Enhanced Humoral Immune Responses against Toxin A and B of Clostridium difficile is Associated with a Milder Disease Manifestation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Specimens’ Handling and Laboratory Methods
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
3.1. Differences in IgA and IgG Levels against TcdA and TcdB between CDI Cases and the Controls
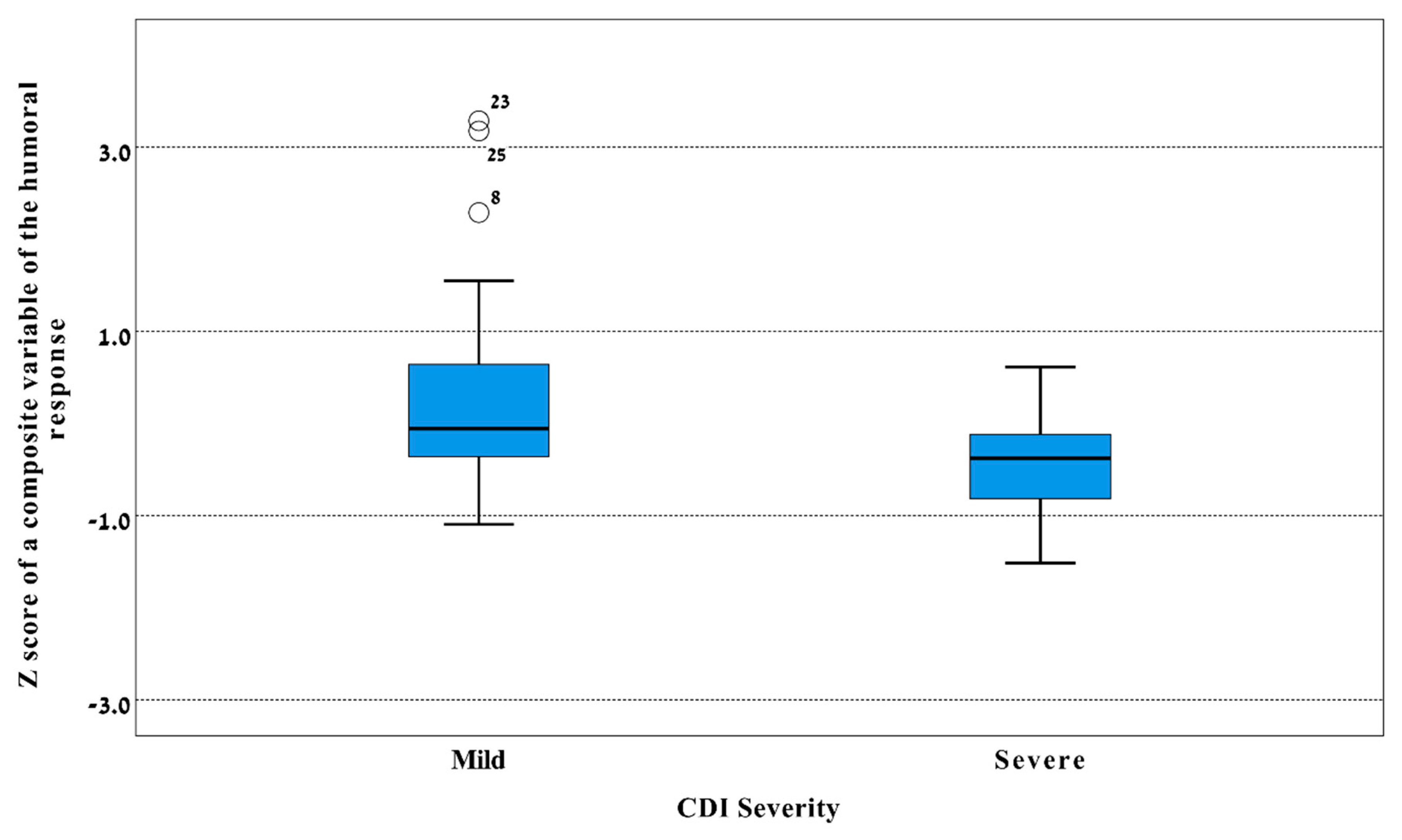
3.2. Differences in IgA and IgG Levels against TcdA and TcdB between Vatients with Mild and Severe CDI
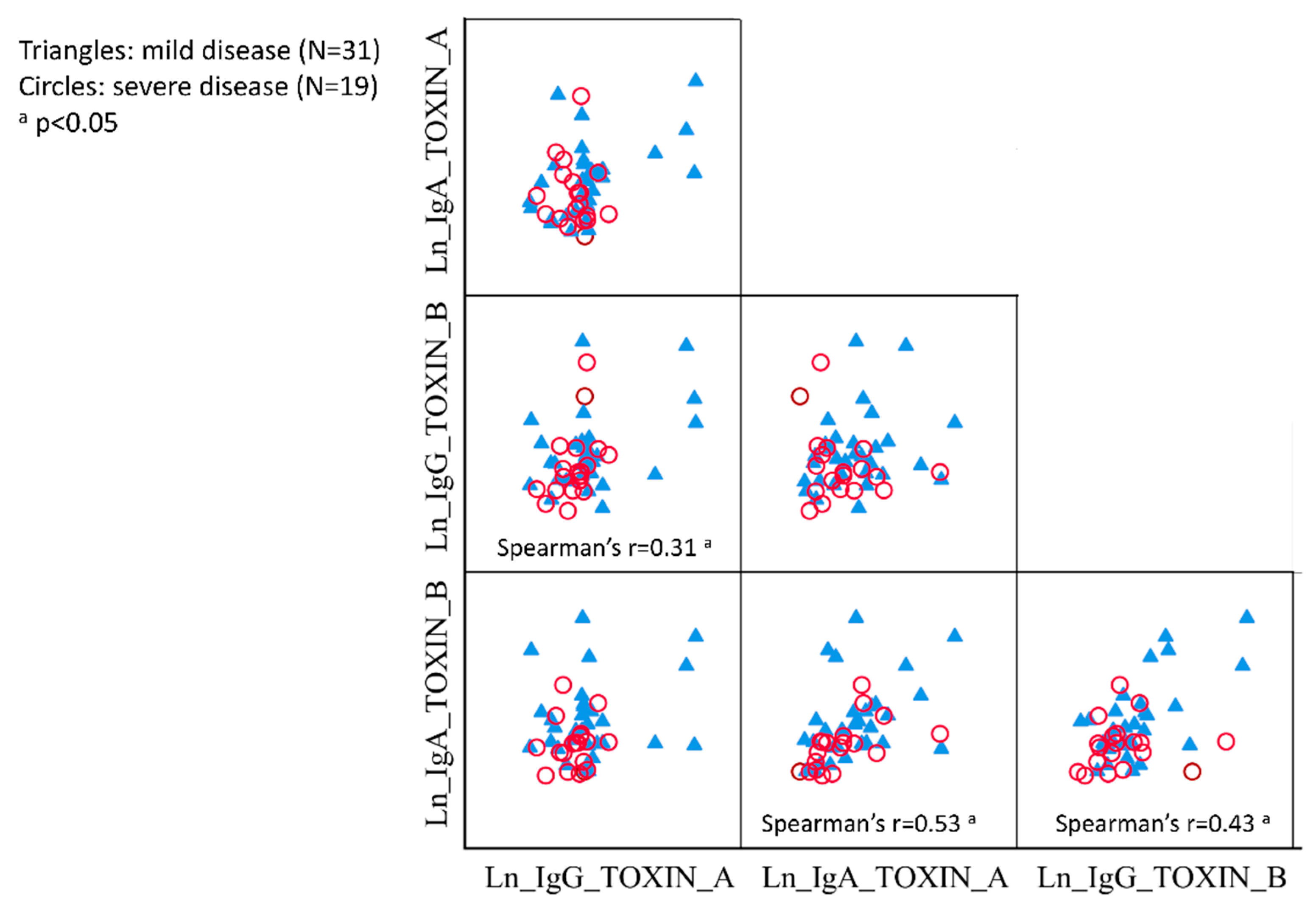
3.3. Correlations between IgG and IgA against TcdA and TcdB
3.4. Factor Analysis of Serum IgG and IgA against TcdA and TcdB
3.5. Paired Sera from CDI Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rupnik, M.; Wilcox, M.H.; Gerding, D.N. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, S.; Ascenzi, P.; Siarakas, S.; Petrosillo, N.; di Masi, A. Clostridium difficile toxins A and B: Insights into pathogenic properties and extraintestinal effects. Toxins 2016, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.P.; LaMont, J.T. Clostridium difficile—More difficult than ever. N. Engl. J. Med. 2008, 359, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Miller, M.A.; Louie, T.J.; Crook, D.W.; Gorbach, S.L. Treatment of first recurrence of Clostridium difficile infection: Fidaxomicin versus vancomycin. Clin. Infect. Dis. 2012, 55 (Suppl. 2), S154–S161. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.P.; Kyne, L. The host immune response to Clostridium difficile. J. Med. Microbiol. 2011, 60, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Kuijper, E.J.; Coignard, B.; Tull, P.; ESCMID Study Group for Clostridium difficile (ESGCD); EU Member States; The European Centre for Disease Prevention and Control (ECDC). Emergence of Clostridium difficile-associated disease in North America and Europe. Clin. Microbiol. Infect. 2006, 12 (Suppl. 6), 2–18. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.J.D.; Dubberke, E.R.D. Trends in Clostridium difficile disease: Epidemiology and intervention. Infect. Med. 2009, 26, 211–220. [Google Scholar]
- Solomon, K.; Martin, A.J.; O’Donoghue, C.; Chen, X.; Fenelon, L.; Fanning, S.; Kelly, C.P.; Kyne, L. Mortality in patients with Clostridium difficile infection correlates with host pro-inflammatory and humoral immune responses. J. Med. Microbiol. 2013, 62, 1453–1460. [Google Scholar] [CrossRef]
- Loo, V.G.; Bourgault, A.M.; Poirier, L.; Lamothe, F.; Michaud, S.; Turgeon, N.; Toye, B.; Beaudoin, A.; Frost, E.H.; Gilca, R.; et al. Host and pathogen factors for Clostridium difficile infection and colonization. N. Engl. J. Med. 2011, 365, 1693–1703. [Google Scholar] [CrossRef]
- Kyne, L.; Warny, M.; Qamar, A.; Kelly, C.P. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 2001, 357, 189–193. [Google Scholar] [CrossRef]
- Kyne, L.; Warny, M.; Qamar, A.; Kelly, C.P. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 2000, 342, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, B.; Granstrom, M.; Mollby, R.; Nord, C.E. Serum antibody response to Clostridium difficile toxins in patients with Clostridium difficile diarrhoea. Infection 1985, 13, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Bacon, A.E., III; Fekety, R. Immunoglobulin G directed against toxins A and B of Clostridium difficile in the general population and patients with antibiotic-associated diarrhea. Diagn. Microbiol. Infect. Dis. 1994, 18, 205–209. [Google Scholar] [CrossRef]
- Islam, J.; Taylor, A.L.; Rao, K.; Huffnagle, G.; Young, V.B.; Rajkumar, C.; Cohen, J.; Papatheodorou, P.; Aronoff, D.M.; Llewelyn, M.J. The role of the humoral immune response to Clostridium difficile toxins A and B in susceptibility to C. difficile infection: A case-control study. Anaerobe 2014, 27, 82–86. [Google Scholar] [CrossRef]
- Steele, J.; Mukherjee, J.; Parry, N.; Tzipori, S. Antibody against TcdB, but not TcdA, prevents development of gastrointestinal and systemic Clostridium difficile disease. J. Infect. Dis. 2013, 207, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, S.A.; Cartman, S.T.; Heap, J.T.; Kelly, M.L.; Cockayne, A.; Minton, N.P. The role of toxin A and toxin B in Clostridium difficile infection. Nature 2010, 467, 711–713. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Kelly, C.P.; Poxton, I.R.; Shen, J.; Wilcox, M.H.; Gerding, D.N.; Zhao, X.; Laterza, O.F.; Railkar, R.; Guris, D.; Dorr, M.B. Effect of endogenous Clostridioides difficile toxin antibodies on recurrence of C. difficile infection. Clin. Infect. Dis. 2020, 71, 81–86. [Google Scholar] [CrossRef]
- Muhsen, K.; Na’amnih, W.; Adler, A.; Carmeli, Y.; Cohen, D. Clostridium difficile-associated disease and Helicobacter pylori seroprevalence: A case-control study. Helicobacter 2020, 25, e12668. [Google Scholar] [CrossRef]
- Na’amnih, W.; Adler, A.; Miller-Roll, T.; Cohen, D.; Carmeli, Y. Incidence and risk factors for community and hospital acquisition of Clostridium difficile infection in the Tel Aviv Sourasky medical center. Infect. Control Hosp. Epidemiol. 2017, 38, 912–920. [Google Scholar] [CrossRef]
- Wullt, M.; Noren, T.; Ljungh, A.; Akerlund, T. IgG antibody response to toxins A and B in patients with Clostridium difficile infection. Clin. Vaccine Immunol. 2012, 19, 1552–1554. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Genser, B.; Cooper, P.J.; Yazdanbakhsh, M.; Barreto, M.L.; Rodrigues, L.C. A guide to modern statistical analysis of immunological data. BMC Immunol. 2007, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Genser, B.; Fischer, J.E.; Figueiredo, C.A.; Alcantara-Neves, N.; Barreto, M.L.; Cooper, P.J.; Amorim, L.D.; Saemann, M.D.; Weichhart, T.; Rodrigues, L.C. Applied immuno-epidemiological research: An approach for integrating existing knowledge into the statistical analysis of multiple immune markers. BMC Immunol. 2016, 17, 11. [Google Scholar] [CrossRef]
- Abramson, J.H. WINPEPI updated: Computer programs for epidemiologists, and their teaching potential. Epidemiol. Perspect. Innov. 2011, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Warny, M.; Vaerman, J.P.; Avesani, V.; Delmee, M. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect. Immun. 1994, 62, 384–389. [Google Scholar] [CrossRef]
- Viscidi, R.; Laughon, B.E.; Yolken, R.; Bo-Linn, P.; Moench, T.; Ryder, R.W.; Bartlett, J.G. Serum antibody response to toxins A and B of Clostridium difficile. J. Infect. Dis. 1983, 148, 93–100. [Google Scholar] [CrossRef]
- Johnson, S.; Gerding, D.N.; Janoff, E.N. Systemic and mucosal antibody responses to toxin A in patients infected with Clostridium difficile. J. Infect. Dis. 1992, 166, 1287–1294. [Google Scholar] [CrossRef]
- Sanchez-Hurtado, K.; Corretge, M.; Mutlu, E.; McIlhagger, R.; Starr, J.M.; Poxton, I.R. Systemic antibody response to Clostridium difficile in colonized patients with and without symptoms and matched controls. J. Med. Microbiol. 2008, 57, 717–724. [Google Scholar] [CrossRef]
- Cohen, D.; Meron-Sudai, S.; Bialik, A.; Asato, V.; Goren, S.; Ariel-Cohen, O.; Reizis, A.; Hochberg, A.; Ashkenazi, S. Serum IgG antibodies to Shigella lipopolysaccharide antigens—A correlate of protection against shigellosis. Hum. Vaccines Immunother. 2019, 15, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Green, M.S.; Block, C.; Rouach, T.; Ofek, I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J. Infect. Dis. 1988, 157, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Ashkenazi, S.; Green, M.S.; Gdalevich, M.; Robin, G.; Slepon, R.; Yavzori, M.; Orr, N.; Block, C.; Ashkenazi, I.; et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997, 349, 155–159. [Google Scholar] [CrossRef]
- Foglia, G.; Shah, S.; Luxemburger, C.; Pietrobon, P.J. Clostridium difficile: Development of a novel candidate vaccine. Vaccine 2012, 30, 4307–4309. [Google Scholar] [CrossRef] [PubMed]
- Bezay, N.; Ayad, A.; Dubischar, K.; Firbas, C.; Hochreiter, R.; Kiermayr, S.; Kiss, I.; Pinl, F.; Jilma, B.; Westritschnig, K. Safety, immunogenicity and dose response of VLA84, a new vaccine candidate against Clostridium difficile, in healthy volunteers. Vaccine 2016, 34, 2585–2592. [Google Scholar] [CrossRef]
- Eliakim-Raz, N.; Bishara, J. Prevention and treatment of Clostridium difficile associated diarrhea by reconstitution of the microbiota. Hum. Vaccines Immunother. 2019, 15, 1453–1456. [Google Scholar] [CrossRef]
- Forster, B.; Chung, P.K.; Crobach, M.J.T.; Kuijper, E.J. Application of antibody-mediated therapy for treatment and prevention of Clostridium difficile infection. Front. Microbiol. 2018, 9, 1382. [Google Scholar] [CrossRef]
| Cases (n = 50) GMT (SD) | Controls (n = 52) GMT (SD) | p Value b | |
|---|---|---|---|
| Toxin A | |||
| Serum IgG antibody | 20.1 (2.5) | 11.6 (2.1) | 0.001 |
| Serum IgA antibody | 6.8 (2.1) | 5.3 (2.0) | 0.08 |
| Toxin B | |||
| Serum IgG antibody | 18.0 (2.6) | 12.0 (2.7) | 0.04 |
| Serum IgA antibody | 7.2 (2.5) | 5.4 (2.2) | 0.09 |
| Toxin A | Toxin B | Toxin A | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | IgG | Mean Difference (95% CI) | p Value a | IgA | Mean Difference (95% CI) | p Value a | IgG | Mean Difference (95% CI) | p Value a | IgA | Mean Difference (95% CI) | p Value a |
| GMT (SD) | GMT (SD) | GMT (SD) | GMT (SD) | |||||||||
| Disease severity | ||||||||||||
| Mild (n = 31) | 23.4 (1.1) | 0.39 (−0.13, 0.92) | 0.12 b | 7.6 (0.9) | 0.30 (−0.13, 0.74) | 0.14 c | 20.5 (1.0) | 0.34 (−0.22, 0.91) | 0.11 d | 9.2 (1.0) | 0.63 (0.10, 1.15) | 0.023 e |
| Severe (n = 19) | 15.8 (0.5) | Reference | 5.6 (0.7) | Reference | 14.6 (0.9) | Reference | 4.9 (0.6) | Reference | ||||
| Disease severity in the time period, 7–14 days | ||||||||||||
| Mild (n = 9) | 35.5 (1.1) | 0.90 (0.07, 1.74) | 0.004 f | 8.1 (0.5) | 0.33 (−0.20, 0.87) | 0.23 g | 30.1 (1.1) | 0.77 (−0.40, 1.95) | 0.036 h | 9.0 (1.2) | 0.63 (−0.32, 1.57) | 0.27 i |
| Severe (n = 8) | 14.4 (0.4) | Reference | 5.8 (0.6) | Reference | 13.9 (1.2) | Reference | 4.8 (0.5) | Reference |
| 1st Sample GMT (SD) | 2nd Sample GMT (SD) | p Value a | |
|---|---|---|---|
| Toxin A, IgG | 15.8 (1.3) | 18.9 (1.3) | 0.05 |
| Toxin A, IgA | 6.9 (1.4) | 7.1 (1.5) | 0.8 |
| Toxin B, IgG | 17.5 (1.3) | 23.8 (1.3) | 0.02 |
| Toxin B, IgA | 6.1 (1.6) | 6.2 (1.6) | 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na’amnih, W.; Carmeli, Y.; Asato, V.; Goren, S.; Adler, A.; Cohen, D.; Muhsen, K. Enhanced Humoral Immune Responses against Toxin A and B of Clostridium difficile is Associated with a Milder Disease Manifestation. J. Clin. Med. 2020, 9, 3241. https://doi.org/10.3390/jcm9103241
Na’amnih W, Carmeli Y, Asato V, Goren S, Adler A, Cohen D, Muhsen K. Enhanced Humoral Immune Responses against Toxin A and B of Clostridium difficile is Associated with a Milder Disease Manifestation. Journal of Clinical Medicine. 2020; 9(10):3241. https://doi.org/10.3390/jcm9103241
Chicago/Turabian StyleNa’amnih, Wasef, Yehuda Carmeli, Valeria Asato, Sophy Goren, Amos Adler, Dani Cohen, and Khitam Muhsen. 2020. "Enhanced Humoral Immune Responses against Toxin A and B of Clostridium difficile is Associated with a Milder Disease Manifestation" Journal of Clinical Medicine 9, no. 10: 3241. https://doi.org/10.3390/jcm9103241
APA StyleNa’amnih, W., Carmeli, Y., Asato, V., Goren, S., Adler, A., Cohen, D., & Muhsen, K. (2020). Enhanced Humoral Immune Responses against Toxin A and B of Clostridium difficile is Associated with a Milder Disease Manifestation. Journal of Clinical Medicine, 9(10), 3241. https://doi.org/10.3390/jcm9103241





