CaRE @ Home: Pilot Study of an Online Multidimensional Cancer Rehabilitation and Exercise Program for Cancer Survivors
Abstract
1. Introduction
2. Methods
2.1. Intervention
- Exercise prescription, Physitrack® and Fitbit™: Prior to beginning the program, participants underwent an in-person fitness assessment conducted by a registered kinesiologist (RKin). Based on this assessment, an individualized exercise prescription was developed and progressed towards the ACSM guidelines of 150 min per week of moderate intensity aerobic exercise, 2 to 3 days of resistance training, and routine large muscle group flexibility training [42,43]. (Updated guidelines were published in 2019.) The individualized exercise prescription was then entered by the RKin into Physitrack®, an online platform that allows customizable exercise prescriptions, tracking of exercise completion, and video tutorials to support the prescribed exercises. In addition, participants were provided with a Fitbit™ device, which serves as a self-monitoring tool and provides further assistance to participants in adhering to recommended physical activity routines and tracking physical activity. Data from the device was also used as a weekly monitoring tool by the health care professionals. The participants were provided with an orientation to the Physitrack® application and Fitbit™ device during their initial in-person fitness assessment.
- E-Learning Modules: Participants were registered into an online learning platform and completed 8 comprehensive self-management e-modules. Modules were locked, meaning they had to complete the prior week before unlocking the next, and took approximately 20–45 min to complete. These modules included: Week 1: Getting Started—Set Your Goals; Week 2: Eat and Cook for Wellness; Week 3: Reduce Fatigue; Week 4: Manage your Emotions; Week 5: Be Mindful; Week 6: Improving Cancer-related Brain Fog and Boosting Your Brain Health; Week 7: Find Ways to Connect and Week 8: Plan for the Future. The topics chosen were based on patient-reported needs and commonly presenting symptoms. The content was developed by a multidisciplinary cancer rehabilitation team, including occupational therapy, physiotherapy, dietetics, neurocognitive psychology, social work, kinesiology, physiatry, and also included behavioral scientists. The modules included health literate design features such as linear navigation, intuitive buttons, and plain language and underwent two rounds of usability testing prior to the launch of the program. Attention was placed on behavior change techniques, ensuring that the interactive elements strengthened any behavior change goals.
- Telephone Health coaching: Participants received one weekly health coaching call with the same health coach in weeks 2–7 (6 total) plus one maintenance call at 1 month and one last call at 2 months post-intervention. Calls were scheduled for 20 min. Coaches were either a RKin or social worker trained in behavioral change and utilizing motivational interviewing skills. They guided participants to reflect upon their last week, plan goals for the week ahead, and identify barriers and solutions in achieving their goals and incorporated assessment and enhancement of motivation, promotion of self-efficacy, and collaborative problem solving. The objective was to enhance sustained behavior change by integrating several active ingredients outlined in the cancer-survivor behavior change literature [44,45] and motivational interviewing (MI) [36]. MI is a collaborative counseling method that elicits and strengthens motivation for change by addressing and resolving ambivalence [46] and has been shown to be effective in increasing physical activity in cancer survivors and those with other chronic conditions [47,48,49,50,51,52]. Health coaches were trained in motivational interviewing and supervised by a certified Motivational Interviewing Network Trainer who was part of the team (MO).
2.2. Participants and Procedure
2.3. Study Outcomes
2.3.1. Demographic and Clinical Data
2.3.2. Feasibility and Acceptability
2.3.3. Exploratory Clinical Outcomes
- Disability was measured using the 12-item World Health Organization’s Disability Assessment Schedule 2.0 (WHO-DAS 2.0) [53,54]. Respondents rate their difficulty in engaging in particular activities on a scale from “none” (no difficulty) to “extreme or cannot do” on six domains of functioning. Scores range from 12 to 60, where higher scores indicate higher disability or loss of function. A WHODAS score of 0–4 is defined as none to mild disability and 5–48 is defined as moderate/high.
- Symptom Severity was measured with the widely used Edmonton Symptom Assessment Schedule revised (ESAS-r) [55,56]. The ESAS-r includes a simple 0–10 severity rating scale (0 being ‘symptom is absent’ and 10 being ‘worst possible severity’) for nine symptoms common in cancer patients: pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, wellbeing, and shortness of breath.
- Physical Activity was measured using the Godin-Shephard Leisure-Time Physical Activity Questionnaire (GSLTPAQ) [57]. The GSLTPAQ is a 3-item questionnaire that includes three questions on the number of times respondents engage in mild, moderate and strenuous leisure time physical activity (LTPA) bouts of at least 15 min duration in a typical week. Participants also report on the average minutes of each session for each intensity level. Examples of LTPA for each intensity category are provided. A Leisure Score Index (LSI) is calculated by taking the number of bouts at each intensity and multiplying by 3, 5, and 9 metabolic equivalents (METs) and then summing the score. In addition, moderate, and strenuous LTPA can be summed and used to categorize respondents as active and insufficiently active based on physical activity guidelines for cancer survivors.
- Work Function was assessed using two items from the iMTA Productivity Cost Questionnaire (iPCQ). Absenteeism was measured in numerical hours by asking participants: “During the past seven days, how many hours did you miss from work because of problems associated with cancer diagnosis and treatment?” Productivity was assessed by asking participants: “During the past seven days, how much did your cancer diagnosis and treatment affect your productivity while you were working?” with answers measured on a 0 to 10 scale, with 0 = problem had no effect on my work and 10 = problem completely prevented me from working [58].
2.4. Data Analysis
2.4.1. Feasibility and Acceptability
2.4.2. Exploratory Clinical Outcomes
3. Results
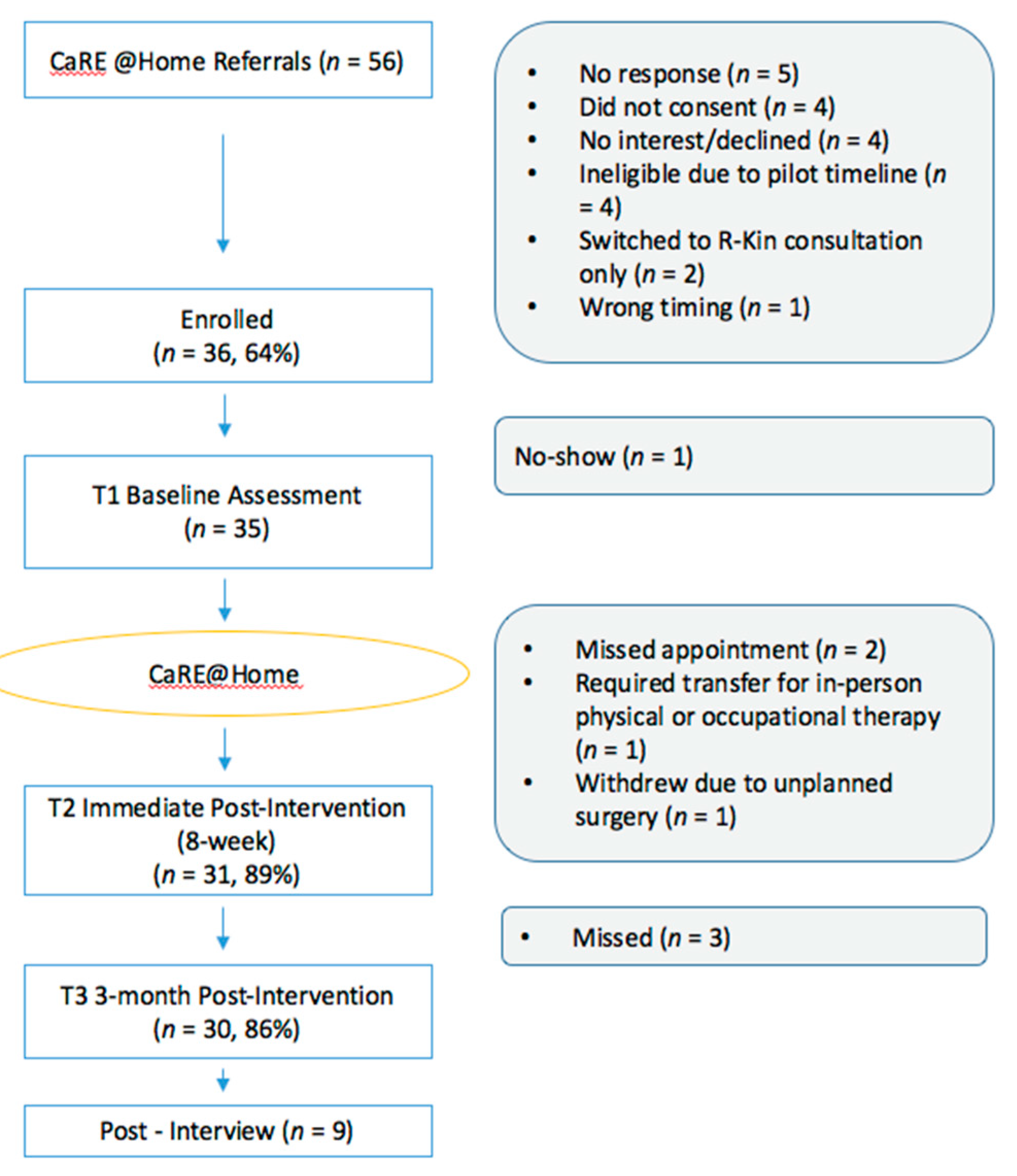
3.1. Feasibility
3.2. Acceptability
3.2.1. Overall Satisfaction with CaRE@Home Program
3.2.2. Benefits of the Program
3.2.3. Areas for Program Improvement
3.3. Exploratory Clinical Outcomes
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.-S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef]
- Siegel, R.L.; Mph, K.D.M.; Jemal, A. Cancer statistics, 2017. CA A Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.E.; Forsythe, L.P.; Reeve, B.B.; Alfano, C.M.; Rodriguez, J.L.; Sabatino, S.A.; Hawkins, N.A.; Rowland, J.H. Mental and Physical Health-Related Quality of Life among U.S. Cancer Survivors: Population Estimates from the 2010 National Health Interview Survey. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2108–2117. [Google Scholar] [CrossRef]
- Alfano, C.M.; Rowland, J.H. Recovery issues in cancer survivorship: A new challenge for supportive care. Cancer J. 2006, 12, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.; Syrjala, K.; Andrykowski, M. Physical and psychological long-term and late effects of cancer. Cancer 2008, 112 (Suppl. S11), 2577–2592. [Google Scholar] [CrossRef]
- Harrington, C.; Hansen, J.; Moskowitz, M.; Todd, B.; Feuerstein, M. It’s not over when it’s over: Long-term symptoms in cancer survivors—A systematic review. Int. J. Psychiatry Med. 2010, 40, 163–181. [Google Scholar] [CrossRef]
- Bernstein, L.J.; McCreath, G.A.; Komeylian, Z.; Rich, J.B. Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: A multilevel meta-analysis. Neurosci. Biobehav. Rev. 2017, 83, 417–428. [Google Scholar] [CrossRef]
- Jones, J.M.; Olson, K.; Catton, P.; Catton, C.N.; Fleshner, N.E.; Krzyzanowska, M.K.; McCready, D.R.; Wong, R.K.S.; Jiang, H.; Howell, D. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J. Cancer Surviv. 2015, 10, 51–61. [Google Scholar] [CrossRef]
- Mewes, J.C.; Steuten, L.M.G.; Ijzerman, M.; Van Harten, W.H. Effectiveness of Multidimensional Cancer Survivor Rehabilitation and Cost-Effectiveness of Cancer Rehabilitation in General: A Systematic Review. Oncologist 2012, 17, 1581–1593. [Google Scholar] [CrossRef]
- Thorsen, L.; Gjerset, G.M.; Loge, J.H.; Kiserud, C.E.; Skovlund, E.; Fløtten, T.; Fosså, S.D. Cancer patients’ needs for rehabilitation services. Acta Oncol. 2011, 50, 212–222. [Google Scholar] [CrossRef]
- Silver, J.K.; Baima, J.; Mayer, R.S. Impairment-driven cancer rehabilitation: An essential component of quality care and survivorship. CA Cancer J. Clin. 2013, 63, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Cheville, A. Adjunctive Rehabilitation Approaches to Oncology; Elsevier: Philadelphia, PA, USA, 2017; Volume 28, pp. 1–215. [Google Scholar]
- Alfano, C.; Cheville, A.; Mustian, K. Developing High-Quality Cancer Rehabilitation Programs: A Timely Need. Am. Soc. Clin. Oncol. Educ. 2018, 36, 241–249. [Google Scholar]
- Scott, D.; Mills, M.; Black, A.; Cantwell, M.; Campbell, A.; Cardwell, C.R.; Porter, S.; Donnelly, M. Multidimensional rehabilitation programmes for adult cancer survivors. Cochrane Database Syst. Rev. 2013, 2013, CD007730. [Google Scholar] [CrossRef] [PubMed]
- Buffart, L.; Kalter, J.; Sweegers, M.G.; Courneya, K.; Newton, R.U.; Aaronson, N.; Jacobsen, P.B.; May, A.M.; Galvão, D.A.; Chinapaw, M.J.M.; et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.N.; McAuley, E.; Trinh, L. Physical activity programming and counseling preferences among cancer survivors: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, A.S.; Hefner, J.E.; Kodish-Wachs, J.E.; Iaccarino, M.A.; Paganoni, S. Telehealth in Physical Medicine and Rehabilitation: A Narrative Review. PMR 2017, 9, S51–S58. [Google Scholar]
- Hardcastle, S.J.; Maxwell-Smith, C.; Kamarova, S.; Lamb, S.; Millar, L.; Cohen, P.A. Factors influencing non-participation in an exercise program and attitudes towards physical activity amongst cancer survivors. Support. Care Cancer 2017, 26, 1289–1295. [Google Scholar] [CrossRef]
- Dalleck, L.C.; Schmidt, L.K.; Lueker, R. Cardiac rehabilitation outcomes in a conventional versus telemedicine-based programme. J. Telemed. Telecare 2011, 17, 217–221. [Google Scholar] [CrossRef]
- Wootton, R. Twenty years of telemedicine in chronic disease management—An evidence synthesis. J. Telemed. Telecare 2012, 18, 211–220. [Google Scholar] [CrossRef]
- Hanlon, P.; Daines, L.; Campbell, C.; McKinstry, B.; Weller, D.; Pinnock, H.; Kaufman, N.; Moy, M.; McLean, S.; Holtz, B. Telehealth Interventions to Support Self-Management of Long-Term Conditions: A Systematic Metareview of Diabetes, Heart Failure, Asthma, Chronic Obstructive Pulmonary Disease, and Cancer. J. Med. Internet Res. 2017, 19, e172. [Google Scholar] [CrossRef]
- Ekeland, A.G.; Bowes, A.; Flottorp, S. Effectiveness of telemedicine: A systematic review of reviews. Int. J. Med. Inform. 2010, 79, 736–771. [Google Scholar] [CrossRef] [PubMed]
- Peretti, A.; Amenta, F.; Tayebati, S.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the State-of-the-Art and Areas of Application. JMIR Rehabil. Assist. Technol. 2017, 4, e7. [Google Scholar] [CrossRef] [PubMed]
- Odeh, S.; Abu Shanab, S.; Anabtawi, M. Augmented Reality Internet Labs versus its Traditional and Virtual Equivalence. Int. J. Emerg. Technol. Learn. (iJET) 2015, 10, 4–9. [Google Scholar] [CrossRef]
- Dorri, S.; Asadi, F.; Olfatbakhsh, A.; Kazemi, A. A Systematic Review of Electronic Health (eHealth) interventions to improve physical activity in patients with breast cancer. Breast Cancer 2019, 27, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.; Kelly, G.; Haberlin, C.; Mockler, D.; Broderick, J. The use of eHealth to promote physical activity in people with mental health conditions: A systematic review [version 3; peer review: 3 approved] Previously titled: The use of eHealth to promote physical activity in patients with mental health conditions: A systematic review. HRB Open Res. 2018, 1, 5. [Google Scholar]
- Agboola, S.; Ju, W.; ElFiky, A.; Kvedar, J.C.; Jethwani, K.; Badger, T.; Nelson, E. The Effect of Technology-Based Interventions on Pain, Depression, and Quality of Life in Patients with Cancer: A Systematic Review of Randomized Controlled Trials. J. Med. Internet Res. 2015, 17, e65. [Google Scholar] [CrossRef]
- Seiler, A.; Klaas, V.; Tröster, G.; Fagundes, C.P. eHealth and mHealth interventions in the treatment of fatigued cancer survivors: A systematic review and meta-analysis. Psycho-Oncology 2017, 26, 1239–1253. [Google Scholar] [CrossRef]
- Harris, D.J.; Buckingham, G.; Wilson, M.R.; Vine, S.J. Virtually the same? How impaired sensory information in virtual reality may disrupt vision for action. Exp. Brain Res. 2019, 237, 2761–2766. [Google Scholar] [CrossRef]
- Fridriksdottir, N.; Gunnarsdottir, S.; Zoëga, S.; Ingadottir, B.; Hafsteinsdottir, E.J.G. Effects of web-based interventions on cancer patients’ symptoms: Review of randomized trials. Support. Care Cancer 2017, 26, 337–351. [Google Scholar] [CrossRef]
- Post, K.E.; Flanagan, J. Web based survivorship interventions for women with breast cancer: An integrative review. Eur. J. Oncol. Nurs. 2016, 25, 90–99. [Google Scholar] [CrossRef]
- Bray, V.J.; Dhillon, H.M.; Bell, M.L.; Kabourakis, M.; Fiero, M.H.; Yip, D.; Boyle, F.; Price, M.A.; Vardy, J. Evaluation of a Web-Based Cognitive Rehabilitation Program in Cancer Survivors Reporting Cognitive Symptoms After Chemotherapy. J. Clin. Oncol. 2017, 35, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Cheville, A.L.; Moynihan, T.; Herrin, J.; Loprinzi, C.; Kroenke, K. Effect of Collaborative Telerehabilitation on Functional Impairment and Pain Among Patients with Advanced-Stage Cancer: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Bernstein, L.; Chafranskaia, A.; Chang, E.; Langelier, D.; Hospod, A.-M.; Lopez, C.; Maganti, M.; Tan, V.; Mina, D.S. Cancer Rehabilitation and Exercise (CaRE): A multicomponent rehabilitation intervention for cancer survivors. J. Psychosoc. Oncol. Res. Pract. 2020, abstract in press. [Google Scholar]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The Behavior Change Technique Taxonomy (v1) of 93 Hierarchically Clustered Techniques: Building an International Consensus for the Reporting of Behavior Change Interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Rollnick, S.; Miller, W.; Butler, C. Motivational Interviewing in Health Care: Helping Patients Change Behavior; Guilford Press: New York, NY, USA, 2007. [Google Scholar]
- Brewin, C. Theoretical foundations of cognitive-behavioral therapy for anxiety and depression. Annu. Rev. Psychol. 1996, 47, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Ajzen, I. The Theory of Planned Behavior. Organ. Behav. Hum. Decis. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Marlatt, G.A.; Gordon, J.R. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors; Guilford Press: New York, NY, USA, 1985. [Google Scholar]
- Parks, G.A.; Anderson, B.K.; Marlatt, G.A. Relapse prevention therapy. In The Handbook of Alcohol Dependence and Problems; Heather, N., Peter, T., Stockwell, T., Eds.; John Wiley & Sons, Ltd.: Sussex, UK, 2001. [Google Scholar]
- Schmitz, K.; Courneya, K.; Matthews, C.E.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Abraham, C.; Michie, S. A taxonomy of behavior change techniques used in interventions. Heal. Psychol. 2008, 27, 379–387. [Google Scholar] [CrossRef]
- Michie, S.; Ashford, S.; Sniehotta, F.F.; Dombrowski, S.U.; Bishop, A.; French, D.P.; Williams, S.L. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychol. Health 2011, 26, 1479–1498. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Rollnick, S. Ten Things that Motivational Interviewing Is Not. Behav. Cogn. Psychother. 2009, 37, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.M.; Frierson, G.M.; Rabin, C.; Trunzo, J.J.; Marcus, B.H. Home-Based Physical Activity Intervention for Breast Cancer Patients. J. Clin. Oncol. 2005, 23, 3577–3587. [Google Scholar] [CrossRef]
- Lundahl, B.W.; Kunz, C.; Brownell, C.; Tollefson, D.; Burke, B.L. A Meta-Analysis of Motivational Interviewing: Twenty-Five Years of Empirical Studies. Res. Soc. Work. Pr. 2010, 20, 137–160. [Google Scholar] [CrossRef]
- Sjöling, M.; Lundberg, K.; Englund, E.; Westman, A.; Jong, M.C. Effectiveness of motivational interviewing and physical activity on prescription on leisure exercise time in subjects suffering from mild to moderate hypertension. BMC Res. Notes 2011, 4, 352. [Google Scholar] [CrossRef]
- Burke, B.L.; Arkowitz, H.; Menchola, M. The efficacy of motivational interviewing: A meta-analysis of controlled clinical trials. J. Consult. Clin. Psychol. 2003, 71, 843–861. [Google Scholar] [CrossRef]
- Brodie, D.A.; Inoue, A. Motivational interviewing to promote physical activity for people with chronic heart failure. J. Adv. Nurs. 2005, 50, 518–527. [Google Scholar] [CrossRef]
- Gallè, F.; Di Onofrio, V.; Miele, A.; Belfiore, P.; Liguori, G. Effects of a community-based exercise and motivational intervention on physical fitness of subjects with type 2 diabetes. Eur. J. Public Health 2018, 29, 281–286. [Google Scholar] [CrossRef]
- Üstün, T.B.; Chatterji, S.; Kostanjsek, N.; Rehm, J.; Kennedy, C.; Epping-Jordan, J.; Saxena, S.; Von Korff, M.; Pull, C. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull. World Health Organ. 2010, 88, 815–823. [Google Scholar] [CrossRef]
- Devis, J.V.L.; Ayuso-Mateos, J.L.; Aguado, J.; Fernández, A.; Serrano-Blanco, A.; Roca, M.; Haro, J.M. The 12-item World Health Organization Disability Assessment Schedule II (WHO-DAS II): A nonparametric item response analysis. BMC Med. Res. Methodol. 2010, 10, 45. [Google Scholar]
- Bruera, E.; Kuehn, N.; Miller, M.J.; Selmser, P.; Macmillan, K. The Edmonton Symptom Assessment System (ESAS): A Simple Method for the Assessment of Palliative Care Patients. J. Palliat. Care 1991, 7, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.; Watanabe, S.; Taube, A. The Edmonton Staging System. J. Pain Symptom. Manag. 1996, 12, 269. [Google Scholar] [CrossRef]
- Godin, G. The Godin-Shephard leisure-time physical activity questionnaire. Health Fit. J. Can. 2001, 4, 18–22. [Google Scholar]
- Bouwmans, C.; Krol, M.; Severens, H.; Koopmanschap, M.; Brouwer, W.; Roijen, L.H.-V. The iMTA Productivity Cost Questionnaire. Value Health 2015, 18, 753–758. [Google Scholar] [CrossRef]
- Brooks, D.; Solway, S.; Gibbons, W.J. ATS statement on six-minute walk test. Am. J. Respir. Crit. Care Med. 2003, 167, 1287. [Google Scholar] [CrossRef]
- Chen, B.P.; Awasthi, R.; Sweet, S.N.; Minnella, E.M.; Bergdahl, A.; Carli, F.; Scheede-Bergdahl, C.; Mina, D.S. Four-week prehabilitation program is sufficient to modify exercise behaviors and improve preoperative functional walking capacity in patients with colorectal cancer. Support. Care Cancer 2016, 25, 33–40. [Google Scholar] [CrossRef]
- Granger, C.L.; Holland, A.; Gordon, I.R.; Denehy, L. Minimal important difference of the 6-minute walk distance in lung cancer. Chronic Respir. Dis. 2015, 12, 146–154. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams and Wikins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Lancaster, G.A.; Dodd, S.; Williamson, P.R. Design and analysis of pilot studies: Recommendations for good practice. J. Eval. Clin. Pract. 2004, 10, 307–312. [Google Scholar] [CrossRef]
- Erlingsson, C.; Brysiewicz, P. A hands-on guide to doing content analysis. Afr. J. Emerg. Med. 2017, 7, 93–99. [Google Scholar] [CrossRef]
- Fewtrell, M.; Kennedy, K.; Singhal, A.; Martin, R.M.; Ness, A.R.; Hadders-Algra, M.; Koletzko, B.; Lucas, A. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch. Dis. Child. 2008, 93, 458–461. [Google Scholar] [CrossRef]
- Neville, L.M.; O’Hara, B.; Milat, A. Computer-tailored physical activity behavior change interventions targeting adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2009, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, S.J.; Galliott, M.; Lynch, B.M.; Nguyen, N.H.; Cohen, P.A.; Mohan, G.R.; Johansen, N.J.; Saunders, C. ‘If I Had Someone Looking Over My Shoulder…’: Exploration of Advice Received and Factors Influencing Physical Activity Among Non-metropolitan Cancer Survivors. Int. J. Behav. Med. 2019, 26, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Blazer, D.; Hoenig, H. Can eHealth Technology Enhance the Patient-Provider Relationship in Rehabilitation? Arch. Phys. Med. Rehabil. 2016, 97, 1403–1406. [Google Scholar] [CrossRef] [PubMed]
- Gell, N.M.; Grover, K.W.; Humble, M.; Sexton, M.; Dittus, K. Efficacy, feasibility, and acceptability of a novel technology-based intervention to support physical activity in cancer survivors. Support. Care Cancer 2016, 25, 1291–1300. [Google Scholar] [CrossRef]
- Rosenberg, R.; Kadokura, E.A.; Bouldin, E.D.; Miyawaki, C.E.; Higano, C.S.; Hartzler, A.L. Acceptability of Fitbit for physical activity tracking within clinical care among men with prostate cancer. AMIA Annu. Symp. Proc. 2017, 2016, 1050–1059. [Google Scholar]
- Vandelanotte, C.; Duncan, M.J.; Maher, C.; Schoeppe, S.; Rebar, A.L.; Power, D.A.; Short, C.E.; Doran, C.M.; Hayman, M.; Alley, S.J.; et al. The Effectiveness of a Web-Based Computer-Tailored Physical Activity Intervention Using Fitbit Activity Trackers: Randomized Trial. J. Med. Internet Res. 2018, 20, e11321. [Google Scholar] [CrossRef]
- Gell, N.M.; Grover, K.W.; Savard, L.; Dittus, K. Outcomes of a text message, Fitbit, and coaching intervention on physical activity maintenance among cancer survivors: A randomized control pilot trial. J. Cancer Surviv. 2019, 14, 80–88. [Google Scholar] [CrossRef]
- Mummah, S.A.; Robinson, T.N.; King, A.C.; Gardner, C.D.; Sutton, S.; Munro, G.; Atienza, A.; Peeples, M. IDEAS (Integrate, Design, Assess, and Share): A Framework and Toolkit of Strategies for the Development of More Effective Digital Interventions to Change Health Behavior. J. Med. Internet Res. 2016, 18, e317. [Google Scholar] [CrossRef]
- Jones, J.M.; Cheng, T.; Jackman, M.; Walton, T.; Haines, S.; Rodin, G.M.; Catton, P. Getting back on track: Evaluation of a brief group psychoeducation intervention for women completing primary treatment for breast cancer. Psycho-Oncology 2011, 22, 117–124. [Google Scholar] [CrossRef]
- Cashman, R.; Bernstein, L.J.; Bilodeau, D.; Bovett, G.; Jackson, B.; Yousefi, M.; Prica, A.; Perry, J. Evaluation of an educational program for the caregivers of persons diagnosed with a malignant glioma. Can. Oncol. Nurs. J. 2007, 17, 6–10. [Google Scholar] [CrossRef]
- Cox, A.; Lucas, G.; Marcu, A.; Piano, M.; Grosvenor, W.; Mold, F.; Maguire, R.; Ream, E.; Pensak, N.A.; Wen, K.-Y. Cancer Survivors’ Experience with Telehealth: A Systematic Review and Thematic Synthesis. J. Med. Internet Res. 2017, 19, e11. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, B.D. Promise of Mobile Health Technology to Reduce Disparities in Patients with Cancer and Survivors. JCO Clin. Cancer Inform. 2018, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Statistics 2008. Available online: http://www.cancer.ca/Canada-wide/About%20cancer/Cancer%20statistics/Canadian%20Cancer%20Statistics.aspx?sc_lang=en (accessed on 22 March 2019).
- Pew Research Center. Mobile Technology and Home Broadband 2019. Available online: https://www.pewresearch.org/internet/2019/06/13/mobile-technology-and-home-broadband-2019/ (accessed on 12 July 2019).
- Nicklin, E.; Velikova, G.; Boele, F. Technology is the future, but who are we leaving behind? Lancet Oncol. 2020, 21, 29. [Google Scholar] [CrossRef]
- Rossen, S.; Kayser, L.; Vibe-Petersen, J.; Ried-Larsen, M.; Christensen, J. Technology in exercise-based cancer rehabilitation: A cross-sectional study of receptiveness and readiness for e-Health utilization in Danish cancer rehabilitation. Acta Oncol. 2019, 58, 610–618. [Google Scholar] [CrossRef]
| Behavior Change Elements | ||
|---|---|---|
| Behavior Change Element (BCT Taxonomy) | Care @ Home Component | Notes/Details |
| Goals and Planning | Goal discussion and agreement during initial assessment Goal setting (Week 1) module completion Weekly action plan completion | Behavior change techniques that are also included here: Problem Solving, Goal setting, Action Planning, Review behavior goal(s), Discrepancy between current behavior and goal, Review outcome goal(s), Behavioral contract, Commitment |
| Feedback and Monitoring | Weekly action plans Physitrack® self- monitoring FitbitTM self-monitoring Follow-up feedback and nudges E-module quizzes and exercises | Patients fill out weekly action plans that are reviewed during their follow-up calls. Patients are given feedback and support during their weekly follow-up calls and visits. Behavior change techniques that are also included here: Monitoring of behavior by others without the feedback, Feedback on behavior, Self-monitoring of behavior, Feedback on outcome(s) of behavior |
| Social Support | Support provided by the program staff and health coach during the various program touch points (assessments, follow-ups) | Behavior change techniques that are also included here: Social support (unspecified), Social support (practical), Social support (emotional). |
| Shaping Knowledge | E-module Content Education during initial assessment, Follow-Up calls and visits | Behavior change techniques that are also included here: Instruction on how to perform the behavior, Information about antecedents, Re-attribution |
| Natural Consequences | E-module content and follow-up calls provide information about the consequences of performing the behavior (exercise, self-management behaviors, etc.) | Behavior change techniques that are also included here: Information about health consequences, Monitoring of emotional consequences, Information about emotional consequences |
| Comparison of Behavior | Physitrack® provides video tutorials of exercises that patients can perform at home | Behavior change techniques that are also included here: Demonstration of the behavior |
| Repetition and Substitution | Weekly exercise prescriptions with repetitive components Goals are increasingly difficult but achievable | Behavior change techniques that are also included here: Behavioral substitution, Habit formation, Habit reversal, Generalization of a target behavior, Graded Tasks |
| Reward and Threat | Initial discussion of motivation and self-incentive/self-reward at onset of program Verbal reward or non-verbal (email) reward when certain tasks are completed, or goals achieved | Behavior change techniques that are also included here: Social Reward, Self-incentive, Self-reward |
| Regulation | Self-management and stress management skills shared throughout the program | Behavior change techniques that are also included here: Reduce negative emotions, Conserving mental resources |
| Antecedents | Advice is provided on how to change the physical and the social environment in order to facilitate the desired behavior | Behavior change techniques that are also included here: Restructuring the physical environment, Restructuring the social environment, Avoidance/reducing exposure to cues for the behavior |
| Identity | E-module curriculum Teaching from Kinesiologist/Health coach | Behavior change techniques that are also included here: Identification of self as role model, Framing/reframing, Valued self-identity |
| Self-belief | Health coach provides reassurance, support, and examples of how to increase confidence in reaching a goal | Behavior change techniques that are also included here: Verbal persuasion about capability, Focus on past success, Self-talk |
| Category | n (%) |
| Sexn (%) | |
| Female | 22 (62.9) |
| Male | 13 (37.1) |
| Age (years) | |
| Mean (SD) | 55 (±15.9) |
| Range | 19–76 |
| Marital Statusn (%) | |
| Married/Common Law | 25 (71.4) |
| Other | 10 (28.6) |
| Educationn (%) | |
| High school | 7 (20.0) |
| University/college | 25 (71.4) |
| Missing/Prefer not to answer | 3 (8.6) |
| Household Incomen (%) | |
| <$40,000 | 2 (5.7) |
| 40,000–75,000 | 7 (20.0) |
| >$75,000 | 14 (40.0) |
| Missing/prefer not to answer | 12 (34.3) |
| Employment Statusn (%) | |
| Employed (full/part time) | 16 (45.7) |
| Not-employed/on disability/retired | 19 (54.3) |
| Cancer Siten (%) | |
| Breast | 12 (34.3) |
| Gastrointestinal | 5 (14.3) |
| Lymphoma and Myeloma | 5 (14.3) |
| Gynecological | 3 (8.6) |
| Endocrine | 3 (8.6) |
| Head and Neck | 2 (5.7) |
| CNS | 2 (5.7) |
| Genitourinary | 1 (2.9) |
| Leukemia | 1 (2.9) |
| Lung | 1 (2.9) |
| Time Since Diagnosis (months) | 24 |
| mean (SD), range | (±25.4), 5–123 |
| Reason for Referral *n (%) | |
| Cancer-related Fatigue | 20 (57.1) |
| Deconditioning and Exercise contraindications | 19 (54.3) |
| Return to work limitations | 10 (28.6) |
| Neurocognitive | 5 (14.3) |
| Lymphedema | 8 (22.9) |
| Musculoskeletal | 5 (14.3) |
| Neurological | 5 (14.3) |
| Balance | 3 (8.6) |
| Activities of Daily Living | 2 (5.7) |
| Category | Analytic Note | Example Quote |
|---|---|---|
| Program structure and delivery | Participants were satisfied with the program and its structure and glad they took part and found the team very supportive. The program length and frequency were reasonable for most participants, with only a few suggesting that they would have liked a longer program or more frequent check-ins as the program progressed. Participants enjoyed being able to take part in the program from home rather than having to travel to the hospital. | “You know when you have problems that you’re trying to resolve but you can’t by yourself it’s extremely important to have support, and I’m not the kind of person to go and ask for help so the fact that I was offered that in such a friendly and considerate manner was very important emotionally for me.” (Female breast cancer survivor, age 62.) “I just wish it was longer.” (Male head and neck cancer survivor, age 58.) “I could certainly have gotten on the subway and made the trek downtown but I just like the convenience and for me it’s just more practical to be able to do them at home.” (Female breast cancer survivor, age 65.) |
| Some participants appreciated having a non-group option, as they didn’t want to be a part of a group session due to the lack of privacy. Finally, some participants had no issue attending the in-person assessment appointments as they held flexible jobs and had flexible schedules. Others found that it was challenging to come to the hospital for midday assessment appointments and suggested that a wider range of time slots be offered to participants | “I feel like I’m eavesdropping when we’re in a group and they’re telling their heart and soul yeah and I always feel like it was none of my business to be listening. […] I always felt sort of awkward.” (Male head and neck cancer survivor, age 58.) “I think some people might find coming in to do the physical assessment might be tricky if they are working and they can’t take the time so fortunately my office is very supportive and that wasn’t an issue for me but um yea it might help if those, the, in person appointments could happen you know either earlier in the morning before people start or sort of much later in the day, at the end of the day.” (Female gynecological cancer survivor, age 53.) |
| Category | Analytic Note | Example Quote |
|---|---|---|
| Satisfaction with the CaRE@Home Program | All interviewed participants were very satisfied with the program and liked how it was organized and structured. They appreciated the support that they received during their program participation. They liked the various topics that were covered, notably the nutrition resources. | “I thought it was very well organized in the sense that it helps put together a number of things which seem basic and things we should know about taking care of ourselves.” (Female breast cancer survivor, age 51.) |
| Participants appreciated that the modules could provide interesting and important information, yet struggled to find time to complete them and to implement the new skills into their daily lives. They would often complete the first few topics, but their engagement would decline as the weeks progressed and their lives became busy. | “I thought the modules were great they were very helpful but as I said for example with when you just read stuff yes it’s very difficult to actually implement.” (Female breast cancer survivor, age 62.) | |
| Health coaching calls were a valuable program component that provided the much-needed human touch element. Participants also reported that the health coaching calls provided structure and support during a time when they often felt alone. The calls also made a few participants realize that they did in fact need the additional support, even though they were not necessarily at first aware. | “I always enjoyed the check-in because it was an opportunity to share insights or ask questions and clarify things. […] I think it’s really extremely extremely valuable and I greatly enjoyed [them].” (Female breast cancer survivor, age 65.) “And the weekly call-in with the kinesiologist. That was also a great motivator, you know, because if I was just given the workbook and the e-classes I’m not sure if I would have stuck with the program, you know, dutifully. Touching base with someone, that was really helpful.” (Female gynecological cancer survivor, age 53.) | |
| Participants liked how the FitbitTM and the Physitrack® technologies motivated them and allowed them to easily see their exercise prescription and progression while at home. They also appreciated the added element of having video tutorials, as well as having their kinesiologists be able to make remote weekly updates to their plans. | “It (Fitbit™) really motivates me and I told him a story about how when at night I noticed I was still short about a hundred steps to hit that magic 10000 it was 11:30 at night and I just walked around the house till I got it so it’s a good thing. I really I love my Fitbit™.” (Female breast cancer survivor, age 65.) “First it (Physitrack®) tells you how to do the exercises if I’ve forgotten how. So yes it’s like a personal trainer. It’s a great little thing and who doesn’t love checking a box right? And saying you’re done for the day. It’s an endorphin fix right there. It’s nice seeing a great big green tick - like well done.” (Male lymphoma cancer survivor, age 37.) | |
| Benefits of the Program | Participants spoke about the benefits of participating in the CaRE@Home program, including managing a cancer diagnosis and taking care of yourself. | “I am eternally grateful that I was part of this program. I don’t know where I would have been in my recovery from cancer had I not joined the program.” (Female breast cancer survivor, age 57.) |
| Many talked about gaining a new appreciation for exercise. They realized that exercise could minimize their symptoms and improve their sleep and/or fatigue. Some participants also learned how to realistically fit exercise into their daily and weekly schedules. Some participants liked that their exercise plan could be modified as the weeks progressed, allowing for additional challenges as they became stronger and/or their functional abilities changed. | “Yea, I just wanted to be stronger you know. I definitely accomplished that. Being assigned fitness exercises and the regular check-in, that helped me keep on track.” (Female gynecological cancer survivor, age 53.) “When that 2 o’clock slump came I worked out and was energized for the rest of the day. Yea. And I used the exercises. To be less fatigued.” (Male lymphoma cancer survivor, age 37.) “I get pretty regular sleep now. I think part of it was the exercise helps. But also part of it was letting go of some things.” (Female breast cancer survivor, age 51.) | |
| Areas for program improvements | Some participants felt that the e-modules were too long and others preferred to be able to complete them on their own timeline rather than a prescribed weekly frequency. Others did not seem to be interested in the e-modules and only wanted to focus on the exercise component of the intervention. | “So the online education thing didn’t really work out well for me at all. But I know yeah it’s unfortunate. It basically just came down to me not having time to do it.” (Male lymphoma cancer survivor, age 37.) “I do think there were times where it would have helped me just have a bit more time to self-reflect on that topic as opposed to moving on to another topic.” (Female breast cancer survivor, age 51.) |
| Participants spoke about the timing of the program and some suggested that it would have been more helpful if they had received it earlier, during treatment, as opposed to after treatment ended. | “If those modules could be made available to people as they’re going through their treatment.” (Female breast cancer survivor, age 51.) | |
| Participants suggested having a train-the-trainer model with remote kinesiologists who could do the assessments in the community rather than having patients come to downtown Toronto. | “If you can look into having remote locations for people to go to instead of coming to downtown Toronto great. It is possible that we will take advantage of. “(Female breast cancer survivor, age 57.) | |
| Participants suggested that some of the health coaching phone check-ins could be done over video conferencing to allow for a more personal touch and to demonstrate exercises etc. | “Rather than phone calls what about doing something where you’re skyping or you can actually see so it’s still remote, but yeah, they would be able to see you and you would see them.” (Female breast cancer survivor, age 57.) | |
| Participants suggested adding in some content and support for the patient’s caregiver. | “I think that it would have been cool that there was one module that included your main support person because they’re going through it too but from a different angle.”(Female breast cancer survivor, age 51.) |
| Outcome Measure | Time Point | n | Mean (SE) | 95% CI | Difference in the Estimates (SE) | p-Value |
|---|---|---|---|---|---|---|
| WHODAS | T1 | 33 | 9.84(1.14) | 7.52–12.2 | - | - |
| T2 | 29 | 8.17(1.01) | 6.13–10.2 | −1.66(0.70) | 0.03 | |
| T3 | 26 | 7.56(1.10) | 5.32–9.80 | −2.28(0.81) | 0.008 | |
| ESAS Measures | ||||||
| Pain | T1 | 35 | 1.94(0.36) | 1.21–2.67 | - | - |
| T2 | 29 | 2.18(0.39) | 1.37–2.98 | −0.23(0.44) | 0.598 | |
| T3 | 28 | 1.81(0.38) | 1.03–2.59 | −0.13(0.35) | 0.719 | |
| Tiredness | T1 | 35 | 3.71(0.41) | 2.88–4.55 | - | - |
| T2 | 29 | 3.15(0.41) | 2.31–3.99 | −0.56(0.34) | 0.105 | |
| T3 | 28 | 3.16(0.35) | 2.45–3.87 | −0.55(0.29) | 0.072 | |
| Drowsiness | T1 | 35 | 2.17(0.40) | 1.36–2.98 | - | - |
| T2 | 29 | 2.15(0.35) | 1.43–2.86 | −0.02(0.37) | 0.948 | |
| T3 | 28 | 2.16(0.41) | 1.33–2.99 | −0.01(0.47) | 0.984 | |
| Depression | T1 | 35 | 2.08(0.39) | 1.28–2.89 | - | - |
| T2 | 29 | 1.90(0.39) | 1.09–2.70 | −0.18(0.26) | 0.478 | |
| T3 | 28 | 1.82(0.37) | 1.05–2.57 | −0.27(0.27) | 0.323 | |
| Anxiety | T1 | 35 | 2.54(0.47) | 1.58–3.50 | - | - |
| T2 | 29 | 2.23(0.42) | 1.38–3.08 | −0.31(0.33) | 0.202 | |
| T3 | 28 | 2.06(0.44) | 1.18–2.95 | −0.48(0.37) | 0.355 | |
| Wellbeing | T1 | 35 | 3.14(0.39) | 2.33–3.95 | - | - |
| T2 | 29 | 3.41(0.46) | 2.47–4.34 | 0.27(0.43) | 0.808 | |
| T3 | 28 | 3.42(0.41) | 2.58–4.26 | 0.28(0.26) | 0.527 | |
| GODIN | ||||||
| Total LSI | T1 | 35 | 18.4(2.22) | 13.9–22.9 | - | - |
| T2 | 31 | 28.4(3.37) | 21.5–35.2 | 9.96(3.45) | 0.007 | |
| T3 | 26 | 30.5(3.30) | 23.8–37.2 | 12.14(2.95) | 0.0002 | |
| Moderate to strenuous LSI | T1 | 35 | 9.26(1.74) | 5.73–12.8 | - | - |
| T2 | 27 | 18.6(3.02) | 12.5–24.7 | 9.34(2.91) | 0.003 | |
| T3 | 25 | 20.0(3.33) | 13.2–26.8 | 10.8(3.17) | 0.002 | |
| Work Status | ||||||
| During the past seven days, how many hours did you miss from work because of problems associated with cancer diagnosis and treatment? | T1 | 16 | 4.83(1.52) | 1.66–8.01 | - | - |
| T2 | 16 | 2.31(0.94) | 0.36–4.27 | −2.52(1.06) | 0.069 | |
| T3 | 17 | 1.88(0.79) | 0.22–3.53 | −2.96(1.27) | 0.074 | |
| During the past seven days, how much did your cancer diagnosis and treatment affect your productivity while you were working? | T1 | 15 | 3.44(0.53) | 2.33–4.54 | - | - |
| T2 | 16 | 2.62(0.65) | 1.27–3.96 | −0.82(0.66) | 0.438 | |
| T3 | 14 | 2.03(0.48) | 1.03–3.03 | −1.41(0.49) | 0.026 | |
| Outcome Measure | Time Point | n | Mean (SE) | 95% CI | Difference in theEstimates (SE) | p-Value |
|---|---|---|---|---|---|---|
| Six Minute Walk Test(Meters) | T1 | 35 | 469.0(16.72) | 435.0–502.9 | - | - |
| T2 | 30 | 510.9(13.74) | 483.0–538.9 | 41.96(8.09) | <0.001 | |
| T3 | 25 | 502.5(19.02) | 463.8–541.1 | 33.48(12.4) | 0.010 | |
| Grip Strength(Kg) | T1 | 35 | 58.5(3.57) | 51.22–65.75 | - | - |
| T2 | 31 | 61.6(3.59) | 54.31–68.92 | 3.13(0.99) | 0.003 | |
| T3 | 27 | 64.1(3.59) | 56.79–71.39 | 5.61(1.16) | <0.001 | |
| Heart Rate(Beats per minute) | T1 | 35 | 70.9(1.74) | 67.34–74.44 | - | - |
| T2 | 31 | 71.7(1.36) | 68.90–74.43 | 0.78(1.47) | 0.597 | |
| T3 | 27 | 73.0(1.39) | 67.36–74.44 | 2.06(1.56) | 0.197 | |
| Systolic Blood Pressure (mm Hg) | T1 | 35 | 122.3(2.09) | 118.0–126.6 | - | - |
| T2 | 31 | 120.3(2.78) | 114.6–125.9 | −2.02(2.25) | 0.377 | |
| T3 | 27 | 118.3(4.26) | 109.6–126.9 | −3.98(4.58) | 0.391 | |
| Diastolic Blood Pressure (mm Hg) | T1 | 35 | 80.7(2.98) | 74.65–86.78 | - | - |
| T2 | 31 | 76.7(1.63) | 73.35–79.99 | −4.05(2.99) | 0.186 | |
| T3 | 27 | 77.2(1.32) | 74.52–79.90 | −3.50(3.18) | 0.279 | |
| BMI(kg/m2) | T1 | 34 | 27.5(1.02) | 25.38–29.53 | - | - |
| T2 | 29 | 27.5(1.04) | 25.41–29.67 | 0.08(0.10) | 0.417 | |
| T3 | 26 | 27.7(1.07) | 25.38–29.53 | 0.19(0.17) | 0.267 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacDonald, A.M.; Chafranskaia, A.; Lopez, C.J.; Maganti, M.; Bernstein, L.J.; Chang, E.; Langelier, D.M.; Obadia, M.; Edwards, B.; Oh, P.; et al. CaRE @ Home: Pilot Study of an Online Multidimensional Cancer Rehabilitation and Exercise Program for Cancer Survivors. J. Clin. Med. 2020, 9, 3092. https://doi.org/10.3390/jcm9103092
MacDonald AM, Chafranskaia A, Lopez CJ, Maganti M, Bernstein LJ, Chang E, Langelier DM, Obadia M, Edwards B, Oh P, et al. CaRE @ Home: Pilot Study of an Online Multidimensional Cancer Rehabilitation and Exercise Program for Cancer Survivors. Journal of Clinical Medicine. 2020; 9(10):3092. https://doi.org/10.3390/jcm9103092
Chicago/Turabian StyleMacDonald, Anne Marie, Aleksandra Chafranskaia, Christian J. Lopez, Manjula Maganti, Lori J. Bernstein, Eugene Chang, David Michael Langelier, Maya Obadia, Beth Edwards, Paul Oh, and et al. 2020. "CaRE @ Home: Pilot Study of an Online Multidimensional Cancer Rehabilitation and Exercise Program for Cancer Survivors" Journal of Clinical Medicine 9, no. 10: 3092. https://doi.org/10.3390/jcm9103092
APA StyleMacDonald, A. M., Chafranskaia, A., Lopez, C. J., Maganti, M., Bernstein, L. J., Chang, E., Langelier, D. M., Obadia, M., Edwards, B., Oh, P., Bender, J. L., Alibhai, S. M., & Jones, J. M. (2020). CaRE @ Home: Pilot Study of an Online Multidimensional Cancer Rehabilitation and Exercise Program for Cancer Survivors. Journal of Clinical Medicine, 9(10), 3092. https://doi.org/10.3390/jcm9103092









