Visual and Topographic Improvement with Epithelium-On, Oxygen-Supplemented, Customized Corneal Cross-Linking for Progressive Keratoconus
Abstract
1. Introduction
2. Methods
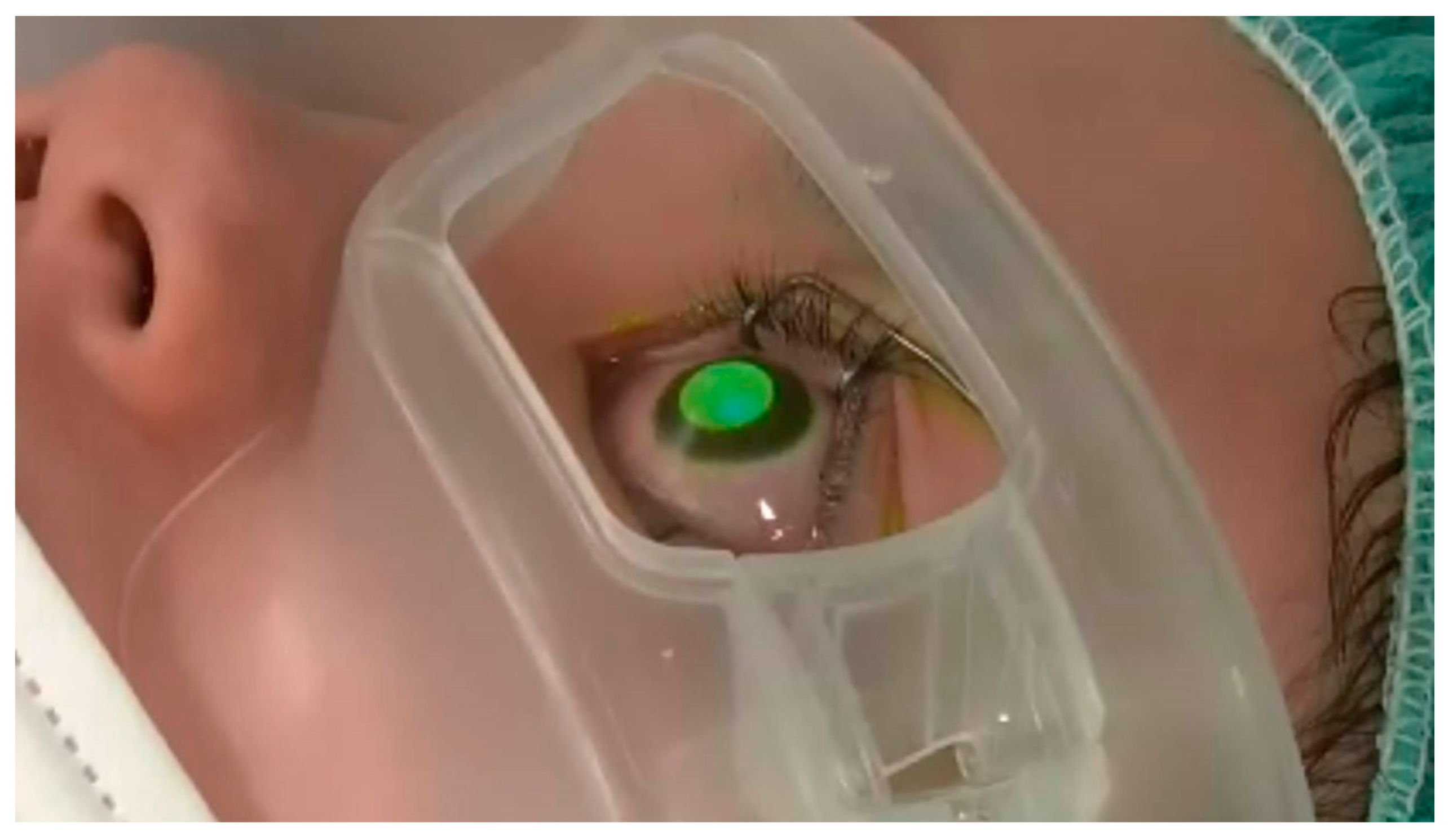
2.1. Cross-Linking Procedure
2.2. Statistical Analysis
3. Results
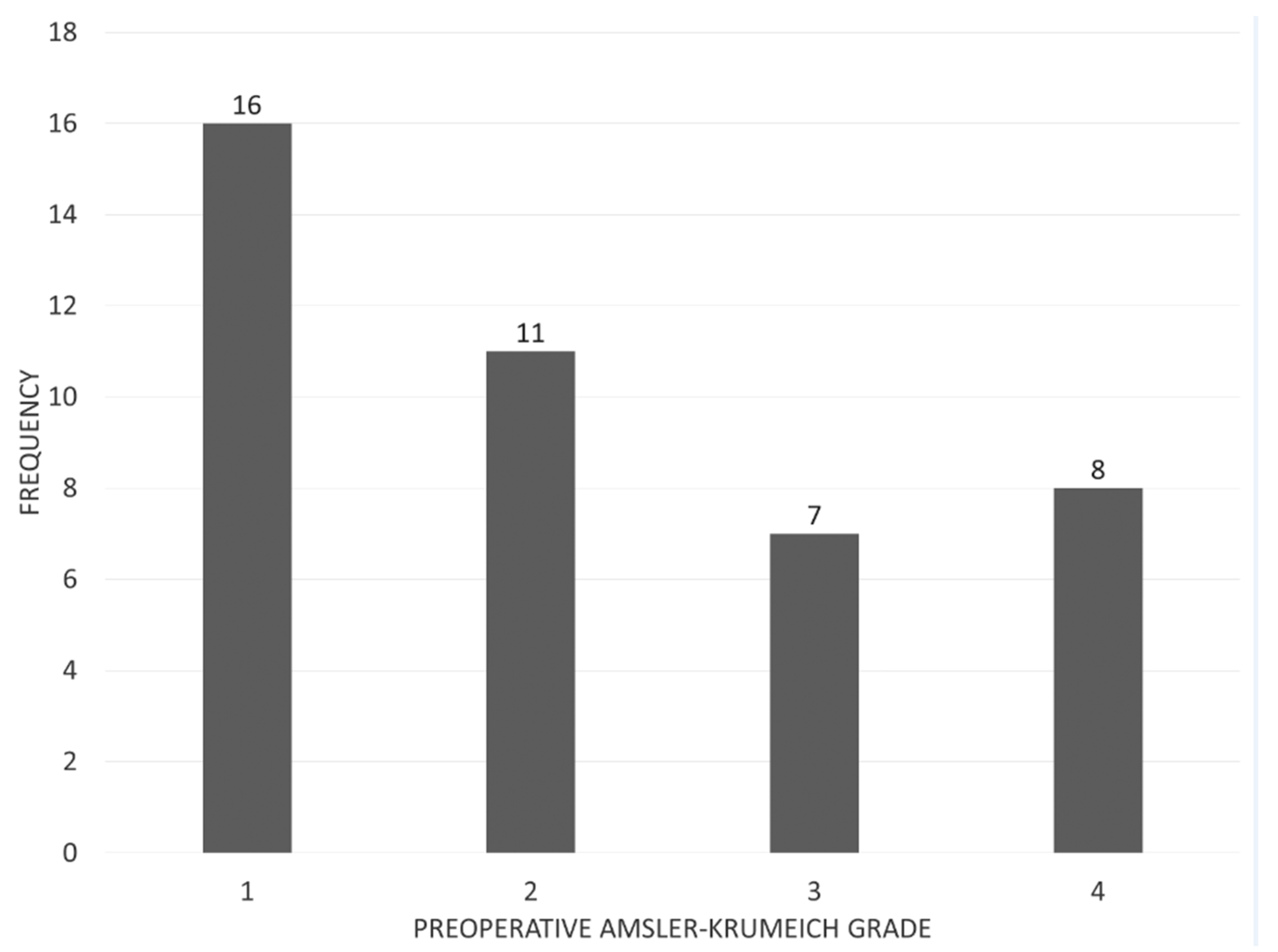
3.1. Study Population
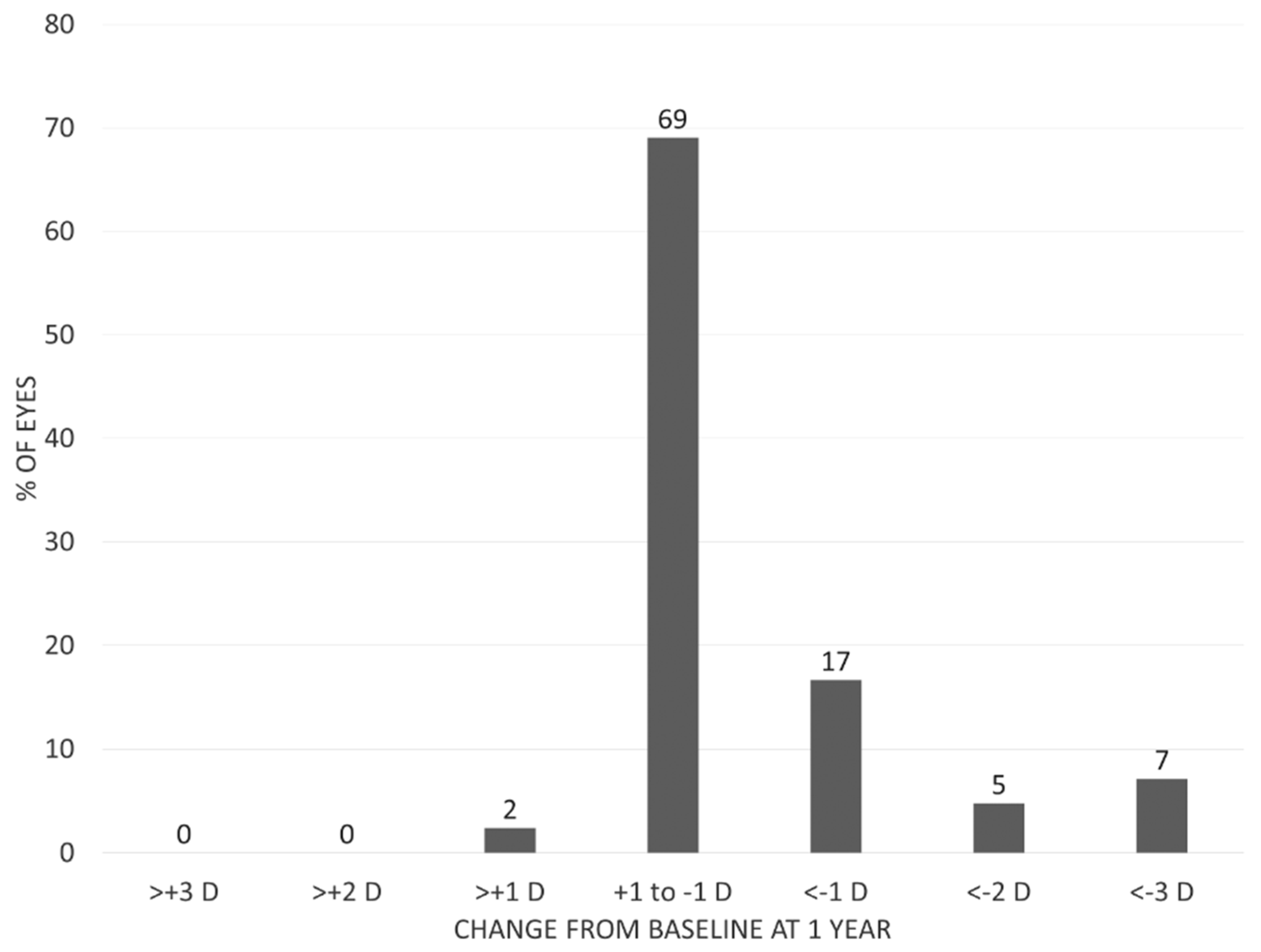
3.2. Visual Outcomes
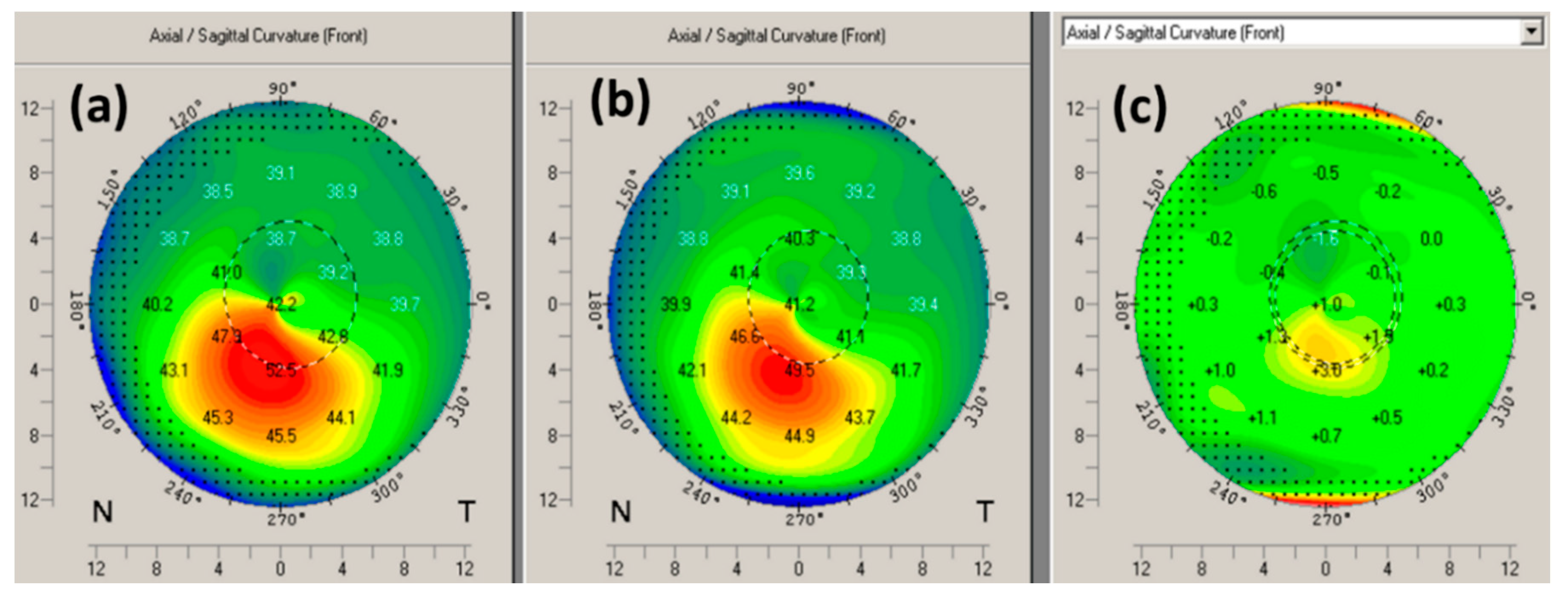
3.3. Corneal Topography
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gomes, J.A.P.; Tan, D.; Rapuano, C.J.; Belin, M.W.; Ambrósio, R., Jr.; Guell, J.L.; Sangwan, V.S. Global Consensus on Keratoconus and Ectatic Diseases. Cornea 2015, 34, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Hersh, P.S.; Stulting, R.D.; Muller, D.; Durrie, D.S.; Rajpal, R.K.; Binder, P.S.; Price, F., Jr. U.S. Multicenter Clinical Trial of Corneal Collagen Crosslinking for Treatment of Corneal Ectasia after Refractive Surgery. Ophthalmology 2017, 124, 1475–1484. [Google Scholar] [CrossRef]
- Lim, L.; Lim, E.W.L. A Review of Corneal Collagen Cross-linking – Current Trends in Practice Applications. Open Ophthalmol. J. 2018, 12 (Suppl. 1), 181–213. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulos, A.J.; Dupps, W.J.; Seven, I.; Asimellis, G. Toric topographically customized transepithelial, pulsed, very high-fluence, higher energy and higher riboflavin concentration collagen cross-linking in keratoconus. Case Rep. Ophthalmol. 2014, 5, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Nethralaya, N.; Pahuja, N.; Roshan, T.; Deshmukh, R.; Francis, M.; Ghosh, A.; Roy, A.S. Customized corneal crosslinking using different UVA beam profiles Customized corneal crosslinking using different UVA beam profiles. J. Refract. Surg. 2017, 33, 676–682. [Google Scholar] [CrossRef]
- Mazzotta, C.; Moramarco, A.; Traversi, C.; Baiocchi, S.; Iovieno, A.; Fontana, L. Accelerated Corneal Collagen Cross-Linking Using Topography-Guided UV-A Energy Emission: Preliminary Clinical and Morphological Outcomes. J. Ophthalmol. 2016, 2016, 2031031. [Google Scholar] [CrossRef]
- Lytle, G. Advances in the Technology of Corneal Cross-Linking for Keratoconus. Eye Contact Lens 2014, 40, 358–364. [Google Scholar] [CrossRef]
- Nordström, M.; Schiller, M.; Fredriksson, A.; Behndig, A. Refractive improvements and safety with topography-guided corneal crosslinking for keratoconus: 1-year results. Br. J. Ophthalmol. 2016, 2016. [Google Scholar] [CrossRef]
- Seiler, T.G.; Fischinger, I.; Koller, T.; Zapp, D.; Frueh, B.E.; Seiler, T. Customized Corneal Cross-linking: One-Year Results. Am. J. Ophthalmol. 2016, 166, 14–21. [Google Scholar] [CrossRef]
- Cassagne, M.; Pierné, K.; Galiacy, S.D.; Asfaux-Marfaing, M.-P.; Fournié, P.; Malecaze, F. Customized Topography-Guided Corneal Collagen Cross-linking for Keratoconus. J. Refract. Surg. 2017, 33, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Koppen, C.; Wouters, K.; Mathysen, D.; Rozema, J.; Tassignon, M.-J. Refractive and topographic results of benzalkonium chloride-assisted transepithelial crosslinking. J. Cataract Refract. Surg. 2012, 38, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Gatzioufas, Z.; Raiskup, F.; O’Brart, D.; Spoerl, E.; Panos, G.D.; Hafezi, F. Transepithelial Corneal Cross-linking Using an Enhanced Riboflavin Solution. J. Refract. Surg. 2016, 32, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Kamaev, P.; Friedman, M.D.; Sherr, E.; Muller, D. Photochemical Kinetics of Corneal Cross-Linking with Riboflavin. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2360–2367. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Liu, C.; Deardorff, P.; Tavakol, B.; Eddington, W.; Thompson, V.; Adler, D.C. Optimization of Oxygen Dynamics, UV-A Delivery, and Drug Formulation for Accelerated Epi-On Corneal Crosslinking. Curr. Eye Res. 2019, 45, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Taneri, S.; Oehler, S.; Lytle, G.; Burkhard Dick, H. Evaluation of epithelial integrity with various transepithelial corneal cross-linking protocols for treatment of keratoconus. J. Ophthalmol. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gore, D.M.; O’Brart, D.; French, P.; Dunsby, C.; Allan, B.D. Transepithelial riboflavin absorption in an ex vivo rabbit corneal model. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5006–5011. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, C.; Bagaglia, S.A.; Vinciguerra, R.; Ferrise, M.; Vinciguerra, P. Enhanced-fluence pulsed-light iontophoresis corneal cross-linking: 1-Year morphological and clinical results. J. Refract. Surg. 2018, 34, 438–444. [Google Scholar] [CrossRef]
- Park, Y.M.; Kim, H.Y.; Lee, J.S. Comparison of 2 Different Methods of Transepithelial Corneal Collagen Cross-Linking: Analysis of Corneal Histology and Hysteresis. Cornea 2017, 36, 860–865. [Google Scholar] [CrossRef]
- Sun, L.; Li, M.; Zhang, X.; Tian, M.; Han, T.; Zhao, J.; Zhou, X. Transepithelial accelerated corneal collagen cross-linking with higher oxygen availability for keratoconus: 1-year results. Int. Ophthalmol. 2017, 38, 2509–2517. [Google Scholar] [CrossRef]
- Sachdev, G.S.; Ramamurthy, S. Photo Essay Clinical presentation following photorefractive intrastromal cross—Linking for myopic correction. Indian J. Ophthalmol. 2018, 66, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Hout, S.E.; Cassagne, M.; De Gauzy, T.S.; Fourni, P. Transepithelial photorefractive intrastromal corneal crosslinking versus photorefractive keratectomy in low myopia. J. Cataract Refract. Surg. 2018, 45, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, C.; Sgheri, A.; Bagaglia, S.A.; Rechichi, M.; Di Maggio, A. Customized corneal crosslinking for treatment of progressive keratoconus: Clinical and OCT outcomes using a transepithelial approach with supplemental oxygen. J. Cataract Refract. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, H.; Rong, S.S.; Ciolino, J.B. Transepithelial versus epithelium-off corneal crosslinking for corneal ectasia. J. Cataract Refract. Surg. 2018, 44, 1507–1516. [Google Scholar] [CrossRef]
- Ferdi, A.C.; Nguyen, V.; Gore, D.M.; Allan, B.D.; Rozema, J.J.; Watson, S.L. Keratoconus Natural Progression: A Systematic Review and Meta-analysis of 11 529 Eyes. Ophthalmology 2019, 126, 935–945. [Google Scholar] [CrossRef]
- Perez-Straziota, C.; Gaster, R.N.; Rabinowitz, Y.S. Corneal Cross-Linking for Pediatric Keratcoconus Review. Cornea 2018, 37, 802–809. [Google Scholar] [CrossRef]
- Ozer, M.D.; Batur, M.; Mesen, S.; Tekın, S.; Seven, E.; Yasar, T. Comparison of the efficacy of accelerated corneal cross-linking therapy in different pediatric age groups having progressive keratoconus. Int. Ophthalmol. 2020, 40, 2651–2658. [Google Scholar] [CrossRef]
- Mazzotta, C.; Hafezi, F.; Kymionis, G.; Caragiuli, S.; Jacob, S.; Traversi, C.; Randleman, J.B. In Vivo Confocal Microscopy after Corneal Collagen Crosslinking. Ocul. Surf. 2015, 13, 298–314. [Google Scholar] [CrossRef]
| Epi-On, Accelerated, Oxygen-Supplemented, Customized Corneal Cross-Linking Methods | |
|---|---|
| Parameter | Variable |
| Treatment target | Keratoconus |
| Fluence (total) (J/cm2) | 15 |
| Soak time and interval (seconds) | (1) 4 min (q60s) + (2) 6 min (q30s) |
| Intensity (mW) | 30 |
| Treatment time (minutes and seconds) | 16:40 (15 J), 11:06 (10 J), 8:00 (7.2 J) |
| Epithelium status | On |
| Chromophore | Riboflavin |
| Chromophore composition | (1) Benzalkonium Chloride/EDTA/Trometamol |
| Hydroxypropylmethylcellulose with NaCl | |
| Phosphate buffered saline solution | |
| (2) With NaCl | |
| Phosphate buffered saline solution | |
| Chromophore osmolality | Iso-osmolar |
| Chromophore concentration | (1) 0.25% (2) 0.22% |
| Light source | Mosaic (Avedro) |
| Irradiation mode | Pulsed (1 s on, 1 s off)) |
| Protocol modifications | Oxygen-supplemented, customized |
| Protocol abbreviation in manuscript | Epi-on customized cross-linking |
| All keratoconus (G1 to 4, n = 42) | Pre- vs. 1 year | |||||
| Parameter | Preoperative | 1 month | 3 months | 6 months | 1 year | p-value |
| Thinnest pachymetry (µm) | 449 ± 42 | 441 ± 38 | 441 ± 38 | 441 ± 38 | 445 ± 40 | 0.244 |
| Kmax (D) | 53.04 ± 7.91 | 52.25 ± 7.31 | 52.32 ± 7.33 | 52.37 ± 7.17 | 52.31 ± 7.50 | <0.001 |
| BSCVA (logMAR) | 0.19 ± 0.36 | 0.10 ± 0.26 | 0.09 ± 0.32 | 0.13 ± 0.36 | 0.11 ± 0.33 | 0.004 |
| UCVA (logMAR) | 0.87 ± 0.53 | 0.78 ± 0.59 | 0.71 ± 0.57 | 0.75 ± 0.59 | 0.78 ± 0.56 | 0.016 |
| Endothelial cell density (cells/mm2) | 2801 ± 232 | 2772 ± 251 | 0.104 | |||
| Mild to moderate keratoconus (G1 to 2, n = 27) | ||||||
| Parameter | Preoperative | 1 month | 3 months | 6 months | 1 year | p-value |
| Thinnest pachymetry (µm) | 464 ± 35 | 456 ± 25 | 454 ± 32 | 455 ± 28 | 458 ± 36 | 0.069 |
| Kmax (D) | 49.36 ± 5.03 | 48.84 ± 4.80 | 48.83 ± 4.75 | 49.10 ± 4.83 | 48.77 ± 4.66 | 0.014 |
| BSCVA (logMAR) | 0.06 ± 0.18 | 0.02 ± 0.17 | 0.00 ± 0.13 | 0.01 ± 0.17 | 0.02 ± 0.16 | 0.041 |
| UCVA (logMAR) | 0.72 ± 0.48 | 0.87 ± 0.54 | 0.87 ± 0.55 | 0.87 ± 0.56 | 0.78 ± 0.56 | 0.018 |
| Severe keratoconus (G3 to 4, n = 15) | ||||||
| Parameter | Preoperative | 1 month | 3 months | 6 months | 1 year | p-value |
| Thinnest pachymetry (µm) | 421 ± 40 | 414 ± 43 | 417 ± 37 | 416 ± 42 | 422 ± 38 | 0.865 |
| Kmax (D) | 59.66 ± 7.95 | 58.38 ± 7.15 | 58.61 ± 7.04 | 58.26 ± 7.02 | 58.69 ± 7.52 | 0.013 |
| BSCVA (logMAR) | 0.42 ± 0.48 | 0.23 ± 0.33 | 0.26 ± 0.48 | 0.33 ± 0.50 | 0.29 ± 0.48 | 0.024 |
| UCVA (logMAR) | 1.15 ± 0.52 | 1.10 ± 0.50 | 1.02 ± 0.57 | 1.04 ± 0.61 | 1.09 ± 0.54 | 0.422 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiya, K.; Kanayama, S.; Takahashi, M.; Shoji, N. Visual and Topographic Improvement with Epithelium-On, Oxygen-Supplemented, Customized Corneal Cross-Linking for Progressive Keratoconus. J. Clin. Med. 2020, 9, 3222. https://doi.org/10.3390/jcm9103222
Kamiya K, Kanayama S, Takahashi M, Shoji N. Visual and Topographic Improvement with Epithelium-On, Oxygen-Supplemented, Customized Corneal Cross-Linking for Progressive Keratoconus. Journal of Clinical Medicine. 2020; 9(10):3222. https://doi.org/10.3390/jcm9103222
Chicago/Turabian StyleKamiya, Kazutaka, Shunsuke Kanayama, Masahide Takahashi, and Nobuyuki Shoji. 2020. "Visual and Topographic Improvement with Epithelium-On, Oxygen-Supplemented, Customized Corneal Cross-Linking for Progressive Keratoconus" Journal of Clinical Medicine 9, no. 10: 3222. https://doi.org/10.3390/jcm9103222
APA StyleKamiya, K., Kanayama, S., Takahashi, M., & Shoji, N. (2020). Visual and Topographic Improvement with Epithelium-On, Oxygen-Supplemented, Customized Corneal Cross-Linking for Progressive Keratoconus. Journal of Clinical Medicine, 9(10), 3222. https://doi.org/10.3390/jcm9103222






