Anxiety, Depression, and Colorectal Cancer Survival: Results from Two Prospective Cohorts
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Measures
2.2.1. Anxiety and Depression
Self-Reported Anxiety/Depression Symptom Scales
Self-Reported Clinically-Diagnosed Depression and Psychotropic Medication
2.2.2. Covariates
2.2.3. Deaths Ascertainment
2.3. Statistical Analysis
2.3.1. Main Analyses
2.3.2. Additional Analyses
2.3.3. Inverse Probability Weighting
3. Results
3.1. Baseline Characteristics
3.2. Associations of Anxiety and Depression with Overall Mortality Risk
3.3. Additional Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Accessibility
Abbreviations
| BMI | Body Mass Index |
| CI | Confident Interval |
| CRC | Colorectal Cancer |
| HPFS | Health Professional Follow-Up Study |
| HR | Hazard Ratio |
| M | Mean |
| NHS | Nurses’ Health Study |
| SD | Standard Deviation |
References
- American Cancer Society. Colorectal Cancer Facts & Figures 2014–2016; American Cancer Society: Atlanta, GA, USA, 2014. [Google Scholar]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, N.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Hansen, I.O.; Jess, P. Possible better long-term survival in left versus right-sided colon cancer—A systematic review. Dan. Med. J. 2012, 59, 4444. [Google Scholar]
- Cohen, B.E.; Edmondson, N.; Kronish, I.M. State of the Art Review: Depression, Stress, Anxiety, and Cardiovascular Disease. Am. J. Hypertens. 2015, 28, 1295–1302. [Google Scholar] [CrossRef]
- Trudel-Fitzgerald, C.; Gilsanz, P.; Mittleman, M.A.; Kubzansky, L.D. Dysregulated Blood Pressure: Can Regulating Emotions Help? Curr. Hypertens. Rep. 2015, 17, 92. [Google Scholar] [CrossRef]
- Trudel-Fitzgerald, C.; Chen, Y.; Singh, A.; Okereke, O.I.; Kubzansky, L.D. Psychiatric, Psychological, and Social Determinants of Health in the Nurses’ Health Study Cohorts. Am. J. Public Health 2016, 106, 1644–1649. [Google Scholar] [CrossRef]
- Hannah, M.; Batty, G.D.; Benzeval, M. Common mental disorders and mortality in the West of Scotland Twenty-07 Study: Comparing the General Health Questionnaire and the Hospital Anxiety and Depression Scale. J. Epidemiol. Community Health 2013, 67, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.O.; Veronese, N.; Sanches, M.; Stubbs, B.; Koyanagi, A.; Thompson, T.; Tzoulaki, I.; Solmi, M.; Vancampfort, D.; Schuch, F.B.; et al. The association of depression and all-cause and cause-specific mortality: An umbrella review of systematic reviews and meta-analyses. BMC Med. 2018, 16, 112. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.; Pikhart, H.; Head, J. Do depressive symptoms predict cancer incidence? J. Psychosom. Res. 2015, 79, 595–603. [Google Scholar] [CrossRef]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef]
- Garssen, B. Letter to the Editor: Depression linked to cancer mortality not convincingly demonstrated. Psychol. Med. 2011, 41, 1338–1342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pinquart, M.; Duberstein, P. Depression and cancer mortality: A meta-analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [CrossRef]
- Pinquart, M.; Duberstein, P.R. The authors reply: Meta-analysis and its discontents. Psychol. Med. 2011, 41, 1338–1342. [Google Scholar]
- Ogino, S.; Fuchs, C.S.; Giovannucci, E. How many molecular subtypes? Implications of the unique tumor principle in personalized medicine. Expert Rev. Mol. Diagn. 2012, 12, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Satin, J.R.; Linden, W.; Phillips, M.J. Depression as a predictor of disease progression and mortality in cancer patients. Cancer 2009, 115, 5349–5361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Fang, F.; Sjölander, A.; Fall, K.; Adami, H.O.; Valdimarsdottir, U.A. First-onset mental disorders after cancer diagnosis and cancer-specific mortality: A nationwide cohort study. Ann. Oncol. 2017, 28, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Batty, G.D.; Russ, T.C.; Stamatakis, E.; Kivimäki, M. Psychological distress in relation to site specific cancer mortality: Pooling of unpublished data from 16 prospective cohort studies. BMJ 2017, 356, j108. [Google Scholar] [CrossRef]
- Schofield, P.; Stockler, M.R.; Zannino, D.; Tebbutt, N.C.; Price, T.J.; Simes, R.J.; Wong, N.; Pavlakis, N.; Ransom, D.; Moylan, E.; et al. Hope, optimism and survival in a randomised trial of chemotherapy for metastatic colorectal cancer. Support. Care Cancer 2015, 24, 401–408. [Google Scholar] [CrossRef]
- Ratjen, I.; Schafmayer, C.; Enderle, J.; Giuseppe, R.; Waniek, S.; Koch, M.; Burmeister, G.; Nöthlings, U.; Hampe, J.; Schlesinger, S.; et al. Health-related quality of life in long-term survivors of colorectal cancer and its association with all-cause mortality: A German cohort study. BMC Cancer 2018, 18, 1156. [Google Scholar] [CrossRef]
- Maisey, N.R.; Norman, A.; Watson, M.; Allen, M.J.; Hill, M.E.; Cunningham, D. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur. J. Cancer 2002, 38, 1351–1357. [Google Scholar] [CrossRef]
- Efficace, F.; Bottomley, A.; Coens, C.; Van Steen, K.; Conroy, T.; Schöffski, P.; Schmoll, H.; Van Cutsem, E.; Köhne, C.-H. Does a patient’s self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer? Eur. J. Cancer 2006, 42, 42–49. [Google Scholar] [CrossRef]
- Sanjida, S.; Janda, M.; Kissane, D.; Shaw, J.; Pearson, S.-A.; Disipio, T.; Couper, J. A systematic review and meta-analysis of prescribing practices of antidepressants in cancer patients. Psycho-Oncology 2016, 25, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Koroukian, S.M.; Sajatovic, M. Increased cancer-specific mortality in individuals developing mental disorders after cancer diagnosis: Biomedical factors versus psychosocial support. Ann. Transl. Med. 2017, 5, 432. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Bayonas, A.; Jimenez-Fonseca, P.; Fernández-Somoano, A.; Álvarez-Manceñido, F.; Castañón, E.; Custodio, A.; De La Peña, F.A.; Payo, R.M.; Valiente, L.P. Top ten errors of statistical analysis in observational studies for cancer research. Clin. Transl. Oncol. 2017, 20, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Manson, J.E.; Hankinson, S.E. The Nurses’ Health Study: 20-Year Contribution to the Understanding of Health Among Women. J. Women’s Health 1997, 6, 49–62. [Google Scholar] [CrossRef]
- Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Giovannucci, E.; Willett, W.C. Effectiveness of various mailing strategies among nonrespondents in a prospective cohort study. Am. J. Epidemiol. 1990, 131, 1068–1071. [Google Scholar] [CrossRef]
- Tsai, A.C.; Lucas, M.; Sania, A.; Kim, D.; Kawachi, I. Social Integration and Suicide Mortality Among Men: 24-Year Cohort Study of U.S. Health Professionals. Ann. Intern. Med. 2014, 161, 85. [Google Scholar] [CrossRef]
- Trudel-Fitzgerald, C.; Zhou, E.S.; Poole, E.M.; Zhang, X.; Michels, K.B.; Eliassen, A.H.; Chen, W.Y.; Holmes, M.D.; Tworoger, S.S.; Schernhammer, E.S. Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses’ Health Study. Br. J. Cancer 2017, 116, 1239–1246. [Google Scholar] [CrossRef]
- Kroenke, C.; Kubzansky, L.D.; Schernhammer, E.S.; Holmes, M.D.; Kawachi, I. Social Networks, Social Support, and Survival after Breast Cancer Diagnosis. J. Clin. Oncol. 2006, 24, 1105–1111. [Google Scholar] [CrossRef]
- Snowden, M.B.; Dhingra, S.S.; Keyes, C.L.M.; Anderson, L.A. Changes in Mental Well-Being in the Transition to Late Life: Findings from MIDUS I and II. Am. J. Public Health 2010, 100, 2385–2388. [Google Scholar] [CrossRef]
- Jones, S.M.W.; Lacroix, A.Z.; Li, W.; Zaslavsky, O.; Wassertheil-Smoller, S.; Weitlauf, J.; Brenes, G.A.; Nassir, R.; Ockene, J.K.; Caire-Juvera, G.; et al. Depression and quality of life before and after breast cancer diagnosis in older women from the Women’s Health Initiative. J. Cancer Surviv. 2015, 9, 620–629. [Google Scholar] [CrossRef]
- Crown, S.; Crisp, A.H. A Short Clinical Diagnostic Self-rating Scale for Psychoneurotic Patients. Br. J. Psychiatry 1966, 112, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.M.; Malmgren, J.A.; Carter, W.B.; Patrick, D.L. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am. J. Prev. Med. 1994, 10, 77–84. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Burnam, M.A.; Wells, K.B.; Leake, B.; Landsverk, J. Development of a Brief Screening Instrument for Detecting Depressive Disorders. Med. Care 1988, 26, 775–789. [Google Scholar] [CrossRef]
- Chang, S.-C.; Wang, W.; Pan, A.; Jones, R.N.; Kawachi, I.; Okereke, O.I. Racial Variation in Depression Risk Factors and Symptom Trajectories among Older Women. Am. J. Geriatr. Psychiatry 2016, 24, 1051–1062. [Google Scholar] [CrossRef]
- Luijendijk, H.J.; Berg, J.F.V.D.; Dekker, M.J.H.J.; Van Tuijl, H.R.; Otte, W.; Smit, F.; Hofman, A.; Stricker, B.H.C.; Tiemeier, H. Incidence and Recurrence of Late-Life Depression. Arch. Gen. Psychiatry 2008, 65, 1394–1401. [Google Scholar] [CrossRef]
- Hamer, M.; Chida, Y.; Molloy, G.J. Psychological distress and cancer mortality. J. Psychosom. Res. 2009, 66, 255–258. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Corsano, K.A.; Stampfer, M.J. Test of the National Death Index and Equifax Nationwide Death Search. Am. J. Epidemiol. 1994, 140, 1016–1019. [Google Scholar] [CrossRef]
- Tufanaru, C.; Munn, Z.; Stephenson, M.; Aromataris, E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int. J. Evid. Based Health 2015, 13, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Banack, H.R.; Kaufman, J.S.; Wactawski-Wende, J.; Troen, B.R.; Stovitz, S.D. Investigating and Remediating Selection Bias in Geriatrics Research: The Selection Bias Toolkit. J. Am. Geriatr. Soc. 2019, 67, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Baillargeon, J.; Kuo, Y.-F.; Lin, Y.-L.; Raji, M.A.; Singh, A.; Goodwin, J.S. Effect of Mental Disorders on Diagnosis, Treatment, and Survival of Older Adults with Colon Cancer. J. Am. Geriatr. Soc. 2011, 59, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Trudel-Fitzgerald, C.; Tworoger, S.; Poole, E.M.; Zhang, L.; Giovannucci, E.L.; Meyerhardt, J.A.; Kubzansky, L.D. Psychological symptoms and subsequent healthy lifestyle after a colorectal cancer diagnosis. Health Psychol. 2018, 37, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Giovannucci, E. Preventable incidence and mortality of carcinoma associated with lifestyle factors among White adults in the United States. JAMA Oncol. 2016, 2, 1154–1161. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Andersen, B.L. Biobehavioral approaches to cancer progression and survival: Mechanisms and interventions. Am. Psychol. 2015, 70, 186–197. [Google Scholar] [CrossRef]
- Trudel-Fitzgerald, C.; Qureshi, F.; Appleton, A.; Kubzansky, L.D. A healthy mix of emotions: Underlying biological pathways linking emotions to physical health. Curr. Opin. Behav. Sci. 2017, 15, 16–21. [Google Scholar] [CrossRef]
- McDonald, P.G.; O’Connell, M.; Lutgendorf, S.K. Psychoneuroimmunology and cancer: A decade of discovery, paradigm shifts, and methodological innovations. Brain Behav. Immun. 2013, 30, S1–S9. [Google Scholar] [CrossRef]
- Bao, Y.; Bertoia, M.L.; Lenart, E.B.; Stampfer, M.J.; Willett, W.C.; Speizer, F.E.; Chavarro, J.E. Origin, Methods, and Evolution of the Three Nurses’ Health Studies. Am. J. Public Health 2016, 106, 1573–1581. [Google Scholar] [CrossRef]
- Mosher, C.E.; Winger, J.G.; Given, B.A.; Helft, P.R.; O’Neil, B.H. Mental health outcomes during colorectal cancer survivorship: A review of the literature. Psycho-Oncology 2015, 25, 1261–1270. [Google Scholar] [CrossRef]
- Trudel-Fitzgerald, C.; Savard, J.; Ivers, H. Evolution of Cancer-Related Symptoms Over an 18-Month Period. J. Pain Symptom Manag. 2013, 45, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.W.; Levy, A.R.; Rosberger, Z.; Edgar, L. Psychological Distress and Cancer Survival. Psychosom. Med. 2003, 65, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Trudel-Fitzgerald, C.; Poole, E.M.; Sawyer, S.; Kubzansky, L.D.; Hankinson, S.E.; Okereke, O.I.; Tworoger, S.S. The Mind–Body Study: Study design and reproducibility and interrelationships of psychosocial factors in the Nurses’ Health Study II. Cancer Causes Control. 2019, 30, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Hoffmeister, M.; Arndt, V.; Haug, U. Response: Re: Protection from Right- and Left-Sided Colorectal Neoplasms After Colonoscopy: Population-Based Study. J. Natl. Cancer Inst. 2010, 102, 990–991. [Google Scholar] [CrossRef][Green Version]
- Lai, S.-M.; Jungk, J.; Garimella, S. Colorectal Cancer Identification Methods Among Kansas Medicare Beneficiaries, 2008–2010. Prev. Chronic Dis. 2015, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- El-Shami, K.; Oeffinger, K.C.; Erb, N.L.; Willis, A.; Bretsch, J.K.; Pratt-Chapman, M.L.; Cannady, R.S.; Wong, S.L.; Rose, J.; Barbour, A.L.; et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA A Cancer J. Clin. 2015, 65, 427–455. [Google Scholar] [CrossRef]

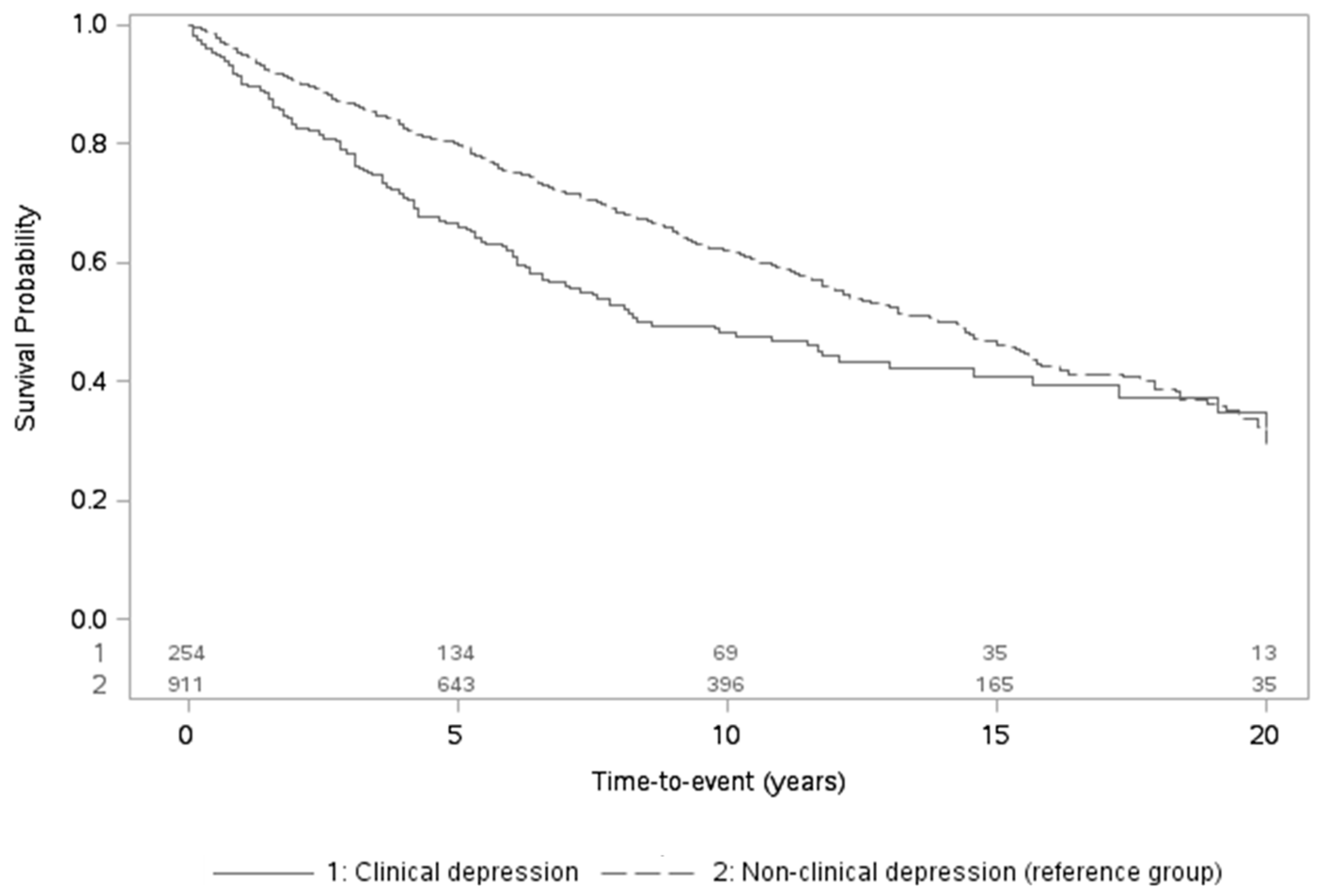
| Clinical Anxiety Levels | Clinical Depression Levels | |||
|---|---|---|---|---|
| No (n = 238) | Yes (n = 97) | No (n = 686) | Yes (n = 207) | |
| Age, mean (SD) § | 74.9 (7.0) | 73.8 (6.1) | 71.7 (7.4) * | 73.1 (7.9) * |
| Married/partnered, % | 60.4 | 63.4 | 64.4 * | 57.1 * |
| Registered nurses degree education level, % | 70.6 | 80.5 | 73.7 | 75.0 |
| Census tract income, mean (SD) | 62,023.1 (21,295.4) | 59,505.9 (21,061.5) | 63,415.4 (22,800.3) | 61,711.3 (22,874.2) |
| Prevalent cardiometabolic disease, % §§ | 25.9 * | 46.1 * | 24.0 * | 41.1 * |
| Lifestyle score, mean (SD) §§§ | 2.4 (1.1) | 2.3 (1.1) | 2.3 (1.1) * | 2.0 (1.0) * |
| Age at diagnosis, mean (SD) | 72.5 (7.0) | 72.7 (6.5) | 70.0 (7.5) | 70.4 (7.9) |
| Proximal tumor location | 40.6 | 39.8 | 48.4 | 41.8 |
| Advanced cancer (stages III-IV), % §§§§ | 30.7 | 28.2 | 32.3 | 35.1 |
| Time in years between diagnosis and analytic baseline, median (interquartile range) | 2.2 (1.1–3.2) | 2.0 (0.9–2.8) | 1.9 (1.0–3.1) | 1.8 (0.9–2.6) |
| Clinical Anxiety Levels | Clinical Depression Levels | |||
|---|---|---|---|---|
| No (n = 181) | Yes (n = 51) | No (n = 225) | Yes (n = 47) | |
| Age, mean (SD) § | 70.9 (9.9) | 72.2 (9.8) | 74.5 (8.7) | 77.0 (8.8) |
| Married/partnered, % | 90.5 | 94.4 | 87.5 | 79.2 |
| Profession | ||||
| Dentist, % | 56.8 | 64.5 | 63.1 | 54.8 |
| Osteopath, % | 5.5 | 1.3 | 4.9 | 6.5 |
| Pharmacist, % | 10.2 | 11.2 | 7.0 | 11.9 |
| Veterinarian, % | 15.4 | 11.0 | 17.3 | 22.1 |
| Other (optometrist or podiatrist), % | 12.0 | 12.1 | 7.6 | 4.7 |
| Prevalent cardiometabolic disease, % §§ | 27.3 | 34.9 | 30.2 | 40.3 |
| Lifestyle score, mean (SD) §§§ | 2.5 (1.1) | 2.5 (1.1) | 2.5 (1.1) * | 2.1 (1.2) * |
| Age at diagnosis, mean (SD) | 69.1 (9.5) | 68.6 (10.1) | 72.3 (8.8) | 72.5 (8.7) |
| Proximal tumor location | 63.9 | 56.2 | 66.3 | 59.7 |
| Advanced cancer (stages III-IV), % §§§§ | 30.3 | 32.9 | 31.5 | 40.7 |
| Time in years between diagnosis and analytic baseline, median (interquartile range) | 1.4 (0.8–2.6) | 1.3 (0.8–2.4) | 2.3 (1.1–3.0) | 2.3 (1.3–3.0) |
| Anxiety | p-Value for Heterogeneity | Depression§ | p-Value for Heterogeneity | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Sample Size (Number of Deaths/Person-Years) | Sample Size (Number of Deaths/Person-Years) | |||||
| Continuous distress level (standardized; per 1-SD) | n = 567 (270/4667) | n = 1009 (458/8718) | ||||
| Model 1 | 1.17 ** | 1.06–1.30 | 0.63 | 1.20 **** | 1.11–1.31 | 0.003 |
| Model 2 | 1.17 ** | 1.05–1.30 | 0.90 | 1.17 **** | 1.08–1.27 | 0.002 |
| Model 3 | 1.16 ** | 1.05–1.29 | 0.92 | 1.16 *** | 1.07–1.26 | 0.003 |
| Dichotomized distress level (clinical versus non-clinical) | Clinical level: n = 148 (82/1261) Non-clinical level: n = 419 (188/3406) | Clinical level: n = 254 (128/1814) Non-clinical level: n = 911 (416/8223) | ||||
| Model 1 | 1.22 | 0.96–1.55 | 0.29 | 1.38 *** | 1.14–1.66 | 0.25 |
| Model 2 | 1.17 | 0.91–1.49 | 0.45 | 1.31 ** | 1.08–1.59 | 0.12 |
| Model 3 | 1.17 | 0.92–1.50 | 0.47 | 1.28 ** | 1.06–1.56 | 0.14 |
| Anxiety | Depression § | |||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Sample Size (Number of Deaths/Person-Years) | Sample Size (Number of Deaths/Person-Years) | |||
| Women (NHS) | ||||
| Continuous distress levels (standardized; per 1-SD) | n = 335 (122/2346) | n = 887 (412/8090) | ||
| Model 1 | 1.21 ** | 1.04–1.40 | 1.15 *** | 1.06–1.26 |
| Model 2 | 1.17 * | 1.01–1.37 | 1.12 ** | 1.03–1.22 |
| Model 3 | 1.17 * | 1.00–1.36 | 1.12 ** | 1.02–1.22 |
| Dichotomized distress levels (clinical versus non-clinical) | Clinical level: n = 97 (45/743) Non-clinical level: n = 238 (77/1603) | Clinical level: n = 207 (103/1573) Non-clinical level: n = 686 (313/6551) | ||
| Model 1 | 1.37 † | 0.99–1.90 | 1.30 * | 1.05–1.61 |
| Model 2 | 1.28 | 0.91–1.79 | 1.22 † | 0.98–1.51 |
| Model 3 | 1.28 | 0.91–1.80 | 1.19 | 0.96–1.48 |
| Men (HPFS) | ||||
| Continuous distress levels (standardized; per 1-SD) | n = 232 (148/2321) | n = 122 (46/628) | ||
| Model 1 | 1.15 † | 0.99–1.32 | 1.70 **** | 1.34–2.17 |
| Model 2 | 1.16 * | 1.00–1.34 | 1.70 **** | 1.33–2.19 |
| Model 3 | 1.16 * | 1.00–1.34 | 1.68 **** | 1.30–2.18 |
| Dichotomized distress levels (clinical versus non-clinical) | Clinical levels: n = 51 (37/518) Non-clinical level: n = 181 (111/1803) | Clinical: n = 47 (25/241) Non-clinical level: n = 225 (103/1672) | ||
| Model 1 | 1.06 | 0.74–1.51 | 1.72 ** | 1.13–2.62 |
| Model 2 | 1.06 | 0.74–1.51 | 1.77 ** | 1.16–2.71 |
| Model 3 | 1.07 | 0.74–1.53 | 1.72 ** | 1.11–2.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trudel-Fitzgerald, C.; Tworoger, S.S.; Zhang, X.; Giovannucci, E.L.; Meyerhardt, J.A.; Kubzansky, L.D. Anxiety, Depression, and Colorectal Cancer Survival: Results from Two Prospective Cohorts. J. Clin. Med. 2020, 9, 3174. https://doi.org/10.3390/jcm9103174
Trudel-Fitzgerald C, Tworoger SS, Zhang X, Giovannucci EL, Meyerhardt JA, Kubzansky LD. Anxiety, Depression, and Colorectal Cancer Survival: Results from Two Prospective Cohorts. Journal of Clinical Medicine. 2020; 9(10):3174. https://doi.org/10.3390/jcm9103174
Chicago/Turabian StyleTrudel-Fitzgerald, Claudia, Shelley S. Tworoger, Xuehong Zhang, Edward L. Giovannucci, Jeffrey A. Meyerhardt, and Laura D. Kubzansky. 2020. "Anxiety, Depression, and Colorectal Cancer Survival: Results from Two Prospective Cohorts" Journal of Clinical Medicine 9, no. 10: 3174. https://doi.org/10.3390/jcm9103174
APA StyleTrudel-Fitzgerald, C., Tworoger, S. S., Zhang, X., Giovannucci, E. L., Meyerhardt, J. A., & Kubzansky, L. D. (2020). Anxiety, Depression, and Colorectal Cancer Survival: Results from Two Prospective Cohorts. Journal of Clinical Medicine, 9(10), 3174. https://doi.org/10.3390/jcm9103174






