [Google Scholar] [PubMed]
- Ozdem, S.; Akcam, M.; Yilmaz, A.; Gultekin, M.; Artan, R. Biochemical Markers of Bone Metabolism in Children with Helicobacter pylori Infection. Dig. Dis. Sci. 2007, 52, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, D.; Nagahara, A.; Shimada, Y.; Matsumoto, K.; Ueyama, H.; Matsumoto, K.; Nakagawa, Y.; Takeda, T.; Tanaka, I.; Sasaki, H.; et al. Risk factors for osteoporosis in Japan: Is it associated with Helicobacter pylori? Clin. Risk Manag. 2015, 11, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Kakehasi, A.M.; Mendes, C.M.C.; Coelho, L.G.V.; Castro, L.P.; Barbosa, A.J.A. The presence of Helicobacter Pylori in postmenopausal women is not a factor to the decrease of bone mineral density. Arq. Gastroenterol. 2007, 44, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Kakehasi, A.M.; Rodrigues, C.B.; Carvalho, A.V.; Barbosa, A.J.A. Chronic Gastritis and Bone Mineral Density in Women. Dig. Dis. Sci. 2008, 54, 819–824. [Google Scholar] [CrossRef]
- Akkaya, N.; Akkaya, S.; Polat, Y.; Turk, M.; Turk, T.; Turhal, E.; Sahin, F. Helicobacter pylori seropositivity in fibromyalgia syndrome. Clin. Rheumatol. 2011, 30, 43–49. [Google Scholar] [CrossRef]
- Asaoka, D.; Nagahara, A.; Hojo, M.; Sasaki, H.; Shimada, Y.; Yoshizawa, T.; Osada, T.; Watanabe, S. The Relationship between H. pyloriInfection and Osteoporosis in Japan. Gastroenterol. Res. Pract. 2014, 2014, 340765. [Google Scholar] [CrossRef]
- Lin, S.-C.; Koo, M.; Tsai, K.-W. Association betweenHelicobacter pyloriInfection and Risk of Osteoporosis in Elderly Taiwanese Women with Upper Gastrointestinal Diseases: A Retrospective Patient Record Review. Gastroenterol. Res. Pract. 2014, 2014, 814756. [Google Scholar] [CrossRef]
- Mizuno, S.; Matsui, D.; Watanabe, I.; Ozaki, E.; Kuriyama, N.; Watanabe, Y. Serologically Determined Gastric Mucosal Condition Is a Predictive Factor for Osteoporosis in Japanese Men. Dig. Dis. Sci. 2015, 60, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Fotouk-Kiai, M.; Hoseini, S.R.; Meftah, N.; Ghadimi, R.; Bijani, A.; Noreddini, H.; Nematollahi, H.; Shokri-Shirvani, J. Relationship between Helicobacter pylori infection (HP) and bone mineral density (BMD) in elderly people. Casp. J. Intern. Med. 2015, 6, 62–66. [Google Scholar]
- Chen, L.-W.; Chen, F.-P.; Hsieh, C.-W.; Kuo, S.-F.; Chien, R.-N. Analysis of the associations among Helicobacter pylori infection, adiponectin, leptin, and 10-year fracture risk using the fracture risk assessment tool: A cross-sectional community-based study. PLoS ONE 2017, 12, e0175365. [Google Scholar] [CrossRef]
- Chinda, D.; Shimoyama, T.; Iino, C.; Matsuzaka, M.; Nakaji, S.; Fukuda, S. Decrease of Estradiol and Several Lifestyle Factors, but Not Helicobacter pylori Infection, Are Significant Risks for Osteopenia in Japanese Females. Digestion 2017, 96, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, N.; Aghaei, M.; Naghdi, M. AB0850 Helicobacter pylori infection and osteoporosis in post monopausal women. Ann. Eur. Congr. Rheumatol. 2017, 76, 1354. [Google Scholar]
- Pan, B.-L.; Huang, C.-F.; Chuah, S.-K.; Chiang, J.-C.; Loke, S.-S. Relationship between Helicobacter pylori infection and bone mineral density: A retrospective cross-sectional study. BMC Gastroenterol. 2018, 18, 54. [Google Scholar] [CrossRef]
- Chinda, D.; Shimoyama, T.; Sawada, K.; Iino, C.; Sakuraba, H.; Nakaji, S.; Fukuda, S. Lifestyle Factors Rather Than Helicobacter pylori Infection or Estradiol Level are Associated With Osteopenia in Japanese Men. Am. J. Men’s Health 2019, 13, 1557988319848219. [Google Scholar] [CrossRef] [PubMed]
- Santavirta, S.; Konttinen, Y.T.; Heliövaara, M.; Knekt, P.; Lüthje, P.; Aromaa, A. Determinants of osteoporotic thoracic vertebral fracture. Acta Orthop. Scand. 1992, 63, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, A.; Regula, A.; Godwod, K.; Debinski, A. Peptic ulcer disease and calcium intake as risk factors of osteoporosis in women. Osteoporos. Int. 2003, 14, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Lewallen, D.G. Association of peptic ulcer disease and pulmonary disease with risk of periprosthetic fracture after primary total knee arthroplasty. Arthritis Rheum. 2011, 63, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Lewallen, D.G. Peptic ulcer disease and heart disease are associated with periprosthetic fractures after total hip replacement. Acta Orthop. 2012, 83, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Tung, Y.-C.; Chaiter, Y.; Lu, Y.-Y.; Su, Y.-F.; Tsai, T.-H.; Kuo, K.-L.; Lin, C.-L. Increased Risk of Osteoporosis in Patients With Peptic Ulcer Disease. Medcine 2016, 95, e3309. [Google Scholar] [CrossRef] [PubMed]
- Yoon, P.H.; An, S.J.; Jeong, S.-H.; Yang, Y.-J.; Hong, Y.-P. Association between Peptic Ulcer Disease and Osteoporosis: The Population-Based Longitudinal Cohort Study in Korea. Int. J. Environ. Res. Public Health 2019, 16, 2777. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Rhim, C.C.; Yoon, J.Y.; Park, B.J.; Min, C.Y.; Lee, S.W. Increased risk of osteoporosis in patients with peptic ulcer: A follow-up study using a national sample cohort. Arch. Osteoporos. 2019, 14, 105. [Google Scholar] [CrossRef]
- Melton, L.J.; Crowson, C.S.; Khosla, S.; O’Fallon, W.M. Fracture risk after surgery for peptic ulcer disease: A population-based cohort study. Bone 1999, 25, 61–67. [Google Scholar] [CrossRef]
- Jeffery, P.L.; McGuckin, M.A.; Linden, S.K. Endocrine impact of Helicobacter pylori: Focus on ghrelin and ghrelin o-acyltransferase. World J. Gastroenterol. 2011, 17, 1249–1260. [Google Scholar] [CrossRef]
- Massironi, S.; Cavalcoli, F.; Rossi, R.E.; Conte, D.; Spampatti, M.P.; Ciafardini, C.; Verga, U.; Beck-Peccoz, P.; Peracchi, M. Chronic autoimmune atrophic gastritis associated with primary hyperparathyroidism: A transversal prospective study. Eur. J. Endocrinol. 2013, 168, 755–761. [Google Scholar] [CrossRef][Green Version]
- Lahner, E.; Annibale, B. Pernicious anemia: New insights from a gastroenterological point of view. World J. Gastroenterol. 2009, 15, 5121–5128. [Google Scholar] [CrossRef]
- Toh, B.-H.; Kyaw, T.; Taylor, R.; Pollock, W.; Schlumberger, W. Parietal cell antibody identified by ELISA is superior to immunofluorescence, rises with age and is associated with intrinsic factor antibody. Autoimmunity 2012, 45, 527–532. [Google Scholar] [CrossRef]
- Tozzoli, R.; Kodermaz, G.; Perosa, A.R.; Tampoia, M.; Zucano, A.; Antico, A.; Bizzaro, N. Autoantibodies to parietal cells as predictors of atrophic body gastritis: A five-year prospective study in patients with autoimmune thyroid diseases. Autoimmun. Rev. 2010, 10, 80–83. [Google Scholar] [CrossRef]
- Abe, T.; Kodama, M.; Murakami, K.; Matsunari, O.; Mizukami, K.; Inoue, K.; Uchida, M.; Okimoto, T.; Fujioka, T.; Uchida, T.; et al. Impact of Helicobacter pylori CagA diversity on gastric mucosal damage: An immunohistochemical study of East-Asian-type CagA. J. Gastroenterol. Hepatol. 2011, 26, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, A.; Lash, R.H.; Genta, R.M. A National Study of Helicobactor pylori Infection in Gastric Biopsy Specimens. Gastroenterology 2010, 139, 1894–1901.e2. [Google Scholar] [CrossRef]
- Heidari, B. Helicobacter pylori infection and osteoporosis in elderly patients. Casp. J. Intern. Med. 2015, 6, 48–50. [Google Scholar]
- Aasarød, K.M.; Mosti, M.P.; Stunes, A. (Astrid); Reseland, J.E.; Basso, T.; Syversen, U.; Fossmark, R. Impaired skeletal health in patients with chronic atrophic gastritis. Scand. J. Gastroenterol. 2016, 51, 774–781. [Google Scholar]
- Kim, H.W.; Kim, Y.-H.; Han, K.; Nam, G.E.; Kim, G.S.; Han, B.-D.; Lee, A.; Ahn, J.Y.; Ko, B.J. Atrophic Gastritis: A Related Factor for Osteoporosis in Elderly Women. PLoS ONE 2014, 9, e101852. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Hadji, P.; Kostev, K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric 2016, 19, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.-S.; Ko, H.-J. Atrophic Gastritis as a Risk Factor for Bone Loss in Premenopausal Women in Their 40s: A Retrospective Cohort Study. Calcif. Tissue Int. 2018, 104, 34–41. [Google Scholar] [CrossRef]
- Muhsen, K.; Sinnreich, R.; Beer-Davidson, G.; Nassar, H.; Cohen, D.; Kark, J.D. Sero-prevalence of Helicobacter pylori CagA immunoglobulin G antibody, serum pepsinogens and haemoglobin levels in adults. Sci. Rep. 2018, 8, 17616. [Google Scholar] [CrossRef]
- Betesh, A.L.; Ana, C.A.S.; Cole, J.A.; Fordtran, J.S. Is achlorhydria a cause of iron deficiency anemia? Am. J. Clin. Nutr. 2015, 102, 9–19. [Google Scholar] [CrossRef]
- Amedei, A.; Bergman, M.P.; Appelmelk, B.J.; Azzurri, A.; Benagiano, M.; Tamburini, C.; Van Der Zee, R.; Telford, J.L.; Vandenbroucke-Grauls, C.M.J.E.; D’Elios, M.M.; et al. Molecular Mimicry between Helicobacter pylori Antigens and H+,K+–Adenosine Triphosphatase in Human Gastric Autoimmunity. J. Exp. Med. 2003, 198, 1147–1156. [Google Scholar] [CrossRef]
- Berman, A.G.; Organ, J.M.; Allen, M.R.; Wallace, J. Muscle contraction induces osteogenic levels of cortical bone strain despite muscle weakness in a mouse model of Osteogenesis Imperfecta. Bone 2020, 132, 115061. [Google Scholar] [CrossRef]
- Weck, M.N.; Gao, L.; Brenner, H. Helicobacter pylori Infection and Chronic Atrophic Gastritis. Epidemiology 2009, 20, 569–574. [Google Scholar] [CrossRef]
- Veijola, L.; Oksanen, A.M.; Sipponen, P.I.; Rautelin, H.I.K. Association of autoimmune type atrophic corpus gastritis with Helicobacter pylori infection. World J. Gastroenterol. 2010, 16, 83–88. [Google Scholar]
- Demiroğlu, H.; Dündar, S. Pernicious anaemia patients should be screened for iron deficiency during follow up. N. Z. Med. J. 1997, 110, 147–148. [Google Scholar]
- Dickey, W. Iron deficiency, gastric atrophy and Helicobacter pylori. Dig. Liver Dis. 2002, 34, 313–315. [Google Scholar] [CrossRef]
- Hershko, C.; Ronson, A.; Souroujon, M.; Maschler, I.; Heyd, J.; Patz, J. Variable hematologic presentation of autoimmune gastritis: Age-related progression from iron deficiency to cobalamin depletion. Blood 2006, 107, 1673–1679. [Google Scholar] [CrossRef]
- Hershko, C.; Patz, J.; Ronson, A. The anemia of achylia gastrica revisited. Blood Cells Mol. Dis. 2007, 39, 178–183. [Google Scholar] [CrossRef]
- Cavalcoli, F.; Zilli, A.; Conte, D.; Massironi, S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: A review. World J. Gastroenterol. 2017, 23, 563–572. [Google Scholar] [CrossRef]
- Çağdaş, K.; Soykan, I. Utility of a laboratory score in the prediction of gastric emptying in autoimmune gastritis patients. Acta Clin. Belg. 2017, 73, 75–79. [Google Scholar]
- Çağdaş, K.; Soykan, I. Polyautoimmunity in autoimmune gastritis. Eur. J. Intern. Med. 2016, 31, 79–83. [Google Scholar] [CrossRef]
- Carmel, R. Cobalamin, the stomach, and aging. Am. J. Clin. Nutr. 1997, 66, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, C.-H.; Park, J.; Lee, K.-U.; Park, C. Effects of vitamin B12 on cell proliferation and cellular alkaline phosphatase activity in human bone marrow stromal osteoprogenitor cells and UMR106 osteoblastic cells. Metabolism 1996, 45, 1443–1446. [Google Scholar] [CrossRef]
- Tucker, K.L.; Hannan, M.T.; Qiao, N.; Jacques, P.F.; Selhub, J.; Cupples, L.A.; Kiel, D.P. Low plasma vitamin B12 is associated with lower BMD: The Framingham Osteoporosis Study. J. Bone Miner. Res. 2005, 20, 152–158. [Google Scholar] [CrossRef]
- Sato, Y.; Honda, Y.; Iwamoto, J.; Kanoko, T.; Satoh, K. Effect of Folate and Mecobalamin on Hip Fractures in Patients with Stroke. JAMA 2005, 293, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R.; Jacques, P.F.; Selhub, J.; Fredman, L.; Tucker, K.L.; Samelson, E.J.; Kiel, D.P.; Cupples, L.A.; Hannan, M.T. Plasma B vitamins, homocysteine, and their relation with bone loss and hip fracture in elderly men and women. J. Clin. Endocrinol. Metab. 2008, 93, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- Swart, K.M.; Van Schoor, N.M.; Lips, P. Vitamin B12, Folic Acid, and Bone. Curr. Osteoporos. Rep. 2013, 11, 213–218. [Google Scholar] [CrossRef]
- Lewerin, C.; Nilsson-Ehle, H.; Jacobsson, S.; Johansson, H.; Sundh, V.; Karlsson, M.K.; Ljunggren, O.; Lorentzon, M.; Kanis, J.; Lerner, U.H.; et al. Low holotranscobalamin and cobalamins predict incident fractures in elderly men: The MrOS Sweden. Osteoporos. Int. 2013, 25, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Koh, W.-P. B-Vitamins and Bone Health–A Review of the Current Evidence. Nutrients 2015, 7, 3322–3346. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K. The Effects of Homocysteine on the Skeleton. Curr. Osteoporos. Rep. 2018, 16, 554–560. [Google Scholar] [CrossRef]
- Su, Y.; Elshorbagy, A.; Turner, C.; Refsum, H.; Chan, R.; Kwok, T.C.Y. Circulating amino acids are associated with bone mineral density decline and ten-year major osteoporotic fracture risk in older community-dwelling adults. Bone 2019, 129, 115082. [Google Scholar] [CrossRef]
- Merriman, N.A.; Putt, M.E.; Metz, D.C.; Yang, Y.-X. Hip Fracture Risk in Patients with a Diagnosis of Pernicious Anemia. Gastroenterology 2010, 138, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Goerss, J.B.; Kim, C.H.; Atkinson, E.J.; Eastell, R.; O’Fallon, W.M.; Melton, L.J. Risk of fractures in patients with pernicious anemia. J. Bone Miner. Res. 2009, 7, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-T.; Zhao, H.-Y.; Kong, Y.; Sun, N.-N.; Dong, A.-Q. Correlation between serum vitamin B12 level and peripheral neuropathy in atrophic gastritis. World J. Gastroenterol. 2018, 24, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Melton, M.E.; Kochman, M.L. Reversal of severe osteoporosis with vitamin B12 and etidronate therapy in a patient with pernicious anemia. Metabolism 1994, 43, 468–469. [Google Scholar] [CrossRef]
- Lopez, M.G.; Baron, J.A.; Omsland, T.K.; Søgaard, A.J.; Meyer, H.E. Homocysteine-Lowering Treatment and the Risk of Fracture: Secondary Analysis of a Randomized Controlled Trial and an Updated Meta-Analysis. JBMR Plus 2018, 2, 295–303. [Google Scholar] [CrossRef]
- Stone, K.L.; Lui, L.-Y.; Christen, W.G.; Troen, A.M.; Bauer, D.C.; Kado, D.; Schambach, C.; Cummings, S.R.; Manson, J.E. Effect of Combination Folic Acid, Vitamin B6, and Vitamin B12Supplementation on Fracture Risk in Women: A Randomized, Controlled Trial. J. Bone Miner. Res. 2017, 32, 2331–2338. [Google Scholar] [CrossRef]
- Massironi, S.; Cavalcoli, F.; Zilli, A.; Del Gobbo, A.; Ciafardini, C.; Bernasconi, S.; Felicetta, I.; Conte, D.; Peracchi, M. Relevance of vitamin D deficiency in patients with chronic autoimmune atrophic gastritis: A prospective study. BMC Gastroenterol. 2018, 18, 172. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Lauretani, F.; Penninx, B.W.H.J.; Bartali, B.; Russo, R.; Cherubini, A.; Woodman, R.; Bandinelli, S.; Guralnik, J.M.; et al. Bone density and hemoglobin levels in older persons: Results from the InCHIANTI study. Osteoporos. Int. 2004, 16, 691–699. [Google Scholar] [CrossRef]
- Laudisio, A.; Marzetti, E.; Pagano, F.; Bernabei, R.; Zuccalà, G. Haemoglobin levels are associated with bone mineral density in the elderly: A population-based study. Clin. Rheumatol. 2008, 28, 145–151. [Google Scholar] [CrossRef]
- Chen, Z.; Thomson, C.A.; Aickin, M.; Nicholas, J.S.; Van Wyck, D.; Lewis, C.E.; Cauley, J.A.; Bassford, T. Short list of Women’s Health Initiative Investigators The relationship between incidence of fractures and anemia in older multiethnic women. J. Am. Geriatr. Soc. 2010, 58, 2337–2344. [Google Scholar] [CrossRef]
- Korkmaz, U.; Korkmaz, N.; Yazıcı, S.; Erkan, M.; Baki, A.E.; Yazici, M.; Özhan, H.; Ataoglu, S.; Yazici, S. Anemia as a risk factor for low bone mineral density in postmenopausal Turkish women. Eur. J. Intern. Med. 2012, 23, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Rutten, E.P.; Franssen, F.M.E.; Spruit, M.A.; Wouters, E.F.M. Anemia is associated with bone mineral density in chronic obstructive pulmonary disease. Copd J. Chronic Obs. Pulm. Dis. 2012, 10, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.H.; Moon, J.H.; Cho, B. Association between Hemoglobin Level and Bone Mineral Density in Korean Adults. J. Bone Metab. 2017, 24, 161–173. [Google Scholar] [CrossRef][Green Version]
- Valderrábano, R.J.; Lee, J.; Lui, L.-Y.; Hoffman, A.R.; Cummings, S.R.; Orwoll, E.S.; Wu, J.Y.; Osteoporotic Fractures in Men (MrOS) Study Research Group. Older Men with Anemia Have Increased Fracture Risk Independent of Bone Mineral Density. J. Clin. Endocrinol. Metab. 2017, 102, 2199–2206. [Google Scholar]
- Chuang, M.-H.; Chuang, T.-L.; Koo, M.; Wang, Y.-F. Low Hemoglobin Is Associated with Low Bone Mineral Density and High Risk of Bone Fracture in Male Adults: A Retrospective Medical Record Review Study. Am. J. Men’s Health 2019, 13, 1557988319850378. [Google Scholar] [CrossRef]
- Valderrábano, R.J.; Wu, J.Y. Bone and blood interactions in human health and disease. Bone 2019, 119, 65–70. [Google Scholar] [CrossRef]
- Lu, M.; Liu, Y.; Shao, M.; Tesfaye, G.C.; Yang, S. Associations of Iron Intake, Serum Iron and Serum Ferritin with Bone Mineral Density in Women: The National Health and Nutrition Examination Survey, 2005–2010. Calcif. Tissue Int. 2019, 106, 232–238. [Google Scholar] [CrossRef]
- Ohkusa, T.; Fujiki, K.; Takashimizu, I.; Kumagai, J.; Tanizawa, T.; Eishi, Y.; Yokoyama, T.; Watanabe, M. Improvement in atrophic gastritis and intestinal metaplasia in patients in whom Helicobacter pylori was eradicated. Ann. Intern. Med. 2001, 134, 380–386. [Google Scholar] [CrossRef]
- Ito, M.; Haruma, K.; Kamada, T.; Mihara, M.; Kim, S.; Kitadai, Y.; Sumii, M.; Tanaka, S.; Yoshihara, M.; Chayama, K. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: A 5-year prospective study of patients with atrophic gastritis. Aliment. Pharm. 2002, 16, 1449–1456. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Kim, N.; Lee, H.S.; Lee, J.B.; Choi, Y.J.; Yoon, H.; Shin, C.M.; Park, Y.S.; Lee, D.H. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication—A prospective study for up to 10 years. Aliment. Pharm. 2018, 47, 380–390. [Google Scholar] [CrossRef]
- Sierra, R.; Une, C.; Ramírez, V.; Alpízar-Alpízar, W.; González, I.M.; Ramírez, J.A.; De Mascarel, A.; Cuenca, P.; Perez, G.I.P.; Mégraud, F. Relation of atrophic gastritis with Helicobacter pylori-CagA+ and interleukin-1 gene polymorphisms. World J. Gastroenterol. 2008, 14, 6481–6487. [Google Scholar] [CrossRef]
- Gao, L.; Weck, M.N.; Nieters, A.; Brenner, H. Inverse association between a pro-inflammatory genetic profile and Helicobacter pylori seropositivity among patients with chronic atrophic gastritis: Enhanced elimination of the infection during disease progression? Eur. J. Cancer 2009, 45, 2860–2866. [Google Scholar] [CrossRef]
- IAR. Schistosomes, Liver Flukes and Helicobacter pylori; International Agency for Research on Cancer: Lyon, France, 1994; Volume 61, pp. 1–241. [Google Scholar]
- Kamangar, F.; Dawsey, S.M.; Blaser, M.J.; Perez-Perez, G.I.; Pietinen, P.; Newschaffer, C.J.; Abnet, C.C.; Albanes, D.; Virtamo, J.; Taylor, P.R. Opposing Risks of Gastric Cardia and Noncardia Gastric Adenocarcinomas Associated with Helicobacter pylori Seropositivity. J. Natl. Cancer Inst. 2006, 98, 1445–1452. [Google Scholar] [CrossRef]
- Amieva, M.; Peek, R.M. Pathobiology of Helicobacter pylori–Induced Gastric Cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef]
- Bakhti, S.Z.; Latifi-Navid, S.; Safaralizadeh, R. Helicobacter pylori-related risk predictors of gastric cancer: The latest models, challenges, and future prospects. Cancer Med. 2020, 9, 4808–4822. [Google Scholar] [CrossRef]
- Vohlonen, I.; Pukkala, E.; Malila, N.; Härkönen, M.; Hakama, M.; Koistinen, V.; Sipponen, P. Risk of gastric cancer in Helicobacter pylori infection in a 15-year follow-up. Scand. J. Gastroenterol. 2016, 51, 1159–1164. [Google Scholar] [CrossRef][Green Version]
- Nomura, A.M.Y.; Lee, J.; Stemmermann, G.N.; Nomura, R.Y.; Perez, G.I.P.; Blaser, M.J. Helicobacter pyloriCagA Seropositivity and Gastric Carcinoma Risk in a Japanese American Population. J. Infect. Dis. 2002, 186, 1138–1144. [Google Scholar] [CrossRef]
- Kusters, J.G.; van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef]
- Park, J.Y.; Forman, D.; Waskito, L.A.; Yamaoka, Y.; Crabtree, J.E. Epidemiology of Helicobacter pylori and CagA-Positive Infections and Global Variations in Gastric Cancer. Toxins 2018, 10, 163. [Google Scholar] [CrossRef]
- Liedman, B.; Henningsson, A.; Mellström, D.; Lundell, L. Changes in Bone Metabolism and Body Composition After Total Gastrectomy. Dig. Dis. Sci. 2000, 45, 819–824. [Google Scholar] [CrossRef]
- Lai, S.-W.; Kuo, Y.-H.; Lai, S.-W. Increased risk of osteoporotic fractures in patients with gastric cancer and post-gastrectomy. Bone 2020, 132, 115185. [Google Scholar] [CrossRef]
- Zittel, T.T.; Zeeb, B.; Maier, G.W.; Kaiser, G.W.; Zwirner, M.; Liebich, H.; Starlinger, M.; Becker, H.D. High prevalence of bone disorders after gastrectomy. Am. J. Surg. 1997, 174, 431–438. [Google Scholar] [CrossRef]
- Kanis, J.; Johnell, O.; Gullberg, B.; Allander, E.; Elffors, L.; Ranstam, J.; Dequeker, J.; Dilsen, G.; Gennari, C.; Vaz, A.L.; et al. Risk Factors for Hip Fracture in Men from Southern Europe: The MEDOS Study. Osteoporos. Int. 1999, 9, 45–54. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, S.B.; Bang, H.Y.; Cheon, G.J.; Lee, J.I. High prevalence of osteoporosis in patients with gastric adenocarcinoma following gastrectomy. World J. Gastroenterol. 2007, 13, 6492–6497. [Google Scholar] [CrossRef]
- Baek, K.H.; Jeon, H.M.; Lee, S.S.; Lim, D.-J.; Oh, K.W.; Lee, W.-Y.; Rhee, E.-J.; Han, J.H.; Cha, B.Y.; Lee, K.W.; et al. Short-term changes in bone and mineral metabolism following gastrectomy in gastric cancer patients. Bone 2008, 42, 61–67. [Google Scholar] [CrossRef]
- Lim, J.S.; Lee, J.-I. Prevalence, Pathophysiology, Screening and Management of Osteoporosis in Gastric Cancer Patients. J. Gastric Cancer 2011, 11, 7–15. [Google Scholar] [CrossRef]
- Krupski, W.; Tatara, M.R.; Bury, P.; Szabelska, A.; Charuta, A.; Maciejewski, R.; Wallner, G.; Dabrowski, A. Negative Effects of Total Gastrectomy on Bone Tissue Metabolism and Volumetric Bone Mineral Density (vBMD) of Lumbar Spine in 1-Year Study in Men. Medcine 2016, 95, e2817. [Google Scholar] [CrossRef]
- Oh, H.J.; Lim, C.-H.; Yoon, B.-H.; Yoon, S.B.; Baeg, M.K.; Kim, W.C.; Choi, M.-G.; Park, J.M.; Choi, M.-G.; Yoo, H.M.; et al. Fracture after gastrectomy for gastric cancer: A long-term follow-up observational study. Eur. J. Cancer 2017, 72, 28–36. [Google Scholar] [CrossRef]
- Yoo, S.H.; Lee, J.A.; Kang, S.Y.; Kim, Y.S.; Sunwoo, S.; Kim, B.S.; Yook, J.-H. Risk of osteoporosis after gastrectomy in long-term gastric cancer survivors. Gastric Cancer 2017, 21, 720–727. [Google Scholar] [CrossRef]
- Noh, H.M.; Yoo, J.-H.; Jeong, J.Y.; Park, Y.S. Bone mineral density after treatment for gastric cancer. Medcine 2018, 97, e9582. [Google Scholar] [CrossRef]
- Seo, G.H.; Kang, H.Y.; Choe, E.K. Osteoporosis and fracture after gastrectomy for stomach cancer. Medcine 2018, 97, e0532. [Google Scholar] [CrossRef]
- Iki, M.; Fujita, Y.; Kouda, K.; Yura, A.; Tachiki, T.; Tamaki, J.; Sato, Y.; Moon, J.-S.; Hamada, M.; Kajita, E.; et al. Increased risk of osteoporotic fracture in community-dwelling elderly men 20 or more years after gastrectomy: The Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Cohort Study. Bone 2019, 127, 250–259. [Google Scholar] [CrossRef]
- Inoue, K.; Shiomi, K.; Higashide, S.; Kan, N.; Nio, Y.; Tobe, T.; Shigeno, C.; Konishi, J.; Okumurat, H.; Yamamuro, T.; et al. Metabolic bone disease following gastrectomy: Assessment by dual energy X-ray absorptiometry. BJS 1992, 79, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, J.T.; Kröger, H.; Pääkkönen, M.; Parviainen, M.T.; Lamberg-Allardt, C.; Alhava, E. Bone mineral metabolism after total gastrectomy. Bone 2001, 28, 123–127. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Shin, N.W.; Lee, J.E.; Jin, S.-M.; Kim, S. Increased Risk of Osteoporosis in Gastric Cancer Survivors Compared to General Population Control: A Study with Representative Korean Population. Cancer Res. Treat. 2018, 51, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.W.; Suh, B.; Lim, H.; Suh, Y.-S.; Choi, Y.J.; Jeong, S.-M.; Yun, J.M.; Song, S.O.; Park, Y. Increased Risk of Osteoporotic Fracture in Postgastrectomy Gastric Cancer Survivors Compared With Matched Controls: A Nationwide Cohort Study in Korea. Am. J. Gastroenterol. 2019, 114, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Tachiki, T.; Kouda, K.; Dongmei, N.; Tamaki, J.; Iki, M.; Kitagawa, J.; Takahira, N.; Sato, Y.; Kajita, E.; Fujita, Y.; et al. Muscle strength is associated with bone health independently of muscle mass in postmenopausal women: The Japanese population-based osteoporosis study. J. Bone Miner. Metab. 2017, 37, 53–59. [Google Scholar] [CrossRef]
- Atsumi, Y.; Rino, Y.; Wada, H.; Kitani, Y.; Ozawa, Y.; Aoyama, T.; Oshima, T.; Yukawa, N.; Yoshikawa, T.; Masuda, M. Changes in bone metabolism after gastric cancer surgery in male patients: A prospective observational study. Gastric Cancer 2018, 22, 237–243. [Google Scholar] [CrossRef]
- Imawari, M.; Kozawa, K.; Akanuma, Y.; Koizumi, S.; Itakura, H.; Kosaka, K. Serum 25-hydroxyvitamin D and vitamin D-binding protein levels and mineral metabolism after partial and total gastrectomy. Gastroenterology 1980, 79, 255–258. [Google Scholar] [CrossRef]
- Ichikawa, C.; Takiguchi, N.; Koda, K.; Oda, K.; Suzuki, H.; Miyazaki, M. Early phase metabolic bone disorders after gastrectomy: Influence of active vitamin D treatment. Dig. Dis. Sci. 2002, 47, 1886–1890. [Google Scholar] [CrossRef]
- Rino, Y.; Takanashi, Y.; Yamamoto, Y.; Inagaki, D.; Kawamoto, M.; Harada, H.; Ashida, A.; Wada, H.; Yamada, R.; Ohshima, T.; et al. Bone disorder and vitamin D after gastric cancer surgery. Hepatogastroenterology 2007, 54, 1596–1600. [Google Scholar]
- Climent, M.; Pera, M.; Aymar, I.; Ramón, J.M.; Grande, L.; Nogués, X. Bone health in long-term gastric cancer survivors: A prospective study of high-dose vitamin D supplementation using an easy administration scheme. J. Bone Miner. Metab. 2017, 36, 462–469. [Google Scholar] [CrossRef]
- Ehrhart, N.; Eurell, J.A.C.; Tommasini, M.; Constable, P.D.; Johnson, A.L.; Feretti, A. Effect of cisplatin on bone transport osteogenesis in dogs. Am. J. Veter Res. 2002, 63, 703–711. [Google Scholar] [CrossRef]
- Xian, C.J.; Cool, J.C.; Pyragius, T.; Foster, B.K. Damage and recovery of the bone growth mechanism in young rats following 5-fluorouracil acute chemotherapy. J. Cell. Biochem. 2006, 99, 1688–1704. [Google Scholar] [CrossRef]
- Stava, C.J.; Jimenez, C.; Hu, M.I.; Vassilopoulou-Sellin, R. Skeletal sequelae of cancer and cancer treatment. J. Cancer Surviv. 2009, 3, 75–88. [Google Scholar] [CrossRef]
- Yaprak, G.; Gemici, C.; Temizkan, S.; Ozdemir, S.; Dogan, B.C.; Seseogullari, O.O. Osteoporosis development and vertebral fractures after abdominal irradiation in patients with gastric cancer. BMC Cancer 2018, 18, 972. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chiang, T.-H.; Chou, C.-K.; Tu, Y.-K.; Liao, W.-C.; Wu, M.-S.; Graham, D.Y. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016, 150, 1113–1124.e5. [Google Scholar] [CrossRef]
- Sugano, K. (Kentaro) Effect of Helicobacter pylori eradication on the incidence of gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2018, 22, 435–445. [Google Scholar] [CrossRef]
- Ding, S.-Z. Global whole family based-Helicobacter pylori eradication strategy to prevent its related diseases and gastric cancer. World J. Gastroenterol. 2020, 26, 995–1004. [Google Scholar] [CrossRef]
- Ford, A.C.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and meta-analysis. Gut 2020. (Epub ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Papagiannakis, P.; Michalopoulos, C.; Papalexi, F.; Dalampoura, D.; Diamantidis, M.D. The role of Helicobacter pylori infection in hematological disorders. Eur. J. Intern. Med. 2013, 24, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Vicari, J.J.; Peek, R.M.; Falk, G.W.; Goldblum, J.R.; Easley, K.A.; Schnell, J.; Perez-Perez, G.I.; Halter, S.A.; Rice, T.W.; Blaser, M.J.; et al. The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology 1998, 115, 50–57. [Google Scholar] [CrossRef]
- Vaezi, M.F.; Falk, G.W.; Peek, R.M.; Vicari, J.J.; Goldblum, J.R.; Perez-Perez, G.I.; Rice, T.W.; Blaser, M.J.; Richter, J.E. CagA-positive strains of Helicobacter pylori may protect against Barrett’s esophagus. Am. J. Gastroenterol. 2000, 95, 2206–2211. [Google Scholar] [CrossRef]
- Queiroz, D.M.M.; Guerra, J.B.; Rocha, G.A.; Rocha, A.M.C.; Santos, A.; De Oliveira, A.G.; Cabral, M.M.D.A.; Nogueira, A.M.M.F.; De Oliveira, C.A. IL1B and IL1RN polymorphic genes and Helicobacter pylori cagA strains decrease the risk of reflux esophagitis. Gastroenterology 2004, 127, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Kubo, A.; Levin, T.R.; Block, G.; Habel, L.; Rumore, G.; Quesenberry, C.; Buffler, P.; Parsonnet, J. Helicobacter pylori and gastroesophageal reflux disease: A case-control study. Helicobacter 2008, 13, 352–360. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Chourasia, D. Gastroesophageal Reflux Disease and Helicobacter pylori: What May Be the Relationship? J. Neurogastroenterol. Motil. 2010, 16, 243–250. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, S.W. Helicobacter pylori Infection in Gastroesophageal Reflux Disease in the Asian Countries. Gastroenterol. Res. Pract. 2015, 2015, 985249. [Google Scholar]
- Ye, W.; Held, M.; Lagergren, J.; Engstrand, L.; Blot, W.J.; McLaughlin, J.K.; Nyrén, O. Helicobacter pylori Infection and Gastric Atrophy: Risk of Adenocarcinoma and Squamous-Cell Carcinoma of the Esophagus and Adenocarcinoma of the Gastric Cardia. J. Natl. Cancer Inst. 2004, 96, 388–396. [Google Scholar] [CrossRef]
- De Martel, C.; Llosa, A.E.; Farr, S.M.; Friedman, G.D.; Vogelman, J.H.; Orentreich, N.; Corley, D.A.; Parsonnet, J. Helicobacter pyloriInfection and the Risk of Development of Esophageal Adenocarcinoma. J. Infect. Dis. 2005, 191, 761–767. [Google Scholar] [CrossRef][Green Version]
- De Martel, C.; Haggerty, T.D.; Corley, A.U.; Vogelman, J.H.; Orentreich, N.; Parsonnet, J. Serum Ghrelin Levels and Risk of Subsequent Adenocarcinoma of the Esophagus. Am. J. Gastroenterol. 2007, 102, 1166–1172. [Google Scholar] [CrossRef]
- Kandulski, A.; Malfertheiner, P. Helicobacter pylori and gastroesophageal reflux disease. Curr. Opin. Gastroenterol. 2014, 30, 402–407. [Google Scholar] [CrossRef]
- Sugimoto, M.; Uotani, T.; Ichikawa, H.; Andoh, A.; Furuta, T. Gastroesophageal Reflux Disease in Time Covering Eradication for All Patients Infected with Helicobacter pylori in Japan. Digestion 2016, 93, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Giordano, F.; Mazzola, M.; Jurjus, R.; Jurjus, A.; Damiani, P.; Nobile, S.; Carini, F.; Leone, A. Helicobacter pylori and Barrett’s esophagus: A protective factor or a real cause? J. Boil. Regul. Homeost. Agents 2017, 31, 9–15. [Google Scholar]
- Sonnenberg, A.; Turner, K.O.; Spechler, S.J.; Genta, R.M. The influence of Helicobacter pylori on the ethnic distribution of Barrett’s metaplasia. Aliment. Pharm. 2016, 45, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Eross, B.; Farkas, N.; Vincze, A.; Tinusz, B.; Szapary, L.; Garami, A.; Balasko, M.; Sarlos, P.; Czopf, L.; Alizadeh, H.; et al. Helicobacter pylori infection reduces the risk of Barrett’s esophagus: A meta-analysis and systematic review. Helicobacter 2018, 23, e12504. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Zeglinas, C.; Artemaki, F.; Doulberis, M.; Kazakos, E.; Katsinelos, P.; Kountouras, J. Helicobacter pylori infection and esophageal adenocarcinoma: A review and a personal view. Ann. Gastroenterol. 2017, 31, 8–13. [Google Scholar] [CrossRef]
- Gao, H.; Li, L.; Zhang, C.; Tu, J.; Geng, X.; Wang, J.; Zhou, X.; Jing, J.; Pan, W.-S. Systematic Review with Meta-analysis: Association of Helicobacter pylori Infection with Esophageal Cancer. Gastroenterol. Res. Pract. 2019, 2019, 1953497. [Google Scholar] [CrossRef]
- Rossi, G.; Gambi, R.; Uncini, R.; Piccinini, R.; Berardi, S.; Pengo, G.; Bassotti, G.; Cerquetella, M. Severe gastritis with double Helicobacter spp. infection associated with Barrett’s esophagus in a cheetah. Helicobacter 2014, 19, 462–464. [Google Scholar] [CrossRef]
- Graham, D.Y. Helicobacter pylori is not and never was “protective” against anything, including GERD. Dig. Dis. Sci. 2003, 48, 629–630. [Google Scholar] [CrossRef]
- Kountouras, J.; Chatzopoulos, D.; Zavos, C. Eradication of Helicobacter pylori might halt the progress to oesophageal adenocarcinoma in patients with gastro-oesophageal reflux disease and Barrett’s oesophagus. Med. Hypotheses 2007, 68, 1174–1175. [Google Scholar] [CrossRef]
- Schwizer, W.; Thumshirn, M.; Dent, J.; Guldenschuh, I.; Menne, D.; Cathomas, G.; Fried, M. Helicobacter pylori and symptomatic relapse of gastro-oesophageal reflux disease: A randomised controlled trial. Lancet 2001, 357, 1738–1742. [Google Scholar] [CrossRef]
- Moayyedi, P.; Soo, S.; Deeks, J.J.; Delaney, B.C.; Harris, A.; Innes, M.; Oakes, R.; Wilson, S.; Roalfe, A.; Bennett, C.; et al. Withdrawn: Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst. Rev. 2011, 2001, CD002096. [Google Scholar]
- Kountouras, J.; Zavos, C.; Chatzopoulos, D.; Romiopoulos, I.; Polyzos, S.A.; Kapetanakis, N.; Tsiaousi, E.; Vardaka, E.; Deretzi, G.; Tsarouchas, G.; et al. Letter: Is Helicobacter pylori behind Barrett’s oesophagus and colorectal neoplasms? Aliment. Pharm. 2013, 37, 837. [Google Scholar] [CrossRef]
- Kountouras, J.; Zavos, C.; Polyzos, S.A.; Katsinelos, P. Helicobacter pylori infection and gastroesophageal reflux disease-Barrett’s esophagus sequence “dilemma”. Ann. Gastroenterol. 2015, 28, 153. [Google Scholar]
- Doulberis, M.; Kountouras, J.; Polyzos, S.A.; Tzivras, D.; Vardaka, E.; Kountouras, C.; Tzilves, D.; Kotronis, G.; Boutsikou, E.; Gialamprinou, D.; et al. Impact of Helicobacter pylori and/or Helicobacter pylori-related metabolic syndrome on gastroesophageal reflux disease—Barrett’s esophagus- esophageal adenocarcinoma sequence. Helicobacter 2018, 23, e12534. [Google Scholar] [CrossRef]
- Schwizer, W.; Menne, D.; Schütze, K.; Vieth, M.; Goergens, R.; Malfertheiner, P.; Leodolter, A.; Fried, M.; Fox, M.R. The effect of Helicobacter pylori infection and eradication in patients with gastro-oesophageal reflux disease: A parallel-group, double-blind, placebo-controlled multicentre study. United Eur. Gastroenterol. J. 2013, 1, 226–235. [Google Scholar] [CrossRef]
- Aghayeva, S.; Mara, K.C.; Katzka, D.A. The impact of Helicobacter pylori on the presence of Barrett’s esophagus in Azerbaijan, a high-prevalence area of infection. Dis. Esophagus 2019, 32. [Google Scholar] [CrossRef]
- Doorakkers, E.; Lagergren, J.; Santoni, G.; Engstrand, L.; Brusselaers, N. Helicobacter pylori eradication treatment and the risk of Barrett’s esophagus and esophageal adenocarcinoma. Helicobacter 2020, 25, e12688. [Google Scholar] [CrossRef]
- Xie, F.-J. Helicobacter pyloriinfection and esophageal cancer risk: An updated meta-analysis. World J. Gastroenterol. 2013, 19, 6098–6107. [Google Scholar] [CrossRef]
- Yaghoobi, M.; Farrokhyar, F.; Yuan, Y.; Hunt, R.H. Is There an Increased Risk of GERD After Helicobacter pylori Eradication?: A Meta-Analysis. Am. J. Gastroenterol. 2010, 105, 1007–1013. [Google Scholar] [CrossRef]
- Saad, A.M.; Choudhary, A.; Bechtold, M.L. Effect of Helicobacter pyloritreatment on gastroesophageal reflux disease (GERD): Meta-analysis of randomized controlled trials. Scand. J. Gastroenterol. 2012, 47, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Ma, S.; Shang, L.; Qian, J.; Zhang, G. Effects of Helicobacter pylori Eradication on Gastroesophageal Reflux Disease. Helicobacter 2011, 16, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Metz, D.C.; Ginsberg, G.G.; Kaplan, D.E.; Goldberg, D.S. Oesophageal and proximal gastric adenocarcinomas are rare after detection of Helicobacter pylori infection. Aliment. Pharm. 2020, 51, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, N.; Kasukawa, Y.; Sasaki, H.; Kamo, K.; Shimada, Y. Impact of spinal kyphosis on gastroesophageal reflux disease symptoms in patients with osteoporosis. Osteoporos. Int. 2008, 20, 1193–1198. [Google Scholar] [CrossRef]
- Yamane, Y.; Yamaguchi, T.; Tsumori, M.; Yamauchi, M.; Yano, S.; Yamamoto, M.; Honda, C.; Kinoshita, Y.; Sugimoto, T. Elcatonin is effective for lower back pain and the symptoms of gastroesophageal reflux disease in elderly osteoporotic patients with kyphosis. Geriatr. Gerontol. Int. 2011, 11, 215–220. [Google Scholar] [CrossRef]
- Sugimoto, M.; Hasegawa, T.; Nishino, M.; Sahara, S.; Uotani, T.; Ichikawa, H.; Kagami, T.; Sugimoto, K.; Yamato, Y.; Togawa, D.; et al. Improvement of gastroesophageal reflux disease in Japanese patients with spinal kyphotic deformity who underwent surgical spinal correction. Dig. Endosc. 2015, 28, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Drake, M.T.; Schleck, C.D.; Johnson, M.L.; Alexander, J.A.; Katzka, D.A.; Iyer, P.G. Incidence and predictors of osteoporotic fractures in patients with Barrett’s oesophagus: A population-based nested case-control study. Aliment. Pharm. 2017, 46, 1094–1102. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Mégraud, F.; O’Morain, A.C.; Atherton, J.; Axon, A.T.R.; Bazzoli, F.; Gensini, G.F.; Gisbert, J.P.; Graham, D.Y.; Rokkas, T.; et al. Management of Helicobacter pyloriinfection—The Maastricht IV/Florence Consensus Report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef]
- Zagari, R.M.; Romano, M.; Ojetti, V.; Stockbrugger, R.; Gullini, S.; Annibale, B.; Farinati, F.; Ierardi, E.; Maconi, G.; Rugge, M.; et al. Guidelines for the management of Helicobacter pylori infection in Italy: The III Working Group Consensus Report 2015. Dig. Liver Dis. 2015, 47, 903–912. [Google Scholar] [CrossRef]
- Pytlik, M.; Cegiela, U.; Nowinska, B.; Folwarczna, J.; Sliwinski, L.; Kaczmarczyk-Sedlak, I.; Pytlik, M.; Bolek, D.; Korzeniowska, H. Bone remodeling after administration of proton pump (H+/K+-ATPase) inhibitors and alendronate in ovariectomized rats. Acta Pol. Pharm. 2012, 69, 113–120. [Google Scholar]
- Hasanin, A.H. Impact of omeprazole on bone remodeling in normal and ovariectomized Wistar rats. Eur. Rev. Med. Pharm. Sci. 2014, 18, 1948–1956. [Google Scholar]
- Matuszewska, A.; Nowak, B.; Rzeszutko, M.; Zduniak, K.; Szandruk, M.; Jêdrzejuk, D.; Landwójtowicz, M.; Bolanowski, M.; Pieśniewska, M.; Kwiatkowska, J.; et al. Effects of long-term administration of pantoprazole on bone mineral density in young male rats. Pharm. Rep. 2016, 68, 1060–1064. [Google Scholar] [CrossRef]
- Yang, Y.-X.; Lewis, J.D.; Epstein, S.; Metz, D.C. Long-term Proton Pump Inhibitor Therapy and Risk of Hip Fracture. JAMA 2006, 296, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Fournier, M.R.; Targownik, E.L.; Leslie, W.D. Proton pump inhibitors, osteoporosis, and osteoporosis-related fractures. Maturitas 2009, 64, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Kubo, A.; Zhao, W.; Quesenberry, C. Proton Pump Inhibitors and Histamine-2 Receptor Antagonists Are Associated with Hip Fractures Among At-Risk Patients. Gastroenterology 2010, 139, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Yeong, J.K.; Loke, Y.K. Meta-analysis: Risk of fractures with acid-suppressing medication. Bone 2011, 48, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Eom, C.-S.; Park, S.M.; Myung, S.-K.; Yun, J.M.; Ahn, J.-S. Use of Acid-Suppressive Drugs and Risk of Fracture: A Meta-analysis of Observational Studies. Ann. Fam. Med. 2011, 9, 257–267. [Google Scholar] [CrossRef]
- Ngamruengphong, S.; Leontiadis, G.I.; Radhi, S.; Dentino, A.; Nugent, K. Proton Pump Inhibitors and Risk of Fracture: A Systematic Review and Meta-Analysis of Observational Studies. Am. J. Gastroenterol. 2011, 106, 1209–1218. [Google Scholar] [CrossRef]
- Ye, X.; Liu, H.; Wu, C.; Qin, Y.; Zang, J.; Gao, Q.; Zhang, X.; He, J. Proton pump inhibitors therapy and risk of hip fracture. Eur. J. Gastroenterol. Hepatol. 2011, 23, 794–800. [Google Scholar] [CrossRef]
- Lau, Y.T.; Ahmed, N.N. Fracture Risk and Bone Mineral Density Reduction Associated with Proton Pump Inhibitors. Pharm. J. Hum. Pharm. Drug Ther. 2012, 32, 67–79. [Google Scholar] [CrossRef]
- Khalili, H.; Huang, E.S.; Jacobson, B.C.; Camargo, A.C.; Feskanich, D.; Chan, A.T. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: A prospective cohort study. BMJ 2012, 344, e372. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.-A.; Leslie, W.D.; Targownik, L.E.; Papaioannou, A.; Adachi, J.D.; CaMos Research Group. The effect of proton pump inhibitors on fracture risk: Report from the Canadian Multicenter Osteoporosis Study. Osteoporos. Int. 2012, 24, 1161–1168. [Google Scholar]
- Lee, J.-Y.; Youn, K.; Choi, N.-K.; Lee, J.-H.; Kang, D.; Song, H.J.; Park, B.-J. A population-based case–control study: Proton pump inhibition and risk of hip fracture by use of bisphosphonate. J. Gastroenterol. 2013, 48, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Cea-Soriano, L.; Ruigómez, A.; Johansson, S.; Rodríguez, L.A.G. Study of the Association Between Hip Fracture and Acid-Suppressive Drug Use in a UK Primary Care Setting. Pharm. J. Hum. Pharm. Drug Ther. 2014, 34, 570–581. [Google Scholar] [CrossRef]
- Ding, J.; Heller, D.A.; Ahern, F.M.; Brown, T.V. The Relationship Between Proton Pump Inhibitor Adherence and Fracture Risk in the Elderly. Calcif. Tissue Int. 2014, 94, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.L.; Black, M.H.; Zhang, J.L.; Shi, J.M.; Jacobsen, S.J. Proton-pump inhibitor use and hip fractures in men: A population-based case-control study. Ann. Epidemiol. 2014, 24, 286–290. [Google Scholar] [CrossRef]
- Cai, D.; Feng, W.; Jiang, Q. Acid-suppressive medications and risk of fracture: An updated meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 8893–8904. [Google Scholar]
- Freedberg, D.E.; Kim, L.S.; Yang, Y.-X. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice from the American Gastroenterological Association. Gastroenterology 2017, 152, 706–715. [Google Scholar] [CrossRef]
- Van Der Hoorn, M.M.; Tett, S.E.; De Vries, O.J.; Dobson, A.J.; Peeters, G. The effect of dose and type of proton pump inhibitor use on risk of fractures and osteoporosis treatment in older Australian women: A prospective cohort study. Bone 2015, 81, 675–682. [Google Scholar] [CrossRef]
- Zhou, B.; Huang, Y.; Li, H.; Sun, W.; Liu, J. Proton pump inhibitors and fracture risk: Response to comments. Osteoporos. Int. 2015, 27, 1673–1674. [Google Scholar] [CrossRef]
- Mössner, J. The Indications, Applications, and Risks of Proton Pump Inhibitors. Dtsch. Aerzteblatt Online 2016, 113, 477–483. [Google Scholar] [CrossRef]
- Andersen, B.N.; Johansen, P.B.; Abrahamsen, B. Proton pump inhibitors and osteoporosis. Curr. Opin. Rheumatol. 2016, 28, 420–425. [Google Scholar] [CrossRef]
- Maes, M.L.; Fixen, D.R.; Linnebur, S.A. Adverse effects of proton-pump inhibitor use in older adults: A review of the evidence. Adv. Drug Saf. 2017, 8, 273–297. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Cao, Y.; Han, Z.; Wang, X.; Atkinson, E.J.; Liua, H.; Amin, S. Proton Pump Inhibitors and the Risk for Fracture at Specific Sites: Data Mining of the FDA Adverse Event Reporting System. Sci. Rep. 2017, 7, 5527. [Google Scholar] [CrossRef]
- Dubcenco, E.; Beers-Block, P.; Kim, L.; Schotland, P.; Levine, J.; McCloskey, C.; Bashaw, E.D. A Proton Pump Inhibitor in the Reformulation Setting: Bioequivalence and Potential Implications for Long-Term Safety. Clin. Transl. Sci. 2017, 10, 387–394. [Google Scholar] [CrossRef]
- Nehra, A.K.; Alexander, J.A.; Loftus, C.G.; Nehra, V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin. Proc. 2018, 93, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Fan, L.; Yang, J.; Wang, J.; Sun, J.; Wang, Z. Proton pump inhibitors therapy and risk of bone diseases: An update meta-analysis. Life Sci. 2019, 218, 213–223. [Google Scholar] [CrossRef]
- Park, J.-H.; Song, Y.; Jung, J.-H.; Han, K. Comparative analysis of the risk of osteoporotic fractures with proton pump inhibitor use and histamine-2 receptor antagonist therapy in elderly women: A nationwide population-based nested case-control study. Bone 2020, 135, 115306. [Google Scholar] [CrossRef]
- Lewis, J.R.; Barre, D.; Zhu, K.; Ivey, K.L.; Lim, E.M.; Hughes, J.; Prince, R.L. Long-Term Proton Pump Inhibitor Therapy and Falls and Fractures in Elderly Women: A Prospective Cohort Study. J. Bone Miner. Res. 2014, 29, 2489–2497. [Google Scholar] [CrossRef]
- Thaler, H.; Sterke, C.; Van Der Cammen, T.J.M. Association of proton pump inhibitor use with recurrent falls and risk of fractures in older women: A study of medication use in older fallers. J. Nutr. Health Aging 2016, 20, 77–81. [Google Scholar] [CrossRef]
- Lai, S.-W. Proton pump inhibitors use and risk of falls. QJM: Int. J. Med. 2018, 112, 317. [Google Scholar] [CrossRef]
- Yu, E.W.; Blackwell, T.; Ensrud, K.E.; Hillier, T.A.; Lane, N.E.; Orwoll, E.; Bauer, D.C. Acid-Suppressive Medications and Risk of Bone Loss and Fracture in Older Adults. Calcif. Tissue Int. 2008, 83, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Laine, L. Proton pump inhibitors and bone fractures? Am. J. Gastroenterol. 2009, 104 (Suppl. 2), S21–S26. [Google Scholar]
- Gray, S.L.; Lacroix, A.Z.; Larson, J.; Robbins, J.; Cauley, J.A.; Manson, J.E.; Chen, Z. Proton Pump Inhibitor Use, Hip Fracture, and Change in Bone Mineral Density in Postmenopausal Women. Arch. Intern. Med. 2010, 170, 765. [Google Scholar] [CrossRef]
- Pouwels, S.; Lalmohamed, A.; Souverein, P.C.; Cooper, C.; Veldt, B.J.; Leufkens, H.G.; De Boer, A.; Van Staa, T.P.; De Vries, F. Use of proton pump inhibitors and risk of hip/femur fracture: A population-based case-control study. Osteoporos. Int. 2010, 22, 903–910. [Google Scholar] [CrossRef]
- Targownik, E.L.; Leslie, W.D. The relationship among proton pump inhibitors, bone disease and fracture. Expert Opin. Drug Saf. 2011, 10, 901–912. [Google Scholar] [CrossRef]
- Targownik, E.L.; Leslie, W.D.; Davison, K.S.; Goltzman, D.; Jamal, S.A.; Kreiger, N.; Josse, R.G.; Kaiser, S.M.; Kovacs, C.S.; Prior, J.C.; et al. The Relationship Between Proton Pump Inhibitor Use and Longitudinal Change in Bone Mineral Density: A Population-Based from the Canadian Multicentre Osteoporosis Study (CaMos). Am. J. Gastroenterol. 2012, 107, 1361–1369. [Google Scholar] [CrossRef]
- Reyes, C.; Formiga, F.; Coderch, M.; Hoyo, J.; Ferriz, G.; Casanovas, J.; Monteserín, R.; Brotons, C.; Rojas, M.; Moral, I. Use of proton pump inhibitors and risk of fragility hip fracture in a Mediterranean region. Bone 2013, 52, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Diem, S.J.; Ruppert, K.; Lian, Y.J.; Liu, C.-C.; Wohlfart, A.; Greendale, G.A.; Finkelstein, J.S. Bone Mineral Density Changes Among Women Initiating Proton Pump Inhibitors or H2 Receptor Antagonists: A SWAN Cohort Study. J. Bone Miner. Res. 2015, 30, 232–239. [Google Scholar] [CrossRef]
- Vaezi, M.F.; Yang, Y.-X.; Howden, C.W. Complications of Proton Pump Inhibitor Therapy. Gastroenterology 2017, 153, 35–48. [Google Scholar] [CrossRef]
- Harding, B.N.; Weiss, N.S.; Walker, R.L.; Larson, E.B.; Dublin, S. Proton pump inhibitor use and the risk of fractures among an older adult cohort. Pharm. Drug Saf. 2018, 27, 596–603. [Google Scholar] [CrossRef]
- Singh, A.; Cresci, G.A.; Kirby, D.F. Proton Pump Inhibitors: Risks and Rewards and Emerging Consequences to the Gut Microbiome. Nutr. Clin. Pract. 2018, 33, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Torvinen-Kiiskinen, S.; Tolppanen, A.-M.; Koponen, M.; Tanskanen, A.; Tiihonen, J.; Hartikainen, S.; Taipale, H. Proton pump inhibitor use and risk of hip fractures among community-dwelling persons with Alzheimer’s disease-a nested case-control study. Aliment. Pharm. 2018, 47, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Xavier, S.; Magalhães, J.; Cotter, J. Proton Pump Inhibitors: Are They a Real Threat to the Patient? Ge Port. J. Gastroenterol. 2018, 25, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-W. Proton Pump Inhibitors and Fracture Risk. Am. J. Gastroenterol. 2019, 114, 1693. [Google Scholar] [CrossRef]
- Ozen, G.; Pedro, S.; Wolfe, F.; Michaud, K. Medications associated with fracture risk in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 1041–1047. [Google Scholar] [CrossRef]
- Hoff, M.; Skovlund, E.; Skurtveit, S.; Meyer, H.; Langhammer, A.; Søgaard, A.; Syversen, U.; Forsmo, S.; Abrahamsen, B.; Schei, B. Proton pump inhibitors and fracture risk. The HUNT study, Norway. Osteoporos. Int. 2019, 31, 109–118. [Google Scholar] [CrossRef]
- Fedida, B.; Schermann, H.; Ankory, R.; Rotman, D.; Shichman, I.; Yoffe, V.; Shlaifer, A.; Luger, E. Fracture risk of young adults receiving proton-pump inhibitors and H2-receptor antagonists. Int. J. Clin. Pract. 2019, 73, e13339. [Google Scholar] [CrossRef]
- Sugiyama, T. Understanding the Current Evidence on Proton Pump Inhibitor Use and Bone Health. Gastroenterology 2019, 157, 585. [Google Scholar] [CrossRef]
- Sugiyama, T. Response to Lai “Proton Pump Inhibitors and Fracture Risk”. Am. J. Gastroenterol. 2019, 114, 1693–1694. [Google Scholar] [CrossRef]
- Nassar, Y.; Richter, S. Proton-pump Inhibitor Use and Fracture Risk: An Updated Systematic Review and Meta-analysis. J. Bone Metab. 2018, 25, 141–151. [Google Scholar] [CrossRef]
- Poly, T.; Islam, M.; Yang, H.-C.; Wu, C.; Li, Y.-C. (Jack) Proton pump inhibitors and risk of hip fracture: A meta-analysis of observational studies. Osteoporos. Int. 2018, 30, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Vinke, P.; Wesselink, E.; Van Orten-Luiten, A.C.B.; Van Norren, K. The Use of Proton Pump Inhibitors May Increase Symptoms of Muscle Function Loss in Patients with Chronic Illnesses. Int. J. Mol. Sci. 2020, 21, 323. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, M.; Setoguchi, S.; Solomon, D.H. Antihistamine Therapy and Bone Mineral Density: Analysis in a Population-Based US Sample. Am. J. Med. 2008, 121, 1085–1091. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cosman, F.; De Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis-2020 Update Executive Summary. Endocr. Pract. 2020. [Google Scholar] [CrossRef]
- Hansen, K.E.; Nieves, J.W.; Nudurupati, S.V.; Metz, D.C.; Perez, M.C. Dexlansoprazole and Esomeprazole Do Not Affect Bone Homeostasis in Healthy Postmenopausal Women. Gastroenterology 2018, 156, 926–934.e6. [Google Scholar] [CrossRef]
- Moayyedi, P.; Eikelboom, J.W.; Bosch, J.; Connolly, S.J.; Dyal, L.; Shestakovska, O.; Leong, D.; Anand, S.S.; Störk, S.; Branch, K.R.; et al. Pantoprazole to Prevent Gastroduodenal Events in Patients Receiving Rivaroxaban and/or Aspirin in a Randomized, Double-Blind, Placebo-Controlled Trial. Gastroenterology 2019, 157, 403–412.e5. [Google Scholar] [CrossRef]
- Rodríguez, L.A.G.; Lagergren, J.; Lindblad, M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: A nested case control study in the UK. Gut 2006, 55, 1538–1544. [Google Scholar] [CrossRef]
- Tamim, H.; Duranceau, A.; Chen, L.-Q.; LeLorier, J. Association between use of acid-suppressive drugs and risk of gastric cancer. A nested case-control study. Drug Saf. 2008, 31, 675–684. [Google Scholar] [CrossRef]
- Suissa, S.; Suissa, A. Proton-pump inhibitors and increased gastric cancer risk: Time-related biases. Gut 2018, 67, 2228–2229. [Google Scholar] [CrossRef]
- Ahn, J.S.; Eom, C.-S.; Jeon, C.Y.; Park, S.M. Acid suppressive drugs and gastric cancer: A meta-analysis of observational studies. World J. Gastroenterol. 2013, 19, 2560–2568. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Wong, I.C.K.; Leung, W.K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut 2017, 67, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-C.; Huang, L.-R.; Lin, C.-L.; Hsu, W.-Y.; Chang, C.-S.; Yeh, H.-Z.; Kao, C.-H. Association between proton pump inhibitors use and risk of gastric cancer in patients with GERD. Gut 2018, 68, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-W.; Lai, H.-C.; Lin, C.-L.; Liao, K.-F. Proton pump inhibitors and risk of gastric cancer in a case–control study. Gut 2018, 68, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.-Y.; Wu, X.-T.; Li, N.; Du, L.; Wu, X. Long-term proton pump inhibitors use and risk of gastric cancer: A meta-analysis of 926 386 participants. Gut 2018, 68, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Tran-Duy, A.; Spaetgens, B.; Hoes, A.W.; De Wit, N.J.; Stehouwer, C.D. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1706–1719.e5. [Google Scholar] [CrossRef]
- Joo, M.K.; Park, J.-J.; Chun, H.J. Proton pump inhibitor: The dual role in gastric cancer. World J. Gastroenterol. 2019, 25, 2058–2070. [Google Scholar] [CrossRef]
- Papamichael, K.X.; Papaioannou, G.; Karga, H.; Roussos, A.; Mantzaris, G.J. Helicobacter pylori infection and endocrine disorders: Is there a link? World J. Gastroenterol. 2009, 15, 2701–2707. [Google Scholar] [CrossRef]
- Pellicano, R.; Franceschi, F.; Saracco, G.; Fagoonee, S.; Roccarina, D.; Gasbarrini, A. Helicobacters and Extragastric Diseases. Helicobacter 2009, 14, 58–68. [Google Scholar] [CrossRef]
- Tan, H.J.; Goh, K.-L. Extragastrointestinal manifestations of Helicobacter pylori infection: Facts or myth? A critical review. J. Dig. Dis. 2012, 13, 342–349. [Google Scholar] [CrossRef]
- Banić, M.; Franceschi, F.; Babic, Z.; Gasbarrini, A. Extragastric Manifestations of Helicobacter pylori Infection. Helicobacter 2012, 17, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, F.; Gasbarrini, A.; Polyzos, S.A.; Kountouras, J. Extragastric Diseases andHelicobacter pylori. Helicobacter 2015, 20 (Suppl. 1), 40–46. [Google Scholar] [CrossRef]
- Sotuneh, N.; Hosseini, S.R.; Shokri-Shirvani, J.; Bijani, A.; Ghadimi, R. Helicobacter Pylori Infection and Metabolic Parameters: Is There an Association in Elderly Population? Int. J. Prev. Med. 2014, 5, 1537–1542. [Google Scholar]
- Wong, F.; Rayner-Hartley, E.; Byrne, M.F. Extraintestinal manifestations of Helicobacter pylori: A concise review. World J. Gastroenterol. 2014, 20, 11950–11961. [Google Scholar] [CrossRef]
- Hagymási, K.; Tulassay, Z. Helicobacter pyloriinfection: New pathogenetic and clinical aspects. World J. Gastroenterol. 2014, 20, 6386–6399. [Google Scholar] [CrossRef] [PubMed]
- Campuzano-Maya, G. Hematologic manifestations of Helicobacter pyloriinfection. World J. Gastroenterol. 2014, 20, 12818–12838. [Google Scholar] [CrossRef]
- Goni, E.; Franceschi, F. Helicobacter pyloriand extragastric diseases. Helicobacter 2016, 21 (Suppl. 1), 45–48. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, Y.; Shi, J.; Song, C.; Zhang, J.; Wang, K.-J. Population attributable burden of Helicobacter pylori-related gastric cancer, coronary heart disease, and ischemic stroke in China. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 36, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Bravo, D.; Hoare, A.; Soto, C.; Valenzuela, M.A.; Quest, A.F.G. Helicobacter pylori in human health and disease: Mechanisms for local gastric and systemic effects. World J. Gastroenterol. 2018, 24, 3071–3089. [Google Scholar] [CrossRef] [PubMed]
- Razuka-Ebela, D.; Giupponi, B.; Franceschi, F. Helicobacter pylori and extragastric diseases. Helicobacter 2018, 23 (Suppl. 1), e12520. [Google Scholar] [CrossRef]
- Tsay, F.-W.; Hsu, P.-I.H. pylori infection and extra-gastroduodenal diseases. J. Biomed. Sci. 2018, 25, 65. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, F.; Covino, M.; Baudron, C.R. Review: Helicobacter pylori and extragastric diseases. Helicobacter 2019, 24 (Suppl. 1), e12636. [Google Scholar] [CrossRef] [PubMed]
- Baudron, C.R.; Franceschi, F.; Salles, N.; Gasbarrini, A. Extragastric Diseases andHelicobacter pylori. Helicobacter 2013, 18 (Suppl. 1), 44–51. [Google Scholar] [CrossRef]
- Dennison, E.M.; Compston, J.E.; Flahive, J.; Siris, E.S.; Gehlbach, S.H.; Adachi, J.D.; Boonen, S.; Chapurlat, R.; Díez-Pérez, A.; Anderson, F.A.; et al. Effect of co-morbidities on fracture risk: Findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 2012, 50, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Bloch, F.; Thibaud, M.; Tournoux-Facon, C.; Brèque, C.; Rigaud, A.-S.; Dugué, B.; Kemoun, G. Estimation of the risk factors for falls in the elderly: Can meta-analysis provide a valid answer? Geriatr. Gerontol. Int. 2012, 13, 250–263. [Google Scholar] [CrossRef]
- Zendehdel, A.; Roham, M. Role of Helicobacter pylori infection in the manifestation of old age-related diseases. Mol. Genet. Genom. Med. 2020, 8, e1157. [Google Scholar] [CrossRef]
- Vranken, L.; Wyers, C.E.; Van Der Velde, R.Y.; Janzing, H.M.; Kaarsemaker, S.; Geusens, P.P.; Bergh, J.P.V.D. Comorbidities and medication use in patients with a recent clinical fracture at the Fracture Liaison Service. Osteoporos. Int. 2017, 29, 397–407. [Google Scholar] [CrossRef]
- Walker-Bone, K. Recognizing and treating secondary osteoporosis. Nat. Rev. Rheumatol. 2012, 8, 480–492. [Google Scholar] [CrossRef]
- Bogoch, E.R.; Elliot-Gibson, V.; Wang, R.Y.C.; Josse, R.G. Secondary Causes of Osteoporosis in Fracture Patients. J. Orthop. Trauma 2012, 26, e145–e152. [Google Scholar] [CrossRef]
- Diab, D.L.; Watts, N.B. Secondary Osteoporosis. Clin. Obs. Gynecol. 2013, 56, 686–693. [Google Scholar] [CrossRef]
- 3Bours, S.P.; Bergh, J.P.V.D.; Van Geel, T.; Geusens, P.P. Secondary osteoporosis and metabolic bone disease in patients 50 years and older with osteoporosis or with a recent clinical fracture. Curr. Opin. Rheumatol. 2014, 26, 430–439. [Google Scholar]
- Mirza, F.S.; Canalis, E. MANAGEMENT OF ENDOCRINE DISEASE: Secondary osteoporosis: Pathophysiology and management. Eur. J. Endocrinol. 2015, 173, R131–R151. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Briot, K. Osteoporosis in 2017: Addressing the crisis in the treatment of osteoporosis. Nat. Rev. Rheumatol. 2018, 14, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaeili, F.; Goh, Y.M.; Kamarehei, M.; Meng, G.Y. A comprehensive overview on osteoporosis and its risk factors. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis; Committee of Scientific Advisors of the International Osteoporosis Foundation. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef]
- Kanis, J.A.; Harvey, N.C.; McCloskey, E.; Bruyere, O.; Veronese, N.; Lorentzon, M.; Cooper, C.; Rizzoli, R.; Adib, G.; Al-Daghri, N.; et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos. Int. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Mégraud, F.; O’Morain, A.C.; Gisbert, J.P.; Kuipers, E.; Axon, A.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pyloriinfection—The Maastricht V/Florence Consensus Report. Gut 2016, 66, 6–30. [Google Scholar] [CrossRef]
- Smyk, D.S. Helicobacter pyloriand autoimmune disease: Cause or bystander. World J. Gastroenterol. 2014, 20, 613–629. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Papatheodorou, A.; Patsiaoura, K.; Katsiki, E.; Zafeiriadou, E.; Zavos, C.; Anastasiadou, K.; Terpos, E. Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism 2013, 62, 121–126. [Google Scholar] [CrossRef]
- Sumida, Y.; Kanemasa, K.; Imai, S.; Mori, K.; Tanaka, S.; Shimokobe, H.; Kitamura, Y.; Fukumoto, K.; Kakutani, A.; Ohno, T.; et al. Helicobacter pylori infection might have a potential role in hepatocyte ballooning in nonalcoholic fatty liver disease. J. Gastroenterol. 2015, 50, 996–1004. [Google Scholar] [CrossRef]
- Upala, S.; Jaruvongvanich, V.; Riangwiwat, T.; Jaruvongvanich, S.; Sanguankeo, A. Association betweenHelicobacter pyloriinfection and metabolic syndrome: A systematic review and meta-analysis. J. Dig. Dis. 2016, 17, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Sinn, D.H.; Min, Y.W.; Son, H.J.; Chang, Y.; Baek, S.-Y.; Ahn, S.H.; Lee, H.; Ryu, S. A cohort study on Helicobacter pylori infection associated with nonalcoholic fatty liver disease. J. Gastroenterol. 2017, 29, 559–1210. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.M.; Kumar, S. The Association Between Helicobacter pylori Infection and Nonalcoholic Fatty Liver Disease. Curr. Gastroenterol. Rep. 2017, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Thongprayoon, C.; Panjawatanan, P.; Manatsathit, W.; Jaruvongvanich, V.; Ungprasert, P. Helicobacter pylori and Risk of Nonalcoholic Fatty Liver Disease. J. Clin. Gastroenterol. 2018, 52, 386–391. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, J.; Song, Z.; Wang, J.; Wu, L. Association of Helicobacter pylori infection with metabolic syndrome in aged Chinese females. Exp. Med. 2019, 17, 4403–4408. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Cai, J.-T.; Song, Z.-Y.; Tong, Y.-L.; Wang, J.-H. The associations among Helicobacter pylori infection, white blood cell count and nonalcoholic fatty liver disease in a large Chinese population. Medicine 2018, 97, e13271. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, X.; Xia, C.; Liu, H.; Yan, H.; Wang, G.; Wu, Z. Association between Helicobacter pylori infection and non-alcoholic fatty liver disease in North Chinese: A cross-sectional study. Sci. Rep. 2019, 9, 4874. [Google Scholar] [CrossRef]
- Mantovani, A.; Turino, T.; Altomari, A.; Lonardo, A.; Zoppini, G.; Valenti, L.; Tilg, H.; Byrne, C.D.; Targher, G. Association between Helicobacter pylori infection and risk of nonalcoholic fatty liver disease: An updated meta-analysis. Metabolism 2019, 96, 56–65. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, H.; Xu, W.; Wang, K.; Guo, P.; Ai, Y. Association between Helicobacter pylori infection and nonalcoholic fatty liver disease: A systematic review and meta-analysis of observational studies. Helicobacter 2019, 24, e12576. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Zavos, C.; Deretzi, G. Helicobacter pylori Infection and insulin resistance. Helicobacter 2012, 18, 165–166. [Google Scholar] [CrossRef]
- He, C.; Yang, Z.; Lu, N.-H. Helicobacter pyloriinfection and diabetes: Is it a myth or fact. World J. Gastroenterol. 2014, 20, 4607–4617. [Google Scholar] [CrossRef]
- Bonfigli, A.R.; Boemi, M.; Festa, R.; Bonazzi, P.; Brandoni, G.; Spazzafumo, L.; Olivieri, F.; Ceriello, A.; Genovese, S.; Testa, R. Randomized, double-blind, placebo-controlled trial to evaluate the effect of Helicobacter pylori eradication on glucose homeostasis in type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Fang, W.-H.; Wang, C.-C.; Kao, T.-W.; Chang, Y.-W.; Wu, C.-J.; Zhou, Y.-C.; Sun, Y.-S.; Chen, W.-L. Helicobacter pylori infection increases risk of incident metabolic syndrome and diabetes: A cohort study. PLoS ONE 2019, 14, e0208913. [Google Scholar] [CrossRef] [PubMed]
- Nodoushan, S.A.H.; Nabavi, A. The Interaction of Helicobacter pylori Infection and Type 2 Diabetes Mellitus. Adv. Biomed. Res. 2019, 8, 15. [Google Scholar] [CrossRef]
- Wang, F.; Fu, Y.; Lv, Z. Association of Helicobacter pyloriinfection with diabetic complications: A meta-analysis. Endocr. Res. 2013, 39, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhong, X.; Chen, J.; Li, C.; Shang, M.; Jiang, C.; Yang, H.; Zhao, W.; Liu, L. Helicobacter pylori infection associated with type 2 diabetic nephropathy in patients with dyspeptic symptoms. Diabetes Res. Clin. Pr. 2015, 110, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Gokturk, H.S.; Ozturk, N.A.; Kulaksizoglu, M.; Serin, E.; Yilmaz, U. Helicobacter pylori Prevalence in Diabetes Mellitus Patients with Dyspeptic Symptoms and its Relationship to Glycemic Control and Late Complications. Dig. Dis. Sci. 2008, 53, 2646–2649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, T.; Chen, X.; Yu, X.; Tu, L.; Zhang, C. Association between Helicobacter pylori infection and overweight or obesity in a Chinese population. J. Infect. Dev. Ctries. 2015, 9, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, W.; Qin, L.; Yang, W.; Yu, G.; Wei, Q. Relationship between Helicobacter pylori infection and obesity in Chinese adults: A systematic review with meta-analysis. PLoS ONE 2019, 14, e0221076. [Google Scholar] [CrossRef]
- Xia, H.H.; Talley, N.J.; Kam, E.P.; Young, L.J.; Hammer, J.; Horowitz, M. Helicobacter pylori infection is not associated with diabetes mellitus, nor with upper gastrointestinal symptoms in diabetes mellitus. Am. J. Gastroenterol. 2001, 96, 1039–1046. [Google Scholar] [CrossRef]
- Stanciu, O.G.; Trifan, A.; Sfarti, C.; Cojocariu, C.; Stanciu, C. Helicobacter pylori infection in patients with diabetes mellitus. Rev. Med. Chir. Soc. Med. Nat. Iasi 2003, 107, 59–65. [Google Scholar] [PubMed]
- Dai, Y.-N.; Yu, W.-L.; Zhu, H.-T.; Ding, J.-X.; Yu, C.-H.; Li, Y.-M. Is Helicobacter pyloriinfection associated with glycemic control in diabetics? World J. Gastroenterol. 2015, 21, 5407–5416. [Google Scholar] [CrossRef] [PubMed]
- Okushin, K.; Takahashi, Y.; Yamamichi, N.; Shimamoto, T.; Enooku, K.; Fujinaga, H.; Tsutsumi, T.; Shintani, Y.; Sakaguchi, Y.; Ono, S.; et al. Helicobacter pylori infection is not associated with fatty liver disease including non-alcoholic fatty liver disease: A large-scale cross-sectional study in Japan. Bmc Gastroenterol. 2015, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Baeg, M.K.; Yoon, S.K.; Ko, S.-H.; Noh, Y.-S.; Lee, I.-S.; Choi, M.-G. Helicobacter pyloriinfection is not associated with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 2592–2600. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Liu, L.; Yuan, B.-S.; Yin, J.; Lu, Q. Association of obesity withHelicobacter pyloriinfection: A retrospective study. World J. Gastroenterol. 2017, 23, 2750–2756. [Google Scholar] [CrossRef]
- Mohammadifard, M.; Saremi, Z.; Rastgoo, M.; Akbari, E. Relevance between Helicobacter pylori Infection and Non-Alcoholic Fatty Liver Disease in Birjand, Iran. J. Med. Life 2019, 12, 168–172. [Google Scholar]
- Lender, N.; Talley, N.J.; Enck, P.; Haag, S.; Zipfel, S.; Morrison, M.; Holtmann, G.J. Review article: Associations betweenHelicobacter pyloriand obesity - an ecological study. Aliment. Pharm. 2014, 40, 24–31. [Google Scholar] [CrossRef]
- Vo, H.D.; Goli, S.; Gill, R.; Anderson, V.; Stefanov, D.G.; Xu, J.; Kulsum-Mecci, N.; Schwarz, S.M.; Rabinowitz, S.S. Inverse Correlation Between Helicobacter pylori Colonization and Obesity in a Cohort of Inner City Children. Helicobacter 2014, 20, 64–68. [Google Scholar] [CrossRef]
- Moran-Lev, H.; Lubetzky, R.; Mandel, D.; Yerushalmy-Feler, A.; Cohen, S. Inverse Correlation between Helicobacter pylori Colonization and Pediatric Overweight: A Preliminary Study. Child. Obes. 2017, 13, 267–271. [Google Scholar] [CrossRef]
- Moyaert, H.; Franceschi, F.; Roccarina, D.; Ducatelle, R.; Haesebrouck, F.; Gasbarrini, A. Extragastric Manifestations of Helicobacter pyloriInfection: OtherHelicobacters. Helicobacter 2008, 13, 47–57. [Google Scholar] [CrossRef]
- Pellicano, R.; Oliaro, E.; Fagoonee, S.; Astegiano, M.; Berrutti, M.; Saracco, G.; Smedile, A.; Repici, A.; Leone, N.; Castelli, A.; et al. Clinical and biochemical parameters related to cardiovascular disease after Helicobacter pylori eradication. Int. Angiol. 2009, 28, 469–473. [Google Scholar] [PubMed]
- Figura, N.; Franceschi, F.; Santucci, A.; Bernardini, G.; Gasbarrini, G.; Gasbarrini, A. Extragastric Manifestations of Helicobacter pylori Infection. Helicobacter 2010, 15 (Suppl. 1), 60–68. [Google Scholar] [CrossRef]
- Christodoulou, D.K.; Milionis, H.J.; Pappa, P.; Katsanos, K.H.; Sigounas, D.; Florentin, M.; Elisaf, M.S.; Tsianos, E.V. Association of Helicobacter pylori infection with cardiovascular disease—Is it just a myth? Eur. J. Intern. Med. 2011, 22, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Rogha, M.; Dadkhah, D.; Pourmoghaddas, Z.; Shirneshan, K.; Nikvarz, M.; Pourmoghaddas, M. Association of helicobacter pylori infection with severity of coronary heart disease. Arya Atheroscler. 2012, 7, 138–141. [Google Scholar] [PubMed]
- Matusiak, A.; Chałubiński, M.; Broncel, M.; Rechcinski, T.; Rudnicka, K.; Miszczyk, E.; Walencka, M.; Strapagiel, D.; Gajewski, A.; Chmiela, M. Putative consequences of exposure to Helicobacter pylori infection in patients with coronary heart disease in terms of humoral immune response and inflammation. Arch. Med. Sci. 2016, 12, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-W.; Tseng, K.-L.; Hsu, C.-N.; Liang, C.-M.; Tai, W.-C.; Ku, M.-K.; Hung, T.-H.; Yuan, L.-T.; Nguang, S.-H.; Yang, S.-C.; et al. Association between Helicobacter pylori eradication and the risk of coronary heart diseases. PLoS ONE 2018, 13, e0190219. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Gattani, S.G.; Farhat, S.A. Helicobacter pylori and cardiovascular complications: A mechanism based review on role of Helicobacter pylori in cardiovascular diseases. Integr. Med. Res. 2016, 5, 244–249. [Google Scholar] [CrossRef]
- Cauley, J.A.; Chalhoub, D.; Kassem, A.M.; Fuleihan, G.E.-H. Geographic and ethnic disparities in osteoporotic fractures. Nat. Rev. Endocrinol. 2014, 10, 338–351. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- Hagino, H.; Sakamoto, K.; Harada, A.; Nakamura, T.; Mutoh, Y.; Mori, S.; Endo, N.; Nakano, T.; Itoi, E.; Kita, K.; et al. Nationwide one-decade survey of hip fractures in Japan. J. Orthop. Sci. 2010, 15, 737–745. [Google Scholar] [CrossRef]
- Court-Brown, C.; Clement, N.; Duckworth, A.; Biant, L.; McQueen, M. The changing epidemiology of fall-related fractures in adults. Injury 2017, 48, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Aspray, T.; Hill, T.R. Osteoporosis and the Ageing Skeleton. Subcell. Biochem. 2019, 91, 453–476. [Google Scholar] [PubMed]
- Weidauer, L.; Binkley, T.; Beare, T.; Minett, M.; McCormack, L.; Wey, A.; Specker, B. Do Sex Differences Exist in Rates of Falls and Fractures in Hutterite, Rural, and Nonrural Populations, Aged 20 to 66 Years? Clin. Orthop. Relat. Res. 2015, 473, 2514–2520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ibrahim, A.; Singh, D.K.A.; Shahar, S. ‘Timed Up and Go’ test: Age, gender and cognitive impairment stratified normative values of older adults. PLoS ONE 2017, 12, e0185641. [Google Scholar] [CrossRef] [PubMed]
- Rankinen, T.; Sarzynski, M.A.; Ghosh, S.; Bouchard, C. Are there genetic paths common to obesity, cardiovascular disease outcomes, and cardiovascular risk factors? Circ. Res. 2015, 116, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, J.; Deng, H.-W. A Statistical Approach to Fine Mapping for the Identification of Potential Causal Variants Related to Bone Mineral Density. J. Bone Miner. Res. 2017, 32, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, J.; Wu, K.; Zhang, L.; Shen, H.; Zhang, J.; Deng, H.-W. Increased detection of genetic loci associated with risk predictors of osteoporotic fracture using a pleiotropic cFDR method. Bone 2017, 99, 62–68. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Luo, Y. Systemic tracking of diagnostic function modules for post-menopausal osteoporosis in a differential co-expression network view. Exp. Med. 2018, 15, 2961–2967. [Google Scholar] [CrossRef]
- Lin, X.; Peng, C.; Greenbaum, J.; Li, Z.-F.; Wu, K.-H.; Ao, Z.-X.; Zhang, T.; Shen, J.; Deng, H.-W. Identifying potentially common genes between dyslipidemia and osteoporosis using novel analytical approaches. Mol. Genet. Genom. 2018, 293, 711–723. [Google Scholar] [CrossRef]
- Trajanoska, K.; Rivadeneira, F. Using Mendelian Randomization to Decipher Mechanisms of Bone Disease. Curr. Osteoporos. Rep. 2018, 16, 531–540. [Google Scholar] [CrossRef]
- Zheng, J.; Frysz, M.; Kemp, J.P.; Evans, D.M.; Smith, G.D.; Tobias, J.H. Use of Mendelian Randomization to Examine Causal Inference in Osteoporosis. Front. Endocrinol. 2019, 10, 807. [Google Scholar] [CrossRef]
- Hu, Y.; Tan, L.-J.; Chen, X.-D.; Greenbaum, J.; Deng, H.-W. Identification of novel variants associated with osteoporosis, type 2 diabetes and potentially pleiotropic loci using pleiotropic cFDR method. Bone 2018, 117, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Yang, Y.; Chen, Y.; Xia, Z.; Zhang, W.; Feng, Y.; Li, Y.; Tan, J.; Xu, C.; Zhang, Q.; et al. Multivariate analysis of genome-wide data to identify potential pleiotropic genes for type 2 diabetes, obesity and coronary artery disease using MetaCCA. Int. J. Cardiol. 2019, 283, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cherny, S.S.; Freidin, M.B.; Williams, F.M.K.; Livshits, G. The analysis of causal relationships between blood lipid levels and BMD. PLoS ONE 2019, 14, e0212464. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-L.; Shen, H.; Liu, A.; Dong, S.-S.; Zhang, L.; Deng, F.-Y.; Zhao, Q.; Deng, H.-W. A road map for understanding molecular and genetic determinants of osteoporosis. Nat. Rev. Endocrinol. 2019, 16, 91–103. [Google Scholar] [CrossRef]
- Gu, H.; Huang, Z.; Chen, G.; Zhou, K.; Zhang, Y.; Chen, J.; Xu, J.; Yin, X. Network and pathway-based analyses of genes associated with osteoporosis. Medcine 2020, 99, e19120. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.D.; Bůžková, P.; Fink, H.A.; Lee, J.S.; Chen, Z.; Ahmed, A.; Parashar, S.; Robbins, J.R. Hip fractures and heart failure: Findings from the Cardiovascular Health Study. Eur. Heart J. 2009, 31, 77–84. [Google Scholar] [CrossRef]
- Szulc, P. Association between cardiovascular diseases and osteoporosis—Reappraisal. Bonekey Rep. 2012, 1, 144. [Google Scholar] [CrossRef][Green Version]
- Fisher, A.; Srikusalanukul, W.; Davis, M.; Smith, P. Cardiovascular diseases in older patients with osteoporotic hip fracture: Prevalence, disturbances in mineral and bone metabolism, and bidirectional links. Clin. Interv. Aging 2013, 8, 239–256. [Google Scholar] [CrossRef]
- Chen, S.-J.; Lin, C.-S.; Lin, C.-L.; Kao, C.-H. Osteoporosis Is Associated with High Risk for Coronary Heart Disease. Medcine 2015, 94, e1146. [Google Scholar] [CrossRef]
- Popp, A.; Meer, S.; Krieg, M.-A.; Perrelet, R.; Hans, D.; Lippuner, K. Bone mineral density (BMD) and vertebral trabecular bone score (TBS) for the identification of elderly women at high risk for fracture: The SEMOF cohort study. Eur. Spine J. 2015, 25, 3432–3438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, B. Systematic review and meta-analysis for the association of bone mineral density and osteoporosis/osteopenia with vascular calcification in women. Int. J. Rheum. Dis. 2016, 20, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Center, J.R. Fracture Burden: What Two and a Half Decades of Dubbo Osteoporosis Epidemiology Study Data Reveal About Clinical Outcomes of Osteoporosis. Curr. Osteoporos. Rep. 2017, 20, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.-L.; Zhang, Y.-P.; Li, X.; Jing, L.-D.; Cairang, Z.-M.; Gou, J.-Q. Exploration on the relationship between the elderly osteoporosis and cardiovascular disease risk factors. Eur. Rev. Med. Pharm. Sci. 2017, 21, 4386–4390. [Google Scholar]
- Veronese, N.; Stubbs, B.; Crepaldi, G.; Solmi, M.; Cooper, C.; Harvey, N.C.; Reginster, J.-Y.; Rizzoli, R.; Civitelli, R.; Schofield, P.; et al. Relationship Between Low Bone Mineral Density and Fractures With Incident Cardiovascular Disease: A Systematic Review and Meta-Analysis. J. Bone Miner. Res. 2017, 32, 1126–1135. [Google Scholar] [CrossRef]
- Chuang, T.-L.; Lin, J.-W.; Wang, Y.-F. Bone Mineral Density as a Predictor of Atherogenic Indexes of Cardiovascular Disease, Especially in Nonobese Adults. Dis. Markers 2019, 2019, 1045098. [Google Scholar] [CrossRef]
- Verheyen, N.; Grübler, M.R.; Catena, C.; Fahrleitner-Pammer, A.; Van Ballegooijen, A.J. The Bone-Cardiovascular Axis: Mechanisms and Clinical Relevance. Int. J. Endocrinol. 2018, 2018, 9689106. [Google Scholar] [CrossRef]
- Arnold, I.C.; Dehzad, N.; Reuter, S.; Martin, H.; Becher, B.; Taube, C.; Müller, A. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J. Clin. Investig. 2011, 121, 3088–3093. [Google Scholar] [CrossRef]
- Engler, D.B.; Reuter, S.; Van Wijck, Y.; Urban, S.; Kyburz, A.; Maxeiner, J.; Martin, H.; Yogev, N.; Waisman, A.; Gerhard, M.; et al. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc. Natl. Acad. Sci. USA 2014, 111, 11810–11815. [Google Scholar] [CrossRef]
- Castano-Rodriguez, N.; Kaakoush, N.O.; Lee, W.S.; Mitchell, H.M. Dual role of Helicobacter and Campylobacter species in IBD: A systematic review and meta-analysis. Gut 2017, 66, 235–249. [Google Scholar] [CrossRef]
- Lebwohl, B.; Blaser, M.J.; Ludvigsson, J.F.; Green, P.H.R.; Rundle, A.; Sonnenberg, A.; Genta, R.M. Decreased risk of celiac disease in patients with Helicobacter pylori colonization. Am. J. Epidemiol. 2013, 178, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K. Helicobacter pylori-Mediated Protection against Extra-Gastric Immune and Inflammatory Disorders: The Evidence and Controversies. Diseases 2015, 3, 34–55. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, A.; Dellon, E.S.; Turner, K.O.; Genta, R.M. The influence of Helicobacter pylori on the ethnic distribution of esophageal eosinophilia. Helicobacter 2016, 22, e12370. [Google Scholar] [CrossRef]
- Lucero, Y.; Oyarzun, A.; O’Ryan, M.; Quera, R.; Espinosa, N.; Valenzuela, R.; Simian, D.; Alcalde, E.; Arce, C.; Farfan, M.J.; et al. Helicobacter pylori cagA+ Is Associated with Milder Duodenal Histological Changes in Chilean Celiac Patients. Front. Cell Infect. Microbiol. 2017, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Narang, M.; Puri, A.S.; Sachdeva, S.; Singh, J.; Kumar, A.; Saran, R.K. Celiac disease and Helicobacter pylori infection in children: Is there any Association? J. Gastroenterol. Hepatol. 2017, 32, 1178–1182. [Google Scholar] [CrossRef]
- Shah, S.C.; Tepler, A.; Peek, R.M.; Colombel, J.-F.; Hirano, I.; Narula, N. Association Between Helicobacter pylori Exposure and Decreased Odds of Eosinophilic Esophagitis—A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 2185–2198.e3. [Google Scholar] [CrossRef]
- Tepler, A.; Narula, N.; Peek, R.M.; Patel, A.; Edelson, C.; Colombel, J.-F.; Shah, S.C. Systematic review with meta-analysis: Association between Helicobacter pylori CagA seropositivity and odds of inflammatory bowel disease. Aliment. Pharm. 2019, 50, 121–131. [Google Scholar] [CrossRef]
- Ness-Jensen, E.; Langhammer, A.; Hveem, K.; Lu, Y. Helicobacter pylori in relation to asthma and allergy modified by abdominal obesity: The HUNT study in Norway. World Allergy Organ. J. 2019, 12, 100035. [Google Scholar] [CrossRef]
- Shimoyama, T.; Higuchi, H.; Matsuzaka, M.; Chinda, D.; Nakaji, S.; Fukuda, S. Helicobacter pylori infection is associated with a decreased risk of tooth loss in healthy Japanese men. Jpn. J. Infect. Dis. 2013, 66, 489–492. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lin, T.-Y.; Shang, S.-T.; Chen, H.-J.; Kao, C.-H.; Wu, C.-C.; Yang, T.-Y. Helicobacter pylori infection increases the risk of adult-onset asthma: A nationwide cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1587–1594. [Google Scholar] [CrossRef]
- Wu, M.-C.; Huang, J.-Y.; Chen, H.-H.; Wei, J.C.-C. Effect of early eradication therapy on systemic lupus erythematosus risk in patients with Helicobacter pylori infection: A nationwide population-based cohort study. Lupus 2020, 29, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, X.; Tan, W.; Zhang, M. Association between Helicobacter pylori infection and Sjögren syndrome. Medcine 2018, 97, e13528. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Kim, T.-Y.; Kim, E.Y.; Jang, E.K.; Jeon, M.J.; Kim, W.B.; Shong, Y.K. Association between thyroid autoimmunity and Helicobacter pylori infection. Korean J. Intern. Med. 2017, 32, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Gavalas, E.; Kountouras, J.; Boziki, M.; Zavos, C.; Polyzos, S.A.; Vlachaki, E.; Venizelos, I.; Tsiptsios, D.; Deretzi, G. Relationship between Helicobacter pylori infection and multiple sclerosis. Ann. Gastroenterol. 2015, 28, 353–356. [Google Scholar] [PubMed]
- Deretzi, G.; Gavalas, E.; Boziki, M.; Tsiptsios, D.; Polyzos, S.A.; Venizelos, I.; Zavos, C.; Koutlas, E.; Tsiptsios, I.; Katsinelos, P.; et al. Impact of Helicobacter pylorion multiple sclerosis-related clinically isolated syndrome. Acta Neurol. Scand. 2015, 133, 268–275. [Google Scholar] [CrossRef]
- den Hollander, W.J.; Broer, L.; Schurmann, C.; Meyre, D.; Hoed, C.M.D.; Mayerle, J.; Hofman, A.; Homuth, G.; Uitterlinden, A.G.; Lerch, M.M.; et al. Helicobacter pylori colonization and obesity—A Mendelian randomization study. Sci. Rep. 2017, 7, 14467. [Google Scholar] [CrossRef]
- Chen, L.-W.; Kuo, S.-F.; Chen, C.-H.; Chien, C.-H.; Lin, C.-L.; Chien, R.-N. A community-based study on the association between Helicobacter pylori Infection and obesity. Sci. Rep. 2018, 8, 10746. [Google Scholar] [CrossRef] [PubMed]
- Grundstrom, A.C.; Guse, C.E.; Layde, P.M. Risk factors for falls and fall-related injuries in adults 85 years of age and older. Arch. Gerontol. Geriatr. 2012, 54, 421–428. [Google Scholar] [CrossRef]
- Boelens, C.; Hekman, E.E.; Verkerke, G.J. Risk factors for falls of older citizens. Technol. Health Care 2013, 21, 521–533. [Google Scholar] [CrossRef]
- Angelousi, A.; Girerd, N.; Benetos, A.; Frimat, L.; Gautier, S.; Weryha, G.; Boivin, J.-M. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality. J. Hypertens. 2014, 32, 1562–1571. [Google Scholar] [CrossRef]
- Alamgir, H.; Wong, N.J.; Hu, Y.; Yu, M.; Marshall, A.; Yu, S. Epidemiology of Falls in Older Adults in Texas. South. Med. J. 2015, 108, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Fedorowski, A.; Radico, F.; Romanello, M.; Tatasciore, A.; Di Nicola, M.; Zimarino, M.; De Caterina, R. Cardiovascular morbidity and mortality related to orthostatic hypotension: A meta-analysis of prospective observational studies. Eur. Heart J. 2015, 36, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Hartog, L.C.; Schrijnders, D.; Landman, G.; Groenier, K.; Kleefstra, N.; Bilo, H.J.; Van Hateren, K.J.J. Is orthostatic hypotension related to falling? A meta-analysis of individual patient data of prospective observational studies. Age Ageing 2017, 46, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, Y.; Slattum, P.W.; Ratliff, S.M. Chronic Health Conditions as a Risk Factor for Falls among the Community-Dwelling US Older Adults: A Zero-Inflated Regression Modeling Approach. Biomed. Res. Int. 2017, 2017, 5146378. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.; Odén, A.; Orwoll, E.; Lapidus, J.; Kwok, T.; Karlsson, M.K.; Rosengren, B.E.; Ljunggren, Ö.; Cooper, C.; McCloskey, E.; et al. Falls Predict Fractures Independently of FRAX Probability: A Meta-Analysis of the Osteoporotic Fractures in Men (MrOS) Study. J. Bone Miner. Res. 2017, 33, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, S.; Adedire, O.; Copsey, B.J.; Boniface, G.J.; Sherrington, C.; Clemson, L.; Close, J.C.T.; Lamb, S.E. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2018, 7, CD012221. [Google Scholar] [CrossRef]
- Guirguis-Blake, J.M.; Michael, Y.; Perdue, L.A.; Coppola, E.L.; Beil, T.L.; Thompson, J.H. Interventions to Prevent Falls in Community-Dwelling Older Adults: A Systematic Review for the US Preventive Services Task Force; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2018.
- Ronthal, M. Gait Disorders and Falls in the Elderly. Med. Clin. N. Am. 2019, 103, 203–213. [Google Scholar] [CrossRef]
- Mol, A.; Hoang, P.T.S.B.; Sharmin, S.; Reijnierse, E.M.; Van Wezel, R.J.; Meskers, C.G.; Maier, A.B. Orthostatic Hypotension and Falls in Older Adults: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2019, 20, 589–597.e5. [Google Scholar] [CrossRef]
- Perng, H.-J.; Chiu, Y.-L.; Chung, C.-H.; Kao, S.; Chien, W.-C. Fall and risk factors for veterans and non-veterans inpatients over the age of 65 years: 14 years of long-term data analysis. BMJ Open 2019, 9, e030650. [Google Scholar] [CrossRef]
- Ganz, D.A.; Latham, N.K. Prevention of Falls in Community-Dwelling Older Adults. N. Engl. J. Med. 2020, 382, 734–743. [Google Scholar] [CrossRef]
- Patel, D.; Ackermann, R.J. Issues in Geriatric Care: Falls. FP Essent. 2018, 468, 18–25. [Google Scholar] [PubMed]
- Peeters, G.; Van Schoor, N.M.; Lips, P. Fall risk: The clinical relevance of falls and how to integrate fall risk with fracture risk. Best Pract. Res. Clin. Rheumatol. 2009, 23, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, A.F.; Cruz, L.; Paul, G. Falls and Fractures: A systematic approach to screening and prevention. Maturitas 2015, 82, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Franse, C.; Carmen, B.; Rietjens, J.A.; Burdorf, A.; Van Grieken, A.; Korfage, I.J.; Van Der Heide, A.; Raso, F.M.; Van Beeck, E.; Raat, H. A prospective study on the variation in falling and fall risk among community-dwelling older citizens in 12 European countries. BMJ Open 2017, 7, e015827. [Google Scholar] [CrossRef] [PubMed]
- Thayer, S.; Stolshek, B.S.; Rey, G.G.; Seare, J.G. Impact of Osteoporosis on High-Cost Chronic Diseases. Value Health 2014, 17, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.T.; Tran, T.S.; Ho-Le, T.P.; Center, J.R.; Eisman, J.A.; Nguyen, T. Two-Thirds of All Fractures Are Not Attributable to Osteoporosis and Advancing Age: Implications for Fracture Prevention. J. Clin. Endocrinol. Metab. 2019, 104, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
- Karasik, D.; Kiel, D.P. Evidence for pleiotropic factors in genetics of the musculoskeletal system. Bone 2010, 46, 1226–1237. [Google Scholar] [CrossRef][Green Version]
- Fisher, F.M.; Maratos–Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef]
- Karstoft, K.; Pedersen, B.K. Skeletal muscle as a gene regulatory endocrine organ. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 270–275. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Huynh, T.V.; Lee, S.-J. Paracrine and endocrine modes of myostatin action. J. Appl. Physiol. 2016, 120, 592–598. [Google Scholar] [CrossRef]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: Past, present and future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.J.; Scott, D.; Hodge, A.; English, D.R.; Giles, G.G.; Ebeling, P.R. Associations between hip bone mineral density, aortic calcification and cardiac workload in community-dwelling older Australians. Osteoporos. Int. 2017, 28, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Thyfault, J.P.; Ruegsegger, G.N.; Toedebusch, R.G. Role of Inactivity in Chronic Diseases: Evolutionary Insight and Pathophysiological Mechanisms. Physiol. Rev. 2017, 97, 1351–1402. [Google Scholar] [CrossRef]
- Rikkonen, T.; Sirola, J.; Salovaara, K.; Tuppurainen, M.; Jurvelin, J.S.; Honkanen, R.; Kröger, H. Muscle Strength and Body Composition Are Clinical Indicators of Osteoporosis. Calcif. Tissue Int. 2012, 91, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kawao, N.; Kaji, H. Interactions Between Muscle Tissues and Bone Metabolism. J. Cell. Biochem. 2015, 116, 687–695. [Google Scholar] [CrossRef]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.-J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef]
- Curtis, E.; Litwic, A.; Cooper, C.; Dennison, E.M. Determinants of Muscle and Bone Aging. J. Cell. Physiol. 2015, 230, 2618–2625. [Google Scholar] [CrossRef]
- Karsenty, G.; Olson, E.N. Bone and Muscle Endocrine Functions: Unexpected Paradigms of Inter-organ Communication. Cell 2016, 164, 1248–1256. [Google Scholar] [CrossRef]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef]
- Zanker, J.; Duque, G. Osteoporosis in Older Persons: Old and New Players. J. Am. Geriatr. Soc. 2018, 67, 831–840. [Google Scholar] [CrossRef]
- Yoo, J.-I.; Kim, H.; Ha, Y.-C.; Kwon, H.-B.; Koo, K.-H. Osteosarcopenia in Patients with Hip Fracture Is Related with High Mortality. J. Korean Med. Sci. 2018, 33, e27. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.-H.; Lee, J.-K.; Choi, D.-S.; Han, S.-H. Prevalence and Associated Risk Factors of Sarcopenia in Female Patients with Osteoporotic Fracture. J. Bone Metab. 2018, 25, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. Use it or lose it to age: A review of bone and muscle communication. Bone 2019, 120, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Locquet, M.; Beaudart, C.; Durieux, N.; Reginster, J.-Y.; Bruyère, O. Relationship between the changes over time of bone mass and muscle health in children and adults: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 413–429. [Google Scholar] [CrossRef]
- Laurent, M.; Dedeyne, L.; Dupont, J.; Mellaerts, B.; Dejaeger, M.; Gielen, E. Age-related bone loss and sarcopenia in men. Maturitas 2019, 122, 51–56. [Google Scholar] [CrossRef]
- Lombardi, G.; Ziemann, E.; Banfi, G. Physical Activity and Bone Health: What Is the Role of Immune System? A Narrative Review of the Third Way. Front. Endocrinol. 2019, 10, 60. [Google Scholar] [CrossRef]
- Kirk, B.; Al Saedi, A.; Duque, G. Osteosarcopenia: A case of geroscience. Aging Med. 2019, 2, 147–156. [Google Scholar] [CrossRef]
- Herrmann, M.; Engelke, K.; Ebert, R.; Müller-Deubert, S.; Rudert, M.; Ziouti, F.; Jundt, F.; Felsenberg, D.; Jakob, F. Interactions between Muscle and Bone—Where Physics Meets Biology. Biomolecules 2020, 10, 432. [Google Scholar] [CrossRef]
- Willems, S.M.; Wright, D.; Day, I.N.M.; Trajanoska, K.; Joshi, P.K.; Morris, J.A.; Matteini, A.M.; Garton, F.C.; Grarup, N.; Oskolkov, N.; et al. Large-scale GWAS identifies multiple loci for hand grip strength providing biological insights into muscular fitness. Nat. Commun. 2017, 8, 16015. [Google Scholar] [CrossRef]
- Medina-Gomez, C.; Kemp, J.P.; Dimou, N.L.; Kreiner, E.; Chesi, A.; Zemel, B.S.; Bønnelykke, K.; Boer, C.G.; Ahluwalia, S.; Bisgaard, H.; et al. Bivariate genome-wide association meta-analysis of pediatric musculoskeletal traits reveals pleiotropic effects at the SREBF1/TOM1L2 locus. Nat. Commun. 2017, 8, 121. [Google Scholar] [CrossRef]
- Trajanoska, K.; Rivadeneira, F.; Kiel, D.P.; Karasik, D. Genetics of Bone and Muscle Interactions in Humans. Curr. Osteoporos. Rep. 2019, 17, 86–95. [Google Scholar] [CrossRef] [PubMed]
- rajanoska, K.; Morris, J.A.; Oei, L.; Zheng, H.-F.; Evans, D.M.; Kiel, U.P.; Ohlsson, C.; Richards, J.B.; Rivadeneira, F. Assessment of the genetic and clinical determinants of fracture risk: Genome wide association and mendelian randomisation study. BMJ 2018, 362, k3225. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Tweed, K.; Chinappen, U. Fall-related risk factors and osteoporosis in older women referred to an open access bone densitometry service. Age Ageing 2005, 34, 67–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verschueren, S.; Gielen, E.; O’Neill, T.W.; Pye, S.R.; Adams, J.E.; Ward, K.A.; Wu, F.C.; Szulc, P.; Laurent, M.; Claessens, F.; et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos. Int. 2012, 24, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Laurent, M.; Dubois, V.; Claessens, F.; Verschueren, S.; Vanderschueren, D.; Gielen, E.; Jardí, F. Muscle-bone interactions: From experimental models to the clinic? A critical update. Mol. Cell. Endocrinol. 2016, 432, 14–36. [Google Scholar] [CrossRef]
- Paintin, J.; Cooper, C.; Dennison, E. Osteosarcopenia. Br. J. Hosp. Med. 2018, 79, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Locquet, M.; Beaudart, C.; Bruyère, O.; Kanis, J.A.; Delandsheere, L.; Reginster, J.-Y. Bone health assessment in older people with or without muscle health impairment. Osteoporos. Int. 2018, 29, 1057–1067. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Yeung, S.S.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachex Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef]
- De Abreu, D.C.C.; Trevisan, D.C.; Reis, J.G.; Costa, G.D.C.D.; Gomes, M.M.; Matos, M.S. Body balance evaluation in osteoporotic elderly women. Arch. Osteoporos. 2009, 4, 25–29. [Google Scholar] [CrossRef][Green Version]
- Chen, H.-T.; Ma, J.-X.; Liu, A.; Cui, Y.; Ma, X. The association between sarcopenia and fracture in middle-aged and elderly people: A systematic review and meta-analysis of cohort studies. Injury 2020, 51, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, W.; Wang, C.; Tao, W.; Dou, Q.; Yang, Y. Sarcopenia as a predictor of hospitalization among older people: A systematic review and meta-analysis. BMC Geriatr. 2018, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, P.; Dou, Q.; Wang, C.; Zhang, W.; Yang, Y.; Wang, J.; Xie, X.; Zhou, J.; Zeng, Y. Falls among older adults with sarcopenia dwelling in nursing home or community: A meta-analysis. Clin. Nutr. 2020, 39, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Cumming, R.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Handelsman, D.J.; Seibel, M.; Waite, L.M.; Hirani, V. Associations of sarcopenic obesity with the metabolic syndrome and insulin resistance over five years in older men: The Concord Health and Ageing in Men Project. Exp. Gerontol. 2018, 108, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Reuben, D.B.; Gazarian, P.; Alexander, N.; Araujo, K.; Baker, D.; Bean, J.F.; Boult, C.; Charpentier, P.; Duncan, P.; Latham, N.; et al. The Strategies to Reduce Injuries and Develop Confidence in Elders Intervention: Falls Risk Factor Assessment and Management, Patient Engagement, and Nurse Co-management. J. Am. Geriatr. Soc. 2017, 65, 2733–2739. [Google Scholar] [CrossRef]
- Ek, S.; Rizzuto, D.; Fratiglioni, L.; Johnell, K.; Xu, W.; Welmer, A.-K. Risk Profiles for Injurious Falls in People Over 60: A Population-Based Cohort Study. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 2017, 73, 233–239. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Ewing, S.K.; Taylor, B.C.; Fink, H.A.; Stone, K.L.; Cauley, J.A.; Tracy, J.K.; Hochberg, M.C.; Rodondi, N.; Cawthon, P.M.; et al. Frailty and Risk of Falls, Fracture, and Mortality in Older Women: The Study of Osteoporotic Fractures. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 2007, 62, 744–751. [Google Scholar] [CrossRef]
- De Vries, O.J.; Peeters, G.M.E.E.; Lips, P.; Deeg, D.J.H. Does frailty predict increased risk of falls and fractures? A prospective population-based study. Osteoporos. Int. 2013, 24, 2397–2403. [Google Scholar] [CrossRef]
- Milte, R.; Crotty, M. Musculoskeletal health, frailty and functional decline. Best Pract. Res. Clin. Rheumatol. 2014, 28, 395–410. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a Predictor of Future Falls Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015, 16, 1027–1033. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a predictor of fractures among community-dwelling older people: A systematic review and meta-analysis. Bone 2016, 90, 116–122. [Google Scholar] [CrossRef]
- Walters, S.; Chan, S.; Goh, L.; Ong, T.; Sahota, O. The Prevalence of Frailty in Patients Admitted to Hospital with Vertebral Fragility Fractures. Curr. Rheumatol. Rev. 2016, 12, 244–247. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, F.E.; Bartosch, P.; Åkesson, K. Musculoskeletal health and frailty. Best Pract. Res. Clin. Rheumatol. 2017, 31, 145–159. [Google Scholar] [CrossRef]
- Kim, H.-J.; Park, S.; Park, S.-H.; Park, J.; Chang, B.-S.; Lee, C.-K.; Yeom, J.S. Prevalence of Frailty in Patients with Osteoporotic Vertebral Compression Fracture and Its Association with Numbers of Fractures. Yonsei Med. J. 2018, 59, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ravindrarajah, R.; Hazra, N.C.; Charlton, J.; Jackson, S.H.D.; Dregan, A.; Gulliford, M.C. Incidence and mortality of fractures by frailty level over 80 years of age: Cohort study using UK electronic health records. BMJ Open 2018, 8, e018836. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.M.H.; Leung, J.C.; Kwok, T.C.Y. The Clinical Potential of Frailty Indicators on Identifying Recurrent Fallers in the Community: The Mr. Os and Ms. OS Cohort Study in Hong Kong. J. Am. Med. Dir. Assoc. 2019, 20, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, M.; Setoguchi, S.; Schneeweiss, S.; Solomon, D.H. Bone mineral density in subjects using central nervous system-active medications. Am. J. Med. 2005, 118, 1414.e7–1414.e12. [Google Scholar] [CrossRef] [PubMed]
- Gonyeau, M.J. Statins and Osteoporosis: A Clinical Review. Pharm. J. Hum. Pharm. Drug 2005, 25, 228–243. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Jain, G.K. Statins and osteoporosis: New role for old drugs. J. Pharm. Pharm. 2006, 58, 3–18. [Google Scholar] [CrossRef]
- Horiuchi, N.; Maeda, T. Statins and bone metabolism. Oral Dis. 2006, 12, 85–101. [Google Scholar] [CrossRef]
- Walsh, J.S.; Newman, C.; Eastell, R. Heart drugs that affect bone. Trends Endocrinol. Metab. 2012, 23, 163–168. [Google Scholar] [CrossRef]
- Hill, K.D.; Wee, R. Psychotropic Drug-Induced Falls in Older People. Drugs Aging 2012, 29, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Aluoch, A.O.; Jessee, R.; Habal, H.; Garcia-Rosell, M.; Shah, R.; Reed, G.; Carbone, L.D. Heart Failure as a Risk Factor for Osteoporosis and Fractures. Curr. Osteoporos. Rep. 2012, 10, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Ilić, K.; Obradović, N.; Vujasinović-Stupar, N. The Relationship Among Hypertension, Antihypertensive Medications, and Osteoporosis: A Narrative Review. Calcif. Tissue Int. 2012, 92, 217–227. [Google Scholar] [CrossRef]
- Ghosh, M.; Majumdar, S.R. Antihypertensive medications, bone mineral density, and fractures: A review of old cardiac drugs that provides new insights into osteoporosis. Endocrine 2014, 46, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, L.L.; Zhou, Q. Effects of drug pharmacokinetic/pharmacodynamic properties, characteristics of medication use, and relevant pharmacological interventions on fall risk in elderly patients. Clin. Risk Manag. 2014, 10, 437–448. [Google Scholar]
- Thorell, K.; Ranstad, K.; Midlöv, P.; Borgquist, L.A.; Halling, A. Is use of fall risk-increasing drugs in an elderly population associated with an increased risk of hip fracture, after adjustment for multimorbidity level: A cohort study. BMC Geriatr. 2014, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.P.; Pratley, R.E. The Impact of Diabetes and Diabetes Medications on Bone Health. Endocr. Rev. 2015, 36, 194–213. [Google Scholar] [CrossRef]
- Lin, S.-I.; Chang, K.-C.; Lee, H.-C.; Yang, Y.-C.; Tsauo, J.-Y. Problems and fall risk determinants of quality of life in older adults with increased risk of falling. Geriatr. Gerontol. Int. 2014, 15, 579–587. [Google Scholar] [CrossRef]
- Ćurković, M.; Dodig-Curković, K.; Erić, A.P.; Kralik, K.; Pivac, N. Psychotropic medications in older adults: A review. Psychiatr. Danub. 2016, 28, 13–24. [Google Scholar]
- Vianna, A.G.D.; Sanches, C.; Barreto, F.C. Review article: Effects of type 2 diabetes therapies on bone metabolism. Diabetol. Metab. Syndr. 2017, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Puttnam, R.; Davis, B.R.; Pressel, S.L.; Whelton, P.K.; Cushman, W.C.; Louis, G.T.; Margolis, K.L.; Oparil, S.; Williamson, J.; Ghosh, A.; et al. Association of 3 Different Antihypertensive Medications With Hip and Pelvic Fracture Risk in Older Adults. JAMA Intern. Med. 2017, 177, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.; Leong, C. Benzodiazepines and Z-Drugs: An Updated Review of Major Adverse Outcomes Reported on in Epidemiologic Research. Drugs R&D 2017, 17, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.; Bracchi, R.; Hewitt, J.; Routledge, P.A.; Carter, B. Benzodiazepines, Z-drugs and the risk of hip fracture: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0174730. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.J.; Pratt, N.L.; Roughead, E.E. Risk of Hip Fracture in Older People Using Selective Serotonin Reuptake Inhibitors and Other Psychoactive Medicines Concurrently: A Matched Case-Control Study in Australia. Drugs Real World Outcomes 2017, 4, 87–96. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Blom, A.W.; Whitehouse, M.R.; Kehoe, P.G.; Laukkanen, J.A. Renin-angiotensin system inhibitors and risk of fractures: A prospective cohort study and meta-analysis of published observational cohort studies. Eur. J. Epidemiol. 2017, 32, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Kwok, T.C.Y.; Leung, J.; Barrett-Connor, E. Osteoporotic Fractures in Men (MrOS) Research Group ARB users exhibit a lower fracture incidence than ACE inhibitor users among older hypertensive men. Age Ageing 2017, 46, 57–64. [Google Scholar]
- Mabilleau, G.; Gobron, B.; Bouvard, B.; Chappard, D. Incretin-based therapy for the treatment of bone fragility in diabetes mellitus. Peptides 2018, 100, 108–113. [Google Scholar] [CrossRef]
- Seppala, L.J.; Van De Glind, E.M.; Daams, J.G.; Ploegmakers, K.J.; De Vries, M.; Wermelink, A.M.; Van Der Velde, N.; Blain, H.; Bousquet, J.; Bucht, G.; et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-analysis: III. Others. J. Am. Med. Dir. Assoc. 2018, 19, 372.e1–372.e8. [Google Scholar] [CrossRef]
- Treves, N.; Perlman, A.; Geron, L.K.; Asaly, A.; Matok, I. Z-drugs and risk for falls and fractures in older adults—A systematic review and meta-analysis. Age Ageing 2017, 47, 201–208. [Google Scholar] [CrossRef]
- Cortet, B.; Lucas, S.; Legroux-Gerot, I.; Penel, G.; Chauveau, C.; Paccou, J. Bone disorders associated with diabetes mellitus and its treatments. Jt. Bone Spine 2019, 86, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P. Drugs Causing Bone Loss. Handb. Exp. Pharm. 2019. [Google Scholar] [CrossRef]
- Wang, J.; Su, K.; Sang, W.; Li, L.; Ma, S. Thiazide Diuretics and the Incidence of Osteoporotic Fracture: A Systematic Review and Meta-Analysis of Cohort Studies. Front. Pharm. 2019, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Taipale, H.; Rysä, J.; Hukkanen, J.; Koponen, M.; Tanskanen, A.; Tiihonen, J.; Kröger, H.; Hartikainen, S.; Tolppanen, A.-M. Long-term thiazide use and risk of low-energy fractures among persons with Alzheimer’s disease—Nested case-control study. Osteoporos. Int. 2019, 30, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Hartung, D.; Turner, J.; Blazina, I.; Chan, B.; Levander, X.; McDonagh, M.; Selph, S.; Fu, R.; Pappas, M. Opioid Treatments for Chronic Pain; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2020.
- Oh, T.K.; Song, I.-A. Metformin therapy and hip fracture risk among patients with type II diabetes mellitus: A population-based cohort study. Bone 2020, 135, 115325. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.J.; Mohamadi, A.; Wright, C.L.; Chan, J.J.; Weaver, M.J.; Von Keudell, A.; Nazarian, A. Medications as a Risk Factor for Fragility Hip Fractures: A Systematic Review and Meta-analysis. Calcif. Tissue Int. 2020, 107, 1–9. [Google Scholar] [CrossRef]
- Barzilay, J.I.; Davis, B.R.; Pressel, S.L.; Ghosh, A.; Puttnam, R.; Margolis, K.L.; Whelton, P.K. The Impact of Antihypertensive Medications on Bone Mineral Density and Fracture Risk. Curr. Cardiol. Rep. 2017, 19, 76. [Google Scholar] [CrossRef]
- Napoli, N.; Chandran, M.; Pierroz, D.D.; Abrahamsen, B.; Schwartz, A.V.; Ferrari, S.L.; On behalf of the IOF Bone and Diabetes Working Group. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2016, 13, 208–219. [Google Scholar] [CrossRef]
- Lai, S.-W.; Cheng, K.-C.; Lin, C.-L.; Lai, S.-W. Furosemide use and acute risk of hip fracture in older people: A nationwide case-control study in Taiwan. Geriatr. Gerontol. Int. 2017, 17, 2552–2558. [Google Scholar] [CrossRef]
- Andersen, C.U.; Lassen, P.O.; Usman, H.Q.; Albertsen, N.; Nielsen, L.P.; Andersen, S. Prevalence of medication-related falls in 200 consecutive elderly patients with hip fractures: A cross-sectional study. BMC Geriatr. 2020, 20, 121. [Google Scholar] [CrossRef]
- Elias, A.M.; Ogunwale, A.N.; Pepin, M.J.; Bailey, J.C.; Adams, A.D.; Colón-Emeric, C.S.; Vognsen, J.D.; Schmader, K.E.; Pavon, J. High Prevalence of Fall-Related Medication Use in Older Veterans at Risk for Falls. J. Am. Geriatr. Soc. 2019, 68, 438–439. [Google Scholar] [CrossRef] [PubMed]
- Cumming, R.G. Epidemiology of medication-related falls and fractures in the elderly. Drugs Aging 1998, 12, 43–53. [Google Scholar] [CrossRef]
- Leipzig, R.M.; Cumming, R.G.; Tinetti, M.E. Drugs and Falls in Older People: A Systematic Review and Meta-analysis: I. Psychotropic Drugs. J. Am. Geriatr. Soc. 1999, 47, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Leipzig, R.M.; Cumming, R.G.; Tinetti, M.E. Drugs and Falls in Older People: A Systematic Review and Meta-analysis: II. Cardiac and Analgesic Drugs. J. Am. Geriatr. Soc. 1999, 47, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Blackwell, T.; Mangione, C.M.; Bowman, P.J.; Bauer, D.C.; Schwartz, A.; Hanlon, J.T.; Nevitt, M.C.; Whooley, M.A. Central Nervous System Active Medications and Risk for Fractures in Older Women. Arch. Intern. Med. 2003, 163, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Woolcott, J.C.; Richardson, K.J.; Wiens, M.O.; Patel, B.; Marin, J.; Khan, K.M.; Marra, A.C. Meta-analysis of the Impact of 9 Medication Classes on Falls in Elderly Persons. Arch. Intern. Med. 2009, 169, 1952–1960. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Kumar, C. The Patient Who Falls. JAMA 2010, 303, 258–266. [Google Scholar] [CrossRef]
- Huang, A.; Mallet, L.; Buckeridge, D.; Huang, A.R.; Rochefort, C.M.; Eguale, T.; Tamblyn, R. Medication-Related Falls in the Elderly. Drugs Aging 2012, 29, 359–376. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Han, L.; Lee, D.S.H.; McAvay, G.J.; Peduzzi, P.; Gross, C.P.; Zhou, B.; Lin, H. Antihypertensive Medications and Serious Fall Injuries in a Nationally Representative Sample of Older Adults. JAMA Intern. Med. 2014, 174, 588–595. [Google Scholar] [CrossRef]
- Ham, A.C.; Swart, K.M.; Enneman, A.W.; van Dijk, S.C.; Oliai Araghi, S.; van Wijngaarden, J.P.; van der Zwaluw, N.; Brower-Brolsma, E.M.; Dhonukshe-Rutten, R.A.M.; van Schoor, N.M.L.; et al. Medication-related fall incidents in an older, ambulant population: The B-PROOF study. Drugs Aging 2014, 31, 917–927. [Google Scholar] [CrossRef]
- Park, H.; Satoh, H.; Miki, A.; Urushihara, H.; Sawada, Y. Medications associated with falls in older people: Systematic review of publications from a recent 5-year period. Eur. J. Clin. Pharm. 2015, 71, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Bennett, K.; Kenny, R.A. Polypharmacy including falls risk-increasing medications and subsequent falls in community-dwelling middle-aged and older adults. Age Ageing 2014, 44, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ruths, S.; Bakken, M.S.; Ranhoff, A.H.; Hunskaar, S.; Engesæter, L.B.; Engeland, A. Risk of hip fracture among older people using antihypertensive drugs: A nationwide cohort study. BMC Geriatr. 2015, 15, 153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Winter, S.; Vanwynsberghe, S.; Foulon, V.; Dejaeger, E.; Flamaing, J.; Sermon, A.; Van Der Linden, L.; Spriet, I. Exploring the relationship between fall risk-increasing drugs and fall-related fractures. Int. J. Clin. Pharm. 2016, 38, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P.; Mehta, Z. Opioids and Chronic Pain: Where Is the Balance? Curr. Oncol. Rep. 2016, 18, 71. [Google Scholar] [CrossRef]
- Petty, S.; Wilding, H.; Wark, J.D. Osteoporosis Associated with Epilepsy and the Use of Anti-Epileptics—A Review. Curr. Osteoporos. Rep. 2016, 14, 54–65. [Google Scholar] [CrossRef]
- Li, X.; Wu, F.; Zhang, Y.; Yang, J.; Shinohara, A.; Kagami, H. Discontinuation of simvastatin leads to a rebound phenomenon and results in immediate peri-implant bone loss. Clin. Exp. Dent. Res. 2016, 2, 65–72. [Google Scholar] [CrossRef]
- Maximos, M.; Chang, F.; Patel, T. Risk of falls associated with antiepileptic drug use in ambulatory elderly populations. Can. Pharm. J. Rev. Des. Pharm. Can. 2017, 150, 101–111. [Google Scholar] [CrossRef]
- Ming, Y.; Zecevic, A. Medications & Polypharmacy Influence on Recurrent Fallers in Community: A Systematic Review. Can. Geriatr. J. 2018, 21, 14–25. [Google Scholar]
- Beunza-Sola, M.; Hidalgo-Ovejero, Á.M.; Martí-Ayerdi, J.; Sánchez-Hernández, J.G.; Menéndez-García, M.; García-Mata, S. Study of fall risk-increasing drugs in elderly patients before and after a bone fracture. Postgrad. Med. J. 2017, 94, 76–80. [Google Scholar] [CrossRef]
- De Vries, M.; Seppala, L.J.; Daams, J.G.; Van De Glind, E.M.; Masud, T.; Van Der Velde, N.; Blain, H.; Bousquet, J.; Bucht, G.; Caballero-Mora, M.A.; et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-Analysis: I. Cardiovascular Drugs. J. Am. Med. Dir. Assoc. 2018, 19, 371.e1–371.e9. [Google Scholar] [CrossRef] [PubMed]
- Axmon, A.; Sandberg, M.; Ahlström, G.; Midlöv, P. Fall-risk-increasing drugs and falls requiring health care among older people with intellectual disability in comparison with the general population: A register study. PLoS ONE 2018, 13, e0199218. [Google Scholar] [CrossRef]
- Banu, Z.; Lim, K.K.; Kwan, Y.H.; Yap, K.Z.; Ang, H.T.; Tan, C.S.; Fong, W.; Thumboo, J.; Lee, K.H.; Ostbye, T.; et al. Anti-hypertensive medications and injurious falls in an older population of low socioeconomic status: A nested case-control study. BMC Geriatr. 2018, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, S.; Ruzicka, M.; Petrcich, W.; McCallum, M.K.; Hundemer, G.L.; Tanuseputro, P.; Manuel, D.; Burns, K.; Edwards, C.; Bugeja, A.; et al. Alpha-Blocker Use and the Risk of Hypotension and Hypotension-Related Clinical Events in Women of Advanced Age. Hypertension 2019, 74, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, S.L.; Springer, S.P.; Zhao, X.; Cunningham, F.E.; Thorpe, C.T.; Semla, T.P.; Shorr, R.I.; Hanlon, J.T. Central Nervous System Medication Burden and Risk of Recurrent Serious Falls and Hip Fractures in Veterans Affairs Nursing Home Residents. J. Am. Geriatr. Soc. 2018, 67, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Toivo, T.; Airaksinen, M.; Dimitrow, M.; Savela, E.; Pelkonen, K.; Kiuru, V.; Suominen, T.; Uunimäki, M.; Kivelä, S.-L.; Leikola, S.; et al. Enhanced coordination of care to reduce medication risks in older home care clients in primary care: A randomized controlled trial. BMC Geriatr. 2019, 19, 332. [Google Scholar] [CrossRef]
- Park, H.; Satoh, H.; Miki, A.; Maki, H.; Asai, K.; Shiraishi, A.; Urushihara, H.; Sawada, Y. Medications and fall risk: A case–control study in nursing home residents in Japan. Aging Clin. Exp. Res. 2019, 32, 885–892. [Google Scholar] [CrossRef]
- Gray, S.L.A.; Marcum, Z.; Dublin, S.; Walker, R.; Golchin, N.; Rosenberg, E.D.; Bowles, E.J.; Crane, P.; Larson, E.B. Association Between Medications Acting on the Central Nervous System and Fall-Related Injuries in Community-Dwelling Older Adults: A New User Cohort Study. J. Gerontol. Ser. A: Boil. Sci. Med. Sci. 2019, 75, 1003–1009. [Google Scholar] [CrossRef]
- Emeny, R.T.; Chang, C.-H.; Skinner, J.; O’Malley, A.J.; Smith, J.; Chakraborti, G.; Rosen, C.J.; Morden, N.E. Association of Receiving Multiple, Concurrent Fracture-Associated Drugs with Hip Fracture Risk. JAMA Netw. Open 2019, 2, e1915348. [Google Scholar] [CrossRef] [PubMed]
- Correa-Pérez, A.; Delgado-Silveira, E.; Martín-Aragón, S.; Rojo-Sanchís, A.M.; Cruz-Jentoft, A. Fall-risk increasing drugs and prevalence of polypharmacy in older patients discharged from an Orthogeriatric Unit after a hip fracture. Aging Clin. Exp. Res. 2018, 31, 969–975. [Google Scholar] [CrossRef]
- Yue, Q.; Ma, Y.; Teng, Y.; Zhu, Y.; Liu, H.; Xu, S.; Liu, J.; Liu, J.; Zhang, X.; Teng, Z. An updated analysis of opioids increasing the risk of fractures. PLoS ONE 2020, 15, e0220216. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, K.; Kerse, N.; Ryan, C.; Teh, R.; Moyes, S.A.; Menzies, O.; Rolleston, A.; Broad, J.; Hughes, C.M. The Association Between Drug Burden Index (DBI) and Health-Related Outcomes: A Longitudinal Study of the ‘Oldest Old’ (LiLACS NZ). Drugs Aging 2020, 37, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.; Larrañaga, A.C.; Welmer, A.-K.; Rizzuto, D.; Wastesson, J.W.; Johnell, K. Polypharmacy and injurious falls in older adults: A nationwide nested case-control study. Clin. Epidemiol. 2019, 11, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Mogun, H.; Garneau, K.; Fischer, A.M. Risk of fractures in older adults using antihypertensive medications. J. Bone Miner. Res. 2011, 26, 1561–1567. [Google Scholar] [CrossRef]
- De Groot, M.H.; Van Campen, J.P.C.M.; Moek, M.A.; Tulner, L.R.; Beijnen, J.H.; Lamoth, C.J.C. The Effects of Fall-Risk-Increasing Drugs on Postural Control: A Literature Review. Drugs Aging 2013, 30, 901–920. [Google Scholar] [CrossRef]
- Butt, A.D.; Mamdani, M.; Gomes, T.; Lix, L.; Lu, H.; Tu, K. Risk of Osteoporotic Fractures with Angiotensin II Receptor Blockers Versus Angiotensin-Converting Enzyme Inhibitors in Hypertensive Community-Dwelling Elderly. J. Bone Miner. Res. 2014, 29, 2483–2488. [Google Scholar] [CrossRef]
- Ang, H.T.; Lim, K.K.; Kwan, Y.H.; Tan, P.S.; Yap, K.Z.; Banu, Z.; Tan, C.S.; Fong, W.; Thumboo, J.; Østbye, T.; et al. A Systematic Review and Meta-Analyses of the Association Between Anti-Hypertensive Classes and the Risk of Falls Among Older Adults. Drugs Aging 2018, 35, 625–635. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, Y.; Wu, Q. Thiazide diuretic usage and risk of fracture: A meta-analysis of cohort studies. Osteoporos. Int. 2018, 29, 1515–1524. [Google Scholar] [CrossRef]
- Solomon, D.H.; Ruppert, K.; Kazlauskaite, R.; Finkelstein, J.S.; Habel, L.A. Blood pressure lowering medication initiation and fracture risk: A SWAN pharmacoepidemiology study. Arch. Osteoporos. 2019, 14, 73. [Google Scholar] [CrossRef]
- Carbone, L.; Vasan, S.; Prentice, R.; Harshfield, G.; Haring, B.; Cauley, J.; Johnson, K. The renin-angiotensin aldosterone system and osteoporosis: Findings from the Women’s Health Initiative. Osteoporos. Int. 2019, 30, 2039–2056. [Google Scholar] [CrossRef]
- Charkos, T.G.; Liu, Y.; Jin, L.; Yang, S. Thiazide Use and Fracture Risk: An updated Bayesian Meta-Analysis. Sci. Rep. 2019, 9, 19754. [Google Scholar] [CrossRef] [PubMed]
- Shea, C.; Witham, M. Association between the Use of Angiotensin-Blocking Medications with Hip Fracture and Death in Older People. J. Frailty Aging 2020, 9, 107–110. [Google Scholar] [PubMed]
- Rejnmark, L.; Vestergaard, P.; Mosekilde, L. Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: A nationwide case–control study. J. Hypertens. 2006, 24, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Billington, E.O.; Grey, A.; Bolland, M.J. The effect of thiazolidinediones on bone mineral density and bone turnover: Systematic review and meta-analysis. Diabetology 2015, 58, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Schwartz, A.V.; Egger, A.; Lecka-Czernik, B. Effects of diabetes drugs on the skeleton. Bone 2016, 82, 93–100. [Google Scholar] [CrossRef]
- Chandran, M. Diabetes Drug Effects on the Skeleton. Calcif. Tissue Int. 2016, 100, 133–149. [Google Scholar] [CrossRef]
- Adil, M.; Khan, R.A.; Kalam, A.; Venkata, S.K.; Kandhare, A.D.; Ghosh, P.; Sharma, M. Effect of anti-diabetic drugs on bone metabolism: Evidence from preclinical and clinical studies. Pharm. Rep. 2017, 69, 1328–1340. [Google Scholar] [CrossRef] [PubMed]
- Wolverton, D.; Blair, M.M. Fracture risk associated with common medications used in treating type 2 diabetes mellitus. Am. J. Health Pharm. 2017, 74, 1143–1151. [Google Scholar] [CrossRef]
- Guja, C.; Guja, L.; Miulescu, R.D. Effect of type 2 diabetes medications on fracture risk. Ann. Transl. Med. 2019, 7, 580. [Google Scholar] [CrossRef]
- Kalaitzoglou, E.; Fowlkes, J.L.; Popescu, I.; Thrailkill, K.M. Diabetes pharmacotherapy and effects on the musculoskeletal system. Diabetes/Metab. Res. Rev. 2018, 35, e3100. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Sheu, B.-S. Metabolic Interaction of Helicobacter pylori Infection and Gut Microbiota. Microorganisms 2016, 4, 15. [Google Scholar] [CrossRef]
- Sohl, E.; Van Schoor, N.M.; De Jongh, R.T.; De Vries, O.J.; Lips, P. The impact of medication on vitamin D status in older individuals. Eur. J. Endocrinol. 2011, 166, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Van Orten-Luiten, A.C.B.; Janse, A.; Dhonukshe-Rutten, R.A.M.; Witkamp, R.F. The Association Between Drugs Frequently Used by the Elderly and Vitamin D Blood Levels: A Review of Observational and Experimental Studies. Drugs Aging 2014, 31, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Van Orten-Luiten, A.C.B.; Janse, A.; Dhonukshe-Rutten, R.A.M.; Witkamp, R.F. Vitamin D deficiency as adverse drug reaction? A cross-sectional study in Dutch geriatric outpatients. Eur. J. Clin. Pharm. 2016, 72, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Nordqvist, O.; Svensson, U.L.; Brudin, L.; Wanby, P.; Carlsson, M. Adherence to risk management guidelines for drugs which cause vitamin D deficiency—Big data from the Swedish health system. Drug Healthc. Patient Saf. 2019, 11, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Oliai Araghi, S.; Kiefte-de Jong, J.C.; Trajanoska, K.; Koromani, F.; Rivadeneira, F.; Zillikens, M.C.; van Schoor, N.M.; de Groot, L.C.P.G.M.; Ikram, M.A.; Uitterlinden, A.G.; et al. Do Vitamin D Level and Dietary Calcium Intake Modify the Association Between Loop Diuretics and Bone Health? Calcif Tissue Int. 2020, 106, 104–114. [Google Scholar] [CrossRef]
- Stein, E.; Shane, E. Secondary osteoporosis. Endocrinol. Metab. Clin. N. Am. 2003, 32, 115–134. [Google Scholar] [CrossRef]
- Painter, S.E.; Kleerekoper, M.; Camacho, P.M. Secondary Osteoporosis: A Review of the Recent Evidence. Endocr. Pract. 2006, 12, 436–445. [Google Scholar] [CrossRef]
- Gielen, E.; Vanderschueren, D.; Callewaert, F.; Boonen, S. Osteoporosis in men. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 321–335. [Google Scholar] [CrossRef]
- Hudec, S.M.D.; Camacho, P.M. Secondary Causes of Osteoporosis. Endocr. Pract. 2013, 19, 120–128. [Google Scholar] [CrossRef]
- Peacock, M.; Turner, C.H.; Econs, M.J.; Foroud, T. Genetics of Osteoporosis. Endocr. Rev. 2002, 23, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.; Brown, A.M. Mapping genes for osteoporosis—Old dogs and new tricks. Bone 2010, 46, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.; Brown, A.M.; Shore, E.M. The Revolution in Human Monogenic Disease Mapping. Genes 2014, 5, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Palvanen, M.; Kaprio, J.; Parkkari, J.; Koskenvuo, M. Genetic factors and osteoporotic fractures in elderly people: Prospective 25 year follow up of a nationwide cohort of elderly Finnish twins. BMJ 1999, 319, 1334–1337. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Melhus, H.; Ferm, H.; Ahlbom, A.; Pedersen, N.L. Genetic Liability to Fractures in the Elderly. Arch. Intern. Med. 2005, 165, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, A.J.; Snieder, H.; Spector, T.D. Genetic factors and osteoporotic fractures in elderly people. Twin data support genetic contribution to risk of fracture. BMJ 2000, 320, 1669. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.-W.; Chen, W.-M.; Recker, S.; Stegman, M.R.; Li, J.-L.; Davies, K.M.; Zhou, Y.; Deng, H.; Heaney, R.; Recker, R.R. Genetic Determination of Colles’ Fracture and Differential Bone Mass in Women With and Without Colles’ Fracture. J. Bone Miner. Res. 2000, 15, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.-W.; Mahaney, M.C.; Williams, J.T.; Li, J.; Conway, T.; Davies, K.M.; Li, J.-L.; Deng, H.; Recker, R.R. Relevance of the genes for bone mass variation to susceptibility to osteoporotic fractures and its implications to gene search for complex human diseases. Genet. Epidemiol. 2001, 22, 12–25. [Google Scholar] [CrossRef]
- Andrew, T.; Antioniades, L.; Scurrah, K.J.; Macgregor, A.J.; Spector, T.D. Risk of wrist fracture in women is heritable and is influenced by genes that are largely independent of those influencing BMD. J. Bone Miner. Res. 2005, 20, 67–74. [Google Scholar] [CrossRef]
- Carmelli, D.; Kelly-Hayes, M.; Wolf, P.A.; Swan, G.E.; Jack, L.M.; Reed, T.; Guralnik, J.M. The contribution of genetic influences to measures of lower-extremity function in older male twins. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 2000, 55, B49–B53. [Google Scholar]
- Roth, S.M. Genetic aspects of skeletal muscle strength and mass with relevance to sarcopenia. Bonekey Rep. 2012, 1, 58. [Google Scholar] [CrossRef] [PubMed]
- Just, K.S.; Schneider, K.L.; Schurig, M.; Stingl, J.; Brockmöller, J. Falls: The adverse drug reaction of the elderly and the impact of pharmacogenetics. Pharmacogenomics 2017, 18, 1281–1297. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R. Pharmacologic treatment to prevent fractures: From markers to patients. Osteoporos. Int. 2009, 20 (Suppl. 3), S255–S256. [Google Scholar] [CrossRef]
- Poudyal, H.; Brown, L. Osteoporosis and its association with non-gonadal hormones involved in hypertension, adiposity and hyperglycaemia. Curr. Drug Targets 2013, 14, 1694–1706. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Shane, E. A Crisis in the Treatment of Osteoporosis. J. Bone Miner. Res. 2016, 31, 1485–1487. [Google Scholar] [CrossRef]
- Viswanathan, M.; Reddy, S.; Berkman, N.; Cullen, K.; Middleton, J.C.; Nicholson, W.K.; Kahwati, L.C. Screening to Prevent Osteoporotic Fractures. JAMA 2018, 319, 2532–2551. [Google Scholar] [CrossRef]
- Zeng, L.-F.; Pan, B.-Q.; Liang, G.-H.; Luo, M.-H.; Cao, Y.; Guo, D.; Chen, H.-Y.; Pan, J.-K.; Huang, H.-T.; Liu, Q.; et al. Does Routine Anti-Osteoporosis Medication Lower the Risk of Fractures in Male Subjects? An Updated Systematic Review With Meta-Analysis of Clinical Trials. Front. Pharm. 2019, 10, 882. [Google Scholar] [CrossRef]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef]
- Pripp, A.H.; Dahl, O.E. The Population Attributable Risk of Nutrition and Lifestyle on Hip Fractures. Hip Int. 2015, 25, 277–281. [Google Scholar] [CrossRef]
- Trajanoska, K.; Rivadeneira, F. The genetic architecture of osteoporosis and fracture risk. Bone 2019, 126, 2–10. [Google Scholar] [CrossRef]
- Larsson, S.C.; Melhus, H.; Michaëlsson, K. Circulating Serum 25-Hydroxyvitamin D Levels and Bone Mineral Density: Mendelian Randomization Study. J. Bone Miner. Res. 2018, 33, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhou, W.; Wang, P.; Zuo, E.; Ying, X.; Chai, S.; Fei, T.; Jin, L.; Chen, C.; Ma, G.; et al. Comprehensive Analysis of the Genetic and Epigenetic Mechanisms of Osteoporosis and Bone Mineral Density. Front. Cell Dev. Biol. 2020, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Nethander, M.; Vandenput, L.; Eriksson, A.L.; Windahl, S.; Funck-Brentano, T.; Ohlsson, C. Evidence of a Causal Effect of Estradiol on Fracture Risk in Men. J. Clin. Endocrinol. Metab. 2018, 104, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, P.; Wen, Y.; Liang, X.; Liu, L.; Cheng, B.; Ding, M.; Zhao, Y.; Ma, M.; Zhang, L.; et al. Evaluating the Correlations Between Osteoporosis and Lifestyle-Related Factors Using Transcriptome-Wide Association Study. Calcif. Tissue Int. 2019, 106, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Cerani, A.; Zhou, S.; Forgetta, V.; Morris, J.A.; Trajanoska, K.; Rivadeneira, F.; Larsson, S.C.; Michaëlsson, K.; Richards, J.B. Genetic predisposition to increased serum calcium, bone mineral density, and fracture risk in individuals with normal calcium levels: Mendelian randomisation study. BMJ 2019, 366, l4410. [Google Scholar] [CrossRef] [PubMed]
- Oei, L.; Campos-Obando, N.; Dehghan, A.; Oei, E.H.G.; Stolk, L.; Van Meurs, J.B.J.; Hofman, A.; Uitterlinden, A.G.; Franco, O.H.; Zillikens, M.C.; et al. Dissecting the relationship between high-sensitivity serum C-reactive protein and increased fracture risk: The Rotterdam Study. Osteoporos. Int. 2013, 25, 1247–1254. [Google Scholar] [CrossRef]
- Huang, J.V.; Schooling, C.M. Inflammation and bone mineral density: A Mendelian randomization study. Sci. Rep. 2017, 7, 8666. [Google Scholar] [CrossRef]
- Gan, W.; Clarke, R.J.; Mahajan, A.; Kulohoma, B.W.; Kitajima, H.; Robertson, N.R.; Rayner, N.W.; Walters, R.G.; Holmes, M.V.; Chen, Z.; et al. Bone mineral density and risk of type 2 diabetes and coronary heart disease: A Mendelian randomization study. Wellcome Open Res. 2017, 2, 68. [Google Scholar] [CrossRef]
- Cui, Z.; Meng, X.; Zhuang, S.; Liu, Z.; Zhou, F.; Tian, Y. Schizophrenia, Bipolar Disorder, and Alzheimer’s Disease are not Causal Factors of Bone Mineral Density: A Mendelian Randomization Analysis. Calcif. Tissue Int. 2019, 106, 131–146. [Google Scholar] [CrossRef]
- Guo, R.; Wu, L.; Fu, Q. Is There Causal Relationship of Smoking and Alcohol Consumption with Bone Mineral Density? A Mendelian Randomization Study. Calcif. Tissue Int. 2018, 103, 546–553. [Google Scholar] [CrossRef]
- Collaborators GBDCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Gaskell, H.; Derry, S.; Moore, R.A.; McQuay, H.J. Prevalence of anaemia in older persons: Systematic review. BMC Geriatr. 2008, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron deficiency anaemia revisited. J. Intern. Med. 2019, 287, 153–170. [Google Scholar] [CrossRef]
- Donaldson, A.I.; Soiza, R.L.; Hands, K.J.; Witham, M.D.; Myint, P.K. Variability in the clinical management of iron deficiency anaemia in older adults: Results from a survey of UK specialists in the care of older people. Adv. Drug Saf. 2019, 10, 2042098619854870. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Curtis, E.M.; Ward, K.A.; Harvey, N.C.; Dennison, E.M.; Cooper, C. Fracture prediction, imaging and screening in osteoporosis. Nat. Rev. Endocrinol. 2019, 15, 535–547. [Google Scholar] [CrossRef]
- Cheung, C.-L.; Tan, K.C.; Kung, A.W.C. Cohort Profile: The Hong Kong Osteoporosis Study and the follow-up study. Int. J. Epidemiol. 2017, 47, 397-8f. [Google Scholar] [CrossRef]
- Cao, L.; Yu, J.; Cao, L.; Yu, J. Effect of Helicobacter pylori Infection on the Composition of Gastric Microbiota in the Development of Gastric Cancer. Gastrointest. Tumors 2015, 2, 14–25. [Google Scholar] [CrossRef]
- Yap, T.W.-C.; Gan, H.M.; Lee, Y.-P.; Leow, A.H.-R.; Azmi, A.N.; Francois, F.; Perez, G.I.P.; Loke, M.F.; Goh, K.-L.; Vadivelu, J. Helicobacter pylori Eradication Causes Perturbation of the Human Gut Microbiome in Young Adults. PLoS ONE 2016, 11, e0151893. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M., Jr. Helicobacter pylori, Cancer, and the Gastric Microbiota. Adv. Exp. Med. Biol. 2016, 908, 393–408. [Google Scholar]
- Parsons, B.N.; Ijaz, U.Z.; D’Amore, R.; Burkitt, M.D.; Eccles, R.; Lenzi, L.; Duckworth, C.A.; Moore, A.R.; Tiszlavicz, L.; Varro, A.; et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 2017, 13, e1006653. [Google Scholar] [CrossRef]
- Alarcón, T.; Llorca, L.; Perez, G.I.P. Impact of the Microbiota and Gastric Disease Development by Helicobacter pylori. Future HIV 1 Ther. 2017, 400, 253–275. [Google Scholar]
- Chen, L.; Xu, W.; Lee, A.; He, J.; Huang, B.; Zheng, W.; Su, T.; Lai, S.; Long, Y.; Chu, H.; et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: An open-label, randomized clinical trial. EBioMedicine 2018, 35, 87–96. [Google Scholar] [CrossRef]
- Schulz, C.; Schütte, K.; Koch, N.; Vilchez-Vargas, R.; Wos-Oxley, M.L.; Oxley, A.A.P.; Vital, M.; Malfertheiner, P.; Pieper, D.H. The active bacterial assemblages of the upper GI tract in individuals with and without Helicobacter infection. Gut 2016, 67, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-J.; Zhang, Y.; Gerhard, M.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.-L.; Bajbouj, M.; Suchanek, S.; Liu, W.-D.; et al. Association Between Gut Microbiota and Helicobacter pylori-Related Gastric Lesions in a High-Risk Population of Gastric Cancer. Front. Cell. Infect. Microbiol. 2018, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Pero, R.; Brancaccio, M.; Laneri, S.; De Biasi, M.G.; Lombardo, B.; Scudiero, O. A Novel View of Human Helicobacter pylori Infections: Interplay between Microbiota and Beta-Defensins. Biomology 2019, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Pichon, M.; Burucoa, C. Impact of the Gastro-Intestinal Bacterial Microbiome on Helicobacter-Associated Diseases. Healthcare 2019, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Dash, N.R.; Khoder, G.; Nada, A.M.; Al Bataineh, M.T. Exploring the impact of Helicobacter pylori on gut microbiome composition. PLoS ONE 2019, 14, e0218274. [Google Scholar] [CrossRef]
- Iino, C.; Shimoyama, T.; Chinda, D.; Sakuraba, H.; Fukuda, S.; Nakaji, S. Influence of Helicobacter pylori Infection and Atrophic Gastritis on the Gut Microbiota in a Japanese Population. Digestion 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Frost, F.; Kacprowski, T.; Rühlemann, M.C.; Bang, C.; Franke, A.; Zimmermann, K.; Nauck, M.; Völker, U.; Völzke, H.; Biffar, R.; et al. Helicobacter pylori infection associates with fecal microbiota composition and diversity. Sci. Rep. 2019, 9, 20100. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Zhong, H.; Ding, Q.; Lin, Y.; Tang, S.; Zong, Y.; Wang, Q.; Zhang, X.; Yang, H.; et al. Alterations in the human gut microbiome associated with Helicobacter pylori infection. FEBS Openbio 2019, 9, 1552–1560. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Xu, J.; Wei, X.; Yang, J.; Liu, Y.; Li, H.; Zhao, C.; Wang, Y.; Zhang, L.; et al. Helicobacter pylori Infection Aggravates Dysbiosis of Gut Microbiome in Children with Gastritis. Front. Cell. Infect. Microbiol. 2019, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Fischer, A.; Plickert, R.; Wiedemann, T.; Loddenkemper, C.; Göbel, U.B.; Bereswill, S.; Rieder, G. Helicobacter pylori Induced Gastric Immunopathology Is Associated with Distinct Microbiota Changes in the Large Intestines of Long-Term Infected Mongolian Gerbils. PLoS ONE 2014, 9, e100362. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Bäckhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef]
- Ohlsson, C.; Sjögren, K. Effects of the gut microbiota on bone mass. Trends Endocrinol. Metab. 2015, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M. Diet, gut microbiome, and bone health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef]
- Hernandez, C.J. Bone Mechanical Function and the Gut Microbiota. Adv. Exp. Med. Biol. 2017, 1033, 249–270. [Google Scholar]
- Wang, J.; Wang, Y.; Gao, W.; Wang, B.; Zhao, H.; Zeng, Y.; Ji, Y.; Hao, D. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ 2017, 5, e3450. [Google Scholar] [CrossRef] [PubMed]
- Quach, D.; Britton, R.A. Gut Microbiota and Bone Health. Polyglutamine Disord. 2017, 1033, 47–58. [Google Scholar]
- Yan, J.; Charles, J.F. Gut Microbiome and Bone: To Build, Destroy, or Both? Curr. Osteoporos. Rep. 2017, 15, 376–384. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Greenbaum, J.; Shen, H.; Deng, H.-W. Association Between Gut Microbiota and Bone Health: Potential Mechanisms and Prospective. J. Clin. Endocrinol. Metab. 2017, 102, 3635–3646. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2017, 102, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Mulle, J.G.; Pacifici, R. Osteomicrobiology: The influence of gut microbiota on bone in health and disease. Bone 2018, 115, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Takakura, A.; Zandi-Nejad, K.; Charles, J.F. Mechanisms of gut microbiota-mediated bone remodeling. Gut Microbes 2017, 9, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Sjögren, K. Osteomicrobiology: A New Cross-Disciplinary Research Field. Calcif. Tissue Int. 2017, 102, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Chen, W.-D.; Wang, Y.-D. Gut Microbiota: An Integral Moderator in Health and Disease. Front. Microbiol. 2018, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Zhao, H.; Li, N.; Lu, Y.; Lian, Z.; Shao, D.; Jin, M.; Li, Q.; Zhao, L.; Shi, J. Origination, change, and modulation of geriatric disease-related gut microbiota during life. Appl. Microbiol. Biotechnol. 2018, 102, 8275–8289. [Google Scholar] [CrossRef]
- Das, M.; Cronin, O.; Keohane, D.M.; Cormac, E.M.; Nugent, H.; Nugent, M.; Molloy, C.; O’Toole, P.W.; Shanahan, F.; Molloy, M.G.; et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology 2019, 58, 2295–2304. [Google Scholar] [CrossRef]
- Hao, M.-L.; Wang, G.-Y.; Zuo, X.-Q.; Qu, C.-J.; Yao, B.-C.; Wang, D.-L. Gut microbiota: An overlooked factor that plays a significant role in osteoporosis. J. Int. Med. Res. 2019, 47, 4095–4103. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The gut-bone axis: How bacterial metabolites bridge the distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef]
- Ibáñez, L.; Rouleau, M.; Wakkach, A.; Blin-Wakkach, C. Gut microbiome and bone. Jt. Bone Spine 2019, 86, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Nutritional influence on bone: Role of gut microbiota. Aging Clin. Exp. Res. 2019, 31, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Biver, E.; Berenbaum, F.; Valdes, A.M.; De Carvalho, I.A.; Bindels, L.B.; Brandi, M.L.; Calder, P.C.; Castronovo, V.; Cavalier, E.; Cherubini, A.; et al. Gut microbiota and osteoarthritis management: An expert consensus of the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO). Ageing Res. Rev. 2019, 55, 100946. [Google Scholar] [CrossRef] [PubMed]
- Ii, D.Y.; Pan, K.; Shendge, V.B.; Liu, J.; Ebraheim, A.N. Linkage of microbiota and osteoporosis: A mini literature review. World J. Orthop. 2019, 10, 123–127. [Google Scholar] [CrossRef]
- Dutta, S.K.; Verma, S.; Jain, V.; Surapaneni, B.K.; Vinayek, R.; Phillips, L.; Nair, P.P. Transplantation. J. Neurogastroenterol. Motil. 2019, 25, 363–376. [Google Scholar] [CrossRef]
- Li, C.; Huang, Q.; Yang, R.; Dai, Y.; Zeng, Y.; Tao, L.; Li, X.; Zeng, J.; Wang, Q. Gut microbiota composition and bone mineral loss—Epidemiologic evidence from individuals in Wuhan, China. Osteoporos. Int. 2019, 30, 1003–1013. [Google Scholar] [CrossRef]
- Hathaway-Schrader, J.D.; Steinkamp, H.M.; Chavez, M.B.; Poulides, N.A.; Kirkpatrick, J.E.; Chew, M.E.; Huang, E.; Alekseyenko, A.; Aguirre, J.; Novince, C.M. Antibiotic Perturbation of Gut Microbiota Dysregulates Osteoimmune Cross Talk in Postpubertal Skeletal Development. Am. J. Pathol. 2019, 189, 370–390. [Google Scholar] [CrossRef]
- Li, L.; Rao, S.; Cheng, Y.; Zhuo, X.; Deng, C.; Xu, N.; Zhang, H.; Yang, L. Microbial osteoporosis: The interplay between the gut microbiota and bones via host metabolism and immunity. MicrobiologyOpen 2019, 8, e00810. [Google Scholar] [CrossRef]
- Xie, W.; Han, Y.; Li, F.; Gu, X.; Su, D.; Yu, W.; Li, Z.; Xiao, J. Neuropeptide Y1 Receptor Antagonist Alters Gut Microbiota and Alleviates the Ovariectomy-Induced Osteoporosis in Rats. Calcif. Tissue Int. 2019, 106, 444–454. [Google Scholar] [CrossRef]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The role of gut microbiota in bone homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef]
- Ohlsson, C.; Engdahl, C.; Fåk, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Movérare-Skrtic, S.; Islander, U.; Sjögren, K. Probiotics Protect Mice from Ovariectomy-Induced Cortical Bone Loss. PLoS ONE 2014, 9, e92368. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.; Pacifici, R. From Osteoimmunology to Osteomicrobiology: How the Microbiota and the Immune System Regulate Bone. Calcif. Tissue Int. 2017, 102, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Wang, R. Gut microbiota modulates drug pharmacokinetics. Drug Metab. Rev. 2018, 50, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Locantore, P.; Del Gatto, V.; Gelli, S.; Paragliola, R.M.; Pontecorvi, A. The Interplay between Immune System and Microbiota in Osteoporosis. Mediat. Inflamm. 2020, 2020, 3686749. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Jackson, M.A.; Verdi, S.; Maxan, M.-E.; Shin, C.M.; Zierer, J.; Bowyer, R.C.E.; Martin, T.C.; Williams, F.M.K.; Menni, C.; Bell, J.T.; et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat. Commun. 2018, 9, 2655. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. Gut Microbiome and Modulation of CNS Function. Compr. Physiol. 2019, 10, 57–72. [Google Scholar]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflammation 2019, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Itoh, H. Hypertension as a Metabolic Disorder and the Novel Role of the Gut. Curr. Hypertens. Rep. 2019, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Ilie, O.D.; Ciobica, A.; McKenna, J.; Doroftei, B.; Mavroudis, I. Minireview on the Relations between Gut Microflora and Parkinson’s Disease: Further Biochemical (Oxidative Stress), Inflammatory, and Neurological Particularities. Oxid. Med. Cell Longev. 2020, 2020, 4518023. [Google Scholar] [CrossRef] [PubMed]
- Preidis, A.G.; Versalovic, J. Targeting the Human Microbiome with Antibiotics, Probiotics, and Prebiotics: Gastroenterology Enters the Metagenomics Era. Gastroenterology 2009, 136, 2015–2031. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Sterbini, F.P.; Palladini, A.; Masucci, L.; Cannistraci, C.V.; Pastorino, R.; Ianiro, G.; Bugli, F.; Martini, C.; Ricciardi, W.; Gasbarrini, A.; et al. Effects of Proton Pump Inhibitors on the Gastric Mucosa-Associated Microbiota in Dyspeptic Patients. Appl. Environ. Microbiol. 2016, 82, 6633–6644. [Google Scholar] [CrossRef]
- Ticinesi, A.; Milani, C.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; Mangifesta, M.; Viappiani, A.; et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci. Rep. 2017, 7, 11102. [Google Scholar] [CrossRef]
- Imhann, F.; Vila, A.V.; Bonder, M.J.; Manosalva, A.G.L.; Koonen, D.P.; Fu, J.; Wijmenga, C.; Zhernakova, A.; Weersma, R.K. The influence of proton pump inhibitors and other commonly used medication on the gut microbiota. Gut Microbes 2017, 8, 351–358. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nat. Cell Biol. 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Le Bastard, Q.; Al-Ghalith, G.A.; Grégoire, M.; Chapelet, G.; Javaudin, F.; Dailly, E.; Batard, E.; Knights, D.; Montassier, E. Systematic review: Human gut dysbiosis induced by non-antibiotic prescription medications. Aliment. Pharm. 2017, 47, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Zaccari, P.; Rocco, G.; Scalese, G.; Panetta, C.; Porowska, B.; Pontone, S.; Severi, C. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J. Gastroenterol. 2019, 25, 2706–2719. [Google Scholar] [CrossRef] [PubMed]
- Hi, Y.-C.; Cai, S.-T.; Tian, Y.-P.; Zhao, H.-J.; Zhang, Y.-B.; Chen, J.; Ren, R.-R.; Luo, X.; Peng, L.-H.; Sun, G.; et al. Effects of Proton Pump Inhibitors on the Gastrointestinal Microbiota in Gastroesophageal Reflux Disease. Genom. Proteom. Bioinform. 2019, 17, 52–63. [Google Scholar]
- Vila, A.V.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Rajilic-Stojanovic, M.; Figueiredo, C.; Smet, A.; Hansen, R.; Kupcinskas, J.; Rokkas, T.; Andersen, L.; Machado, J.C.; Ianiro, G.; Gasbarrini, A.; et al. Systematic review: Gastric microbiota in health and disease. Aliment. Pharm. 2020, 51, 582–602. [Google Scholar] [CrossRef]
- Clooney, A.G.; Bernstein, C.N.; Leslie, W.D.; Vagianos, K.; Sargent, M.; Laserna-Mendieta, E.J.; Claesson, M.J.; Targownik, L.E. A comparison of the gut microbiome between long-term users and non-users of proton pump inhibitors. Aliment. Pharm. 2016, 43, 974–984. [Google Scholar] [CrossRef]
- Minalyan, A.; Gabrielyan, L.; Scott, D.; Jacobs, J.; Pisegna, J.R. The Gastric and Intestinal Microbiome: Role of Proton Pump Inhibitors. Curr. Gastroenterol. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Tsuchiya, S.; Okayama, T.; Dohi, O.; Yoshida, N.; et al. The influence of long-term use of proton pump inhibitors on the gut microbiota: An age-sex-matched case-control study. J. Clin. Biochem. Nutr. 2018, 62, 100–105. [Google Scholar] [CrossRef]
- Hojo, M.; Asahara, T.; Nagahara, A.; Takeda, T.; Matsumoto, K.; Ueyama, H.; Matsumoto, K.; Asaoka, D.; Takahashi, T.; Nomoto, K.; et al. Gut Microbiota Composition Before and After Use of Proton Pump Inhibitors. Dig. Dis. Sci. 2018, 63, 2940–2949. [Google Scholar] [CrossRef]
- Naito, Y.; Kashiwagi, K.; Takagi, T.; Andoh, A.; Inoue, R. Intestinal Dysbiosis Secondary to Proton-Pump Inhibitor Use. Digestion 2018, 97, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, A.S.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2015, 65, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Macke, L.; Schulz, C.; Koletzko, L.; Malfertheiner, P. Systematic review: The effects of proton pump inhibitors on the microbiome of the digestive tract-evidence from next-generation sequencing studies. Aliment. Pharm. 2020, 51, 505–526. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Peng, C.; Wang, H.; Ouyang, Y.; Zhu, Z.; Shu, X.; Zhu, Y.; Lu, N.-H. The eradication of Helicobacter pylori restores rather than disturbs the gastrointestinal microbiota in asymptomatic young adults. Helicobacter 2019, 24, e12590. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Ade, P.; Marten, B.; Weber, P.; Timm, W.; Aςil, Y.; Glüer, C.-C.; Schrezenmeir, J. Prebiotics, Probiotics, and Synbiotics Affect Mineral Absorption, Bone Mineral Content, and Bone Structure. J. Nutr. 2007, 137, 838S–846S. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef]
- McCabe, L.R.; Britton, R.A.; Parameswaran, N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr. Osteoporos. Rep. 2015, 13, 363–371. [Google Scholar] [CrossRef]
- Schepper, J.D.; Irwin, R.; Kang, J.; Dagenais, K.; Lemon, T.; Shinouskis, A.; Parameswaran, N.; McCabe, L.R. Probiotics in Gut-Bone Signaling. Adv. Exp. Med. Biol. 2017, 1033, 225–247. [Google Scholar]
- Collins, F.L.; Schepper, J.D.; Rios-Arce, N.D.; Steury, M.D.; Kang, H.J.; Mallin, H.; Schoenherr, D.; Camfield, G.; Chishti, S.; McCabe, L.R.; et al. Immunology of Gut-Bone Signaling. Adv. Exp. Med. Biol. 2017, 1033, 59–94. [Google Scholar]
- McCabe, L.R.; Parameswaran, N. Advances in Probiotic Regulation of Bone and Mineral Metabolism. Calcif. Tissue Int. 2018, 102, 480–488. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Martín-Núñez, G.M.; Roca-Rodríguez, M.M.; Cardona, F.; Coin-Aragüez, L.; Sanchez-Alcoholado, L.; Gutiérrez-Repiso, C.; Muñoz-Garach, A.; Fernández-García, J.C.; Queipo-Ortuño, M.I.; et al. H. pylori Eradication Treatment Alters Gut Microbiota and GLP-1 Secretion in Humans. J. Clin. Med. 2019, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Castillo, L.F. Prebiotics, Bone and Mineral Metabolism. Calcif. Tissue Int. 2017, 102, 443–479. [Google Scholar] [CrossRef] [PubMed]
- Lucas, G. Gut thinking: The gut microbiome and mental health beyond the head. Microb. Ecol. Health Dis. 2018, 29, 1548250. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, T.; Hatanaka, M.; Hoshino, T.; Takara, T.; Tanaka, K.; Shimizu, A.; Morita, H.; Nakamura, T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: A randomized, placebo-controlled, double-blind clinical trial. Biosci. Microbiota Food Health 2018, 37, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Kiousi, D.E.; Karapetsas, A.; Karolidou, K.; Panayiotidis, M.I.; Pappa, A.; Galanis, A. Probiotics in Extraintestinal Diseases: Current Trends and New Directions. Nutrients 2019, 11, 788. [Google Scholar] [CrossRef]
- Curtis, E.M.; Moon, R.J.; Harvey, N.C.; Cooper, C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone 2017, 104, 29–38. [Google Scholar] [CrossRef]
- Court-Brown, C.M.; Duckworth, A.D.; Clement, N.D.; McQueen, M.M. Fractures in older adults. A view of the future? Injury 2018, 49, 2161–2166. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Leader, D.; Weiss, R.; Williams, S.A. Challenges in osteoporosis awareness and management: Results from a survey of US postmenopausal women. J. Drug Assess. 2019, 8, 25–31. [Google Scholar] [CrossRef]
- Liu, J.; Curtis, E.M.; Cooper, C.; Harvey, N. State of the art in osteoporosis risk assessment and treatment. J. Endocrinol. Investig. 2019, 42, 1149–1164. [Google Scholar] [CrossRef]
- Hagen, G.; Magnussen, J.; Tell, G.; Omsland, T. Estimating the future burden of hip fractures in Norway. A NOREPOS study. Bone 2020, 131, 115156. [Google Scholar] [CrossRef]
- Schuit, S.; Van Der Klift, M.; Weel, A.; De Laet, C.; Burger, H.; Seeman, E.; Hofman, A.; Uitterlinden, A.; Van Leeuwen, J.; Pols, H. Fracture incidence and association with bone mineral density in elderly men and women: The Rotterdam Study. Bone 2004, 34, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Siris, E.S.; Chen, Y.-T.; Abbott, T.A.; Barrett-Connor, E.; Miller, P.D.; Wehren, L.E.; Berger, M.L. Bone Mineral Density Thresholds for Pharmacological Intervention to Prevent Fractures. Arch. Intern. Med. 2004, 164, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.; Adachi, J.D.; Cooper, C.; Clark, P.; Cummings, S.R.; Diaz-Curiel, M.; Harvey, N.; Hiligsmann, M.; Papaioannou, A.; et al.; The Epidemiology and Quality of Life Working Group of IOF Standardising the descriptive epidemiology of osteoporosis: Recommendations from the Epidemiology and Quality of Life Working Group of IOF. Osteoporos. Int. 2013, 24, 2763–2764. [Google Scholar] [CrossRef] [PubMed]
- Lespessailles, E.; Cortet, B.; Legrand, E.; Guggenbuhl, P.; Roux, C. Low-trauma fractures without osteoporosis. Osteoporos. Int. 2017, 28, 1771–1778. [Google Scholar] [CrossRef]
- Wu, Q.; Xiao, X.; Xu, Y. Evaluating the Performance of the WHO International Reference Standard for Osteoporosis Diagnosis in Postmenopausal Women of Varied Polygenic Score and Race. J. Clin. Med. 2020, 9, 499. [Google Scholar] [CrossRef]
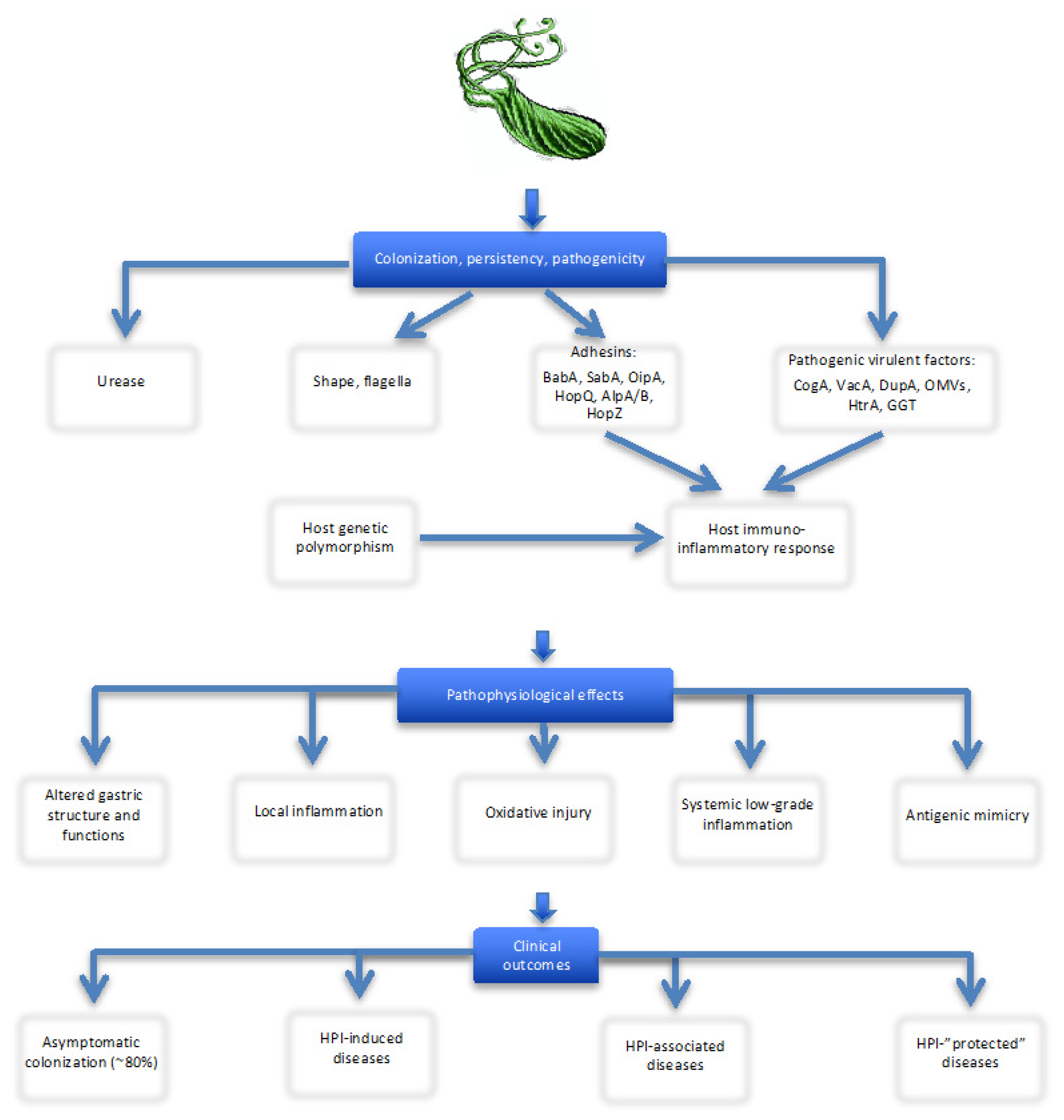
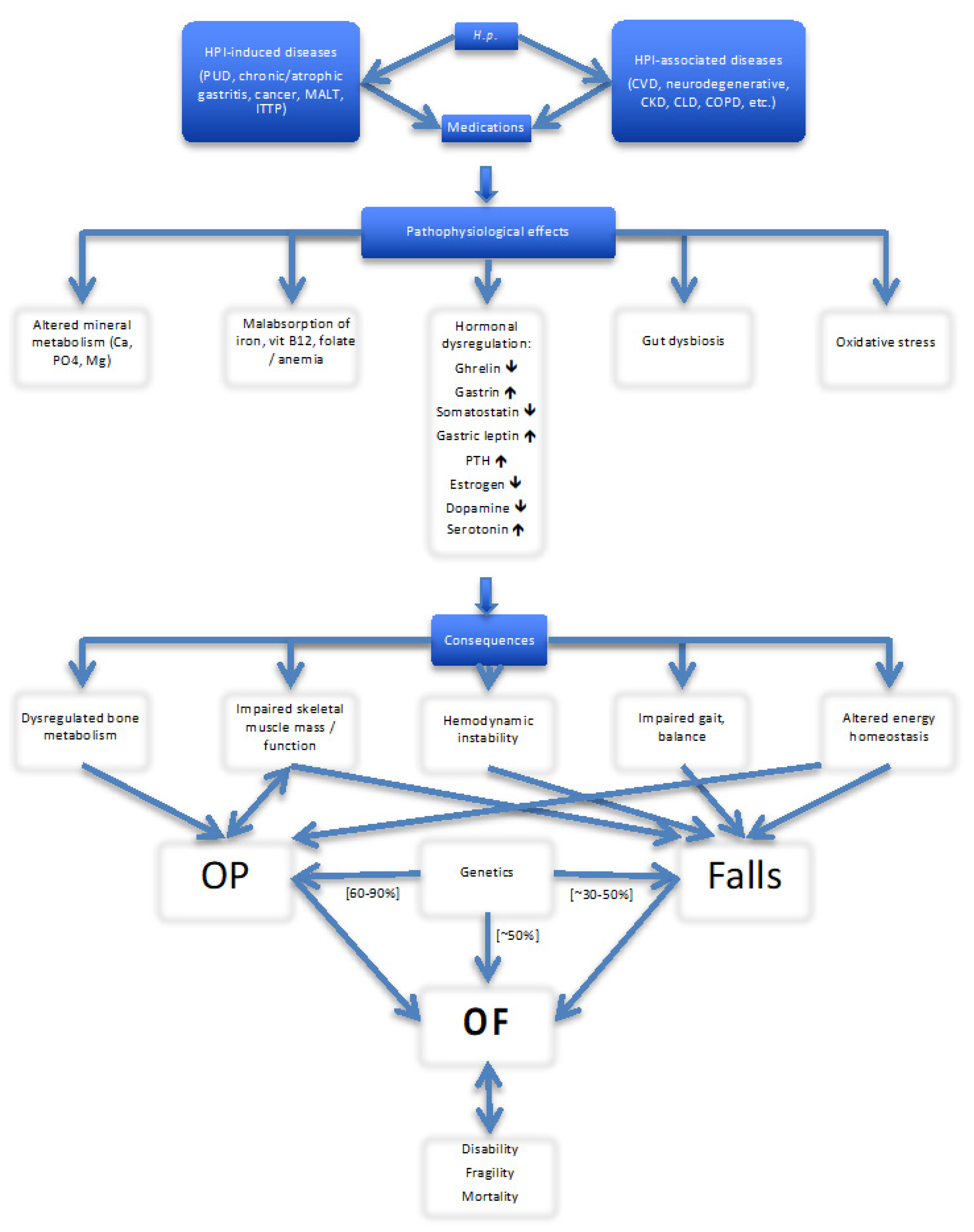
| First Author, Year, Reference No, Country/Region | Population, Gender (F/M) | Mean Age, Years | HPI Detection Method | HPI Strain Identification | Bone Status Assessment Methods, Skeletal Location | HPI+ and OP, n (%) | HPI+ and Non -OP/Controls | Association (Yes/No) |
|---|---|---|---|---|---|---|---|---|
| Figura N, 2005 [98], Italy | 240, M | 65 (55–82) | Serum antibody | CagA | DEXA, LS, FN; Urinary cross-laps, serum bALP, PTH, Ca, PO4, 25(OH)D | 51/80 (63.7%); CagA+: 30 (58.8%) | 107/160 (66.8%); CagA+: 43 (40.1%) | Yes, if CagA+; increased levels of urinary cross-laps |
| Ozdem S, 2007 [105], Turkey | 61, 36/25 | 11.8 ± 3 (F) 10.1 ± 3 (M) | RUT, histology | Serum P1NP, βCTX, OC, ALP, PTH, Ca, PO4 | No | |||
| Kakehasi A, 2007 [107], Brazil | 50, F | 61.7 ± 7 (50–70) | RUT, histology, 13C-UBT | DEXA, LS | 10/18 (55%) | 24/32 (75%) | No | |
| Kakehasi A, 2009 [108], Brazil | 85, F | 63.7 ± 7.3 (HPI+); 62.5 ± 7.0 (HPI−) | RUT, histology, 13C-UBT | DEXA, LS, H | No | |||
| Figura N, 2010 [99], Italy | 1118, 935/183 | 62.5 ± 6 (F); 65.9 ± 6 (M) | Serum antibody | CagA | DEXA | 41.5%; CagA + 30% | 43.9%; CagA + 21% | Yes, if CagA+ |
| Akkaya N, 2011 [109], Turkey | 105, F | 65.3 ± 6.1 (OP+) 63.6 ± 6.5 (OP−) | Serum antibodies | DEXA, LS, H | 41/58 (IgG+) (70.7%) | 35/47 (IgG+) (74.5%) | No | |
| Asaoka D, 2014 [110], Japan | 200, 105/95 | 62.8 ± 7.7 (M) 63.4 ± 9 (F) | Serum antibody, 13C-UBT | DEXA, LS; serum bALP, NTX | 25/41 (61.0%) | 57/159 (35.8%) | Yes, OR 5.33 (1.73–16.42) in PUD | |
| Lin S, 2014 [111], Taiwan | 365, F | 77.3 (65–97) | RUT, histology | DEXA or osteoporosis medication use | 77/101 (76.2%) | 24/101 (23.8%) | Yes, OR 2.03 (1.14–3.62) | |
| Asaoka D, 2015 [106], Japan | 255, 135/120 | 63.2 ± 8.5 | Serum antibody, 13C-UBT | DEXA, LS; serum bALP, NTX | 25/43 (58.1%) | 69/212 (32.5%) | Yes, OR 3.0 (1.31–6.88) | |
| Mizuro S, 2015 [112], Japan | 230, M | >50; 62.1 ± 5.0 (TBD low), 58.4 ± 5.4 (TBD normal) | Serum antibody | QUS, radius | 61/116 (52.5%) | 38/114 (33.3%) | Yes, OR 1.83 (1.04–3.21) | |
| Fotouk-Kiai M, 2015 [113], Iran | 967, 392/575; H.p.+ 758, H.p.− 209 (controls) | 68.3 ± 6.8 (H.p.+) 69.3 ± 7.4 (H.p.−) | Serum antibody | DEXA, LS, FN | 236/758 (31.1%) | 522/758 (68.9%) | No, OR 0.76 (0.55–1.05) | |
| Chung Y, 2015 [97], Korea | I, 126, M H.p.+ 657 H.p.− 469 | 54.4 ± 10.7 (H.p.+) 51.9 ± 12.1 (H.p.−) | Serum antibody | DEXA, LS (L1-L4) | 173 */657 (26.3%) (LS); 114/657 (17.4%) (FN) | 484/657 (73.7%) (LS); 543/657 (82.6) (FN) | Yes, only for lumbar BMD (not for total femur or femoral neck) | |
| Kalantarhormozi M, 2016 [96], Iran | 250, F; 16 (OP), 234 (controls) | 58.9 ± 8.0 | Serum antibody | DEXA, LS, F; bone turnover markers, OPG, RANK, Ca, PO4 | No | |||
| Shih H, 2016 [95], Taiwan | 5447 (H.p.+), 21,788 (controls) | >20 | H.p. eradication treatment for PUD | DEXA | Yes, HR 1.62 (1.06–2.47) | |||
| Chen L, 2017 [114], Taiwan | 2689, 1792/897 | >40 | 13C-UBT | FRAX (without BMD) | F: 177/324↑ (54.6%); M: 54/93 (58.1%) | No, for 10-year fracture risk prediction | ||
| Chinda D, 2017 [115], Japan | 473 F (healthy) | 52.2 ± 15.2 | Serum antibody (IgG), H.p. antigen in stool sample | QUS, calcaneus | 65 */118 (55.1%) | 53/118 (44.9%) | No, OR 0.95 (0.55–1.63) for osteopenia | |
| Abdolahi N, 2017 [116], Iran | 107 F, 34 with OP, 73 controls | Post- menopausal | Serum antibodies (IgA, IgG) | 70.6% IgA+ 82.0% IgG+ | 54.8% IgA+ 75.3% IgG+ | No | ||
| Lu L, 2018 [19], China | 1867, 393/1474 | 13C-UBT | QUS, calcaneus | No, for BMD | ||||
| Pan B, 2018 [117], Taiwan | 867, 299/568 | 55.9 ± 11.3 | RUT | DEXA | 257/556 (46.2%) | 124/311 (39.9%) | Yes, OR 1.62, (1.12–2.35) for decreased BMD | |
| Chinda D, 2019 [118], Japan | 268 M (healthy) | 49.1 ± 15.1 | Serum antibody (IgG), H.p. antigen in stool sample, serum pepsinogens | QUS, calcaneus | No, OR 1.31 (0.54–3.21) for atrophic gastritis, OR 0.74 (0.29–1.90) without gastritis |
| Iron deficiency and iron deficient anemia |
| Vitamin B12 deficiency and vitamin B12 deficient anemia |
| Immune thrombocytopenia |
| Cardiovascular diseases (CAD, myocardial infarction, hypertension, CHF) |
| Cerebrovascular diseases (stroke, TIA) |
| Neurodegenerative diseases (Alzheimer’s and vascular dementia, Parkinson’s disease) |
| Chronic kidney disease |
| Diabetes mellitus |
| Metabolic syndrome |
| Chronic liver disease (MAFLD; liver cirrhosis, hepatic encephalopathy) |
| Chronic obstructive pulmonary disease |
| Depression, anxiety |
| Rheumatologic and autoimmune diseases (rheumatoid arthritis [?], ankylosing spondylitis, psoriatic arthritis, systemic vasculitis, autoimmune thyroid diseases, multiple sclerosis [?]) |
| Eye diseases (open-angle glaucoma, neuromyelitis optica) |
| Malignant tumors (breast, colorectal and prostate cancers) |
| Malnutrition/low body weight |
| Malabsorption |
| Vitamin D insufficiency/deficiency |
| Dysregulation of gastrointestinal microbiota (dysbiosis) |
| Chronic inflammatory bowel diseases [?] |
| Celiac disease [?] |
| Obesity (morbid) [?] |
| Prostatitis |
| Pre-eclampsia |
| General/Common RF | HPI-Induced Diseases and Disorders |
| Advanced age | Anemia, iron deficiency |
| Menopause/male hypogonadism | Vitamin B12 deficiency |
| Body mass loss | Immune thrombocytopenia |
| Low BMD | Chronic/atrophic gastritis |
| Previous fragility fracture | PUD |
| History of falls | Gastric malignancy |
| Family history of OP/OFs | HPI- associated chronic diseases and disorders |
| Ethnicity (Caucasian and Asian vs. black populations) | Cardiovascular diseases (CAD, CHF, AF, hypertension) |
| Impaired balance, gait and mobility, need of assistive device * | Hemodynamic instability (orthostatic and/or postprandial hypotension, dizziness) # |
| Low physical activity/immobilization | Cerebrovascular diseases (stroke, TIA) |
| Low body mass index | Neurodegenerative diseases (dementia, Parkinson’s disease) |
| Hemodynamic instability (orthostatic and/or postprandial hypotension, dizziness) # | COPD |
| Visual impairment | CKD |
| Vitamin D deficiency/insufficiency | Diabetes mellitus |
| Vitamin K deficiency | Metabolic syndrome |
| Hyperparathyroidism | CLD |
| Urinary incontinence # | Depression, anxiety |
| Low calcium intake | Rheumatologic diseases |
| Fear of falling # | Eye diseases (open-angle glaucoma, neuromyelitis optica) |
| Prolonged use of certain medications | Gut dysbiosis |
| Corticosteroids, antidepressants (especially, SSRIs, SSNRIs), opioids, anxiolytics, hypnotics, sedatives (benzodiazepines), antiparkinsonian (dopaminergic) medications, antipsychotics, antiepileptics, glitazones, antiarrhythmics, PPIs, thyroxine, aromatase inhibitors, gonadotropin releasing hormone antagonists, immunosuppressive agents, polypharmacy | Malignant tumors (breast, lung, colorectal, prostate cancers) |
| Environmental, lifestyle and socio-economic RF | |
| Cigarette smoking, excess alcohol consumption, diet, urbanization, poor sanitation conditions, air pollution. |
| Step 1. Assess, in addition to evaluation common risk factors for OP/OF, presence or history of HPI-induced and HPI-associated diseases and disorders and check the appropriateness of concomitant treatments (the potential role of drugs used in regard to OP and falls) (see Table 3). |
| Step 2. If indicated, assess current HPI status, the microbe’s virulent characteristics, predominant site, type and severity of gastritis. |
| Step 3. Assess the bone-mineral status (BMD, bone turnover markers, serum levels of vitamin D, vitamin B12, PTH, calcium, phosphate and magnesium). |
| Step 4. Evaluate for and address HPI-related specific conditions/complications associated with OP and/or falls (e.g., iron deficiency/anemia, gut dysbiosis, hemodynamic instability, gait disturbance, frailty, etc.). |
| Step 5. Introduce a personalized and holistic care plan of preventive and/or therapeutic management according the identified disorders and their combinations. This may include: (1) HPI eradication; (2) adequate antiosteoporotic treatment; (3) elimination/minimization falls-related factors (alleviation effects of chronic diseases and/or drugs causing hemodynamic instability, gait disturbances, muscle loss, frailty); (4) review and optimization of all medications used; (5) correction the metabolic alterations (vitamin D, iron and vitamin B12), hormonal status, anemia and gut dysbiosis (e.g., pre- and probiotic therapy); (6) nutritional support; and (7) modulation of lifestyle factors (physical activity, tobacco smoking and alcohol consumption). |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, L.; Fisher, A.; Smith, P.N. Helicobacter pylori Related Diseases and Osteoporotic Fractures (Narrative Review). J. Clin. Med. 2020, 9, 3253. https://doi.org/10.3390/jcm9103253
Fisher L, Fisher A, Smith PN. Helicobacter pylori Related Diseases and Osteoporotic Fractures (Narrative Review). Journal of Clinical Medicine. 2020; 9(10):3253. https://doi.org/10.3390/jcm9103253
Chicago/Turabian StyleFisher, Leon, Alexander Fisher, and Paul N Smith. 2020. "Helicobacter pylori Related Diseases and Osteoporotic Fractures (Narrative Review)" Journal of Clinical Medicine 9, no. 10: 3253. https://doi.org/10.3390/jcm9103253
APA StyleFisher, L., Fisher, A., & Smith, P. N. (2020). Helicobacter pylori Related Diseases and Osteoporotic Fractures (Narrative Review). Journal of Clinical Medicine, 9(10), 3253. https://doi.org/10.3390/jcm9103253





