Trajectories of Hospitalization in COVID-19 Patients: An Observational Study in France
Abstract
1. Introduction
2. Experimental Section
2.1. Data
2.2. Statistical Analysis
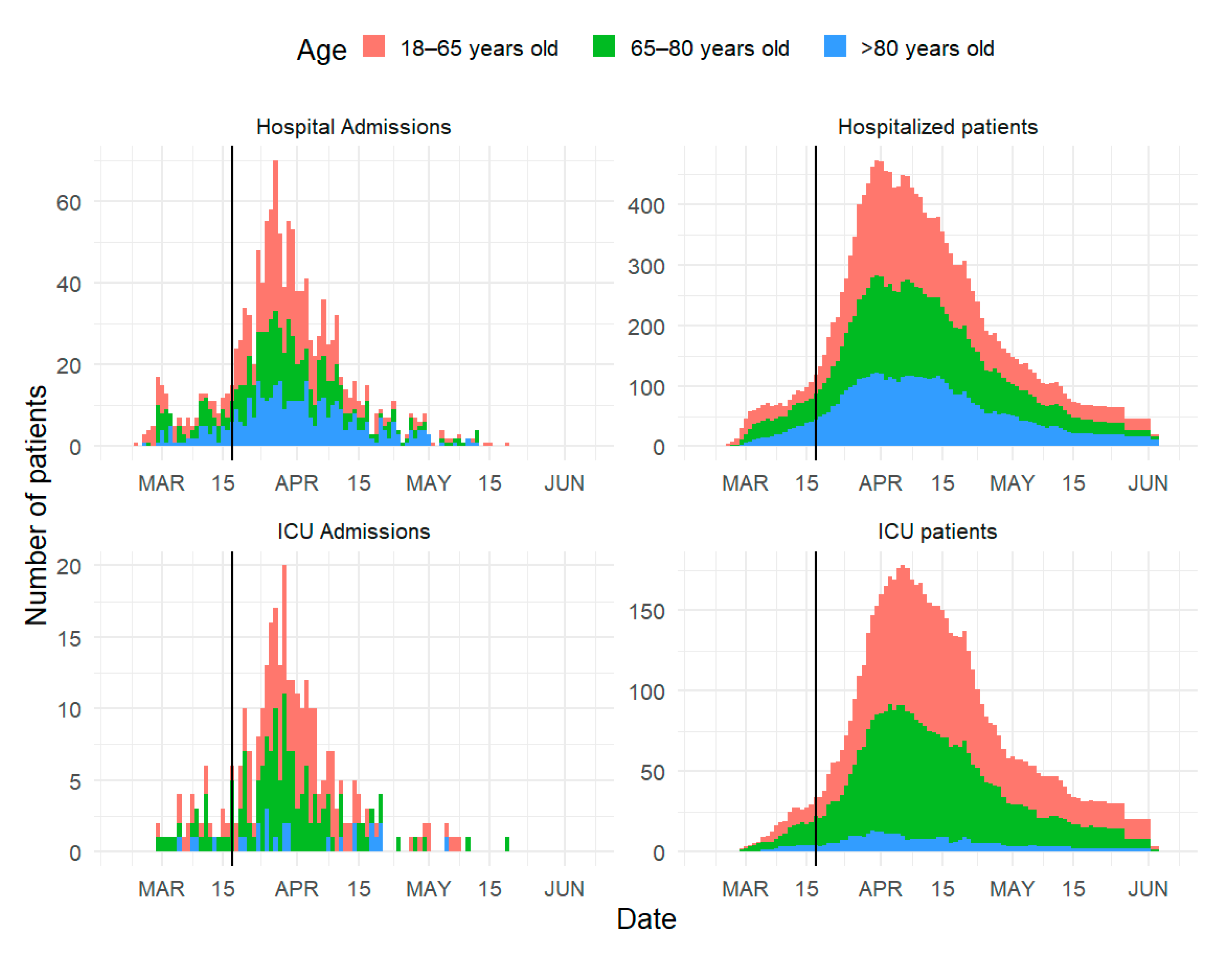
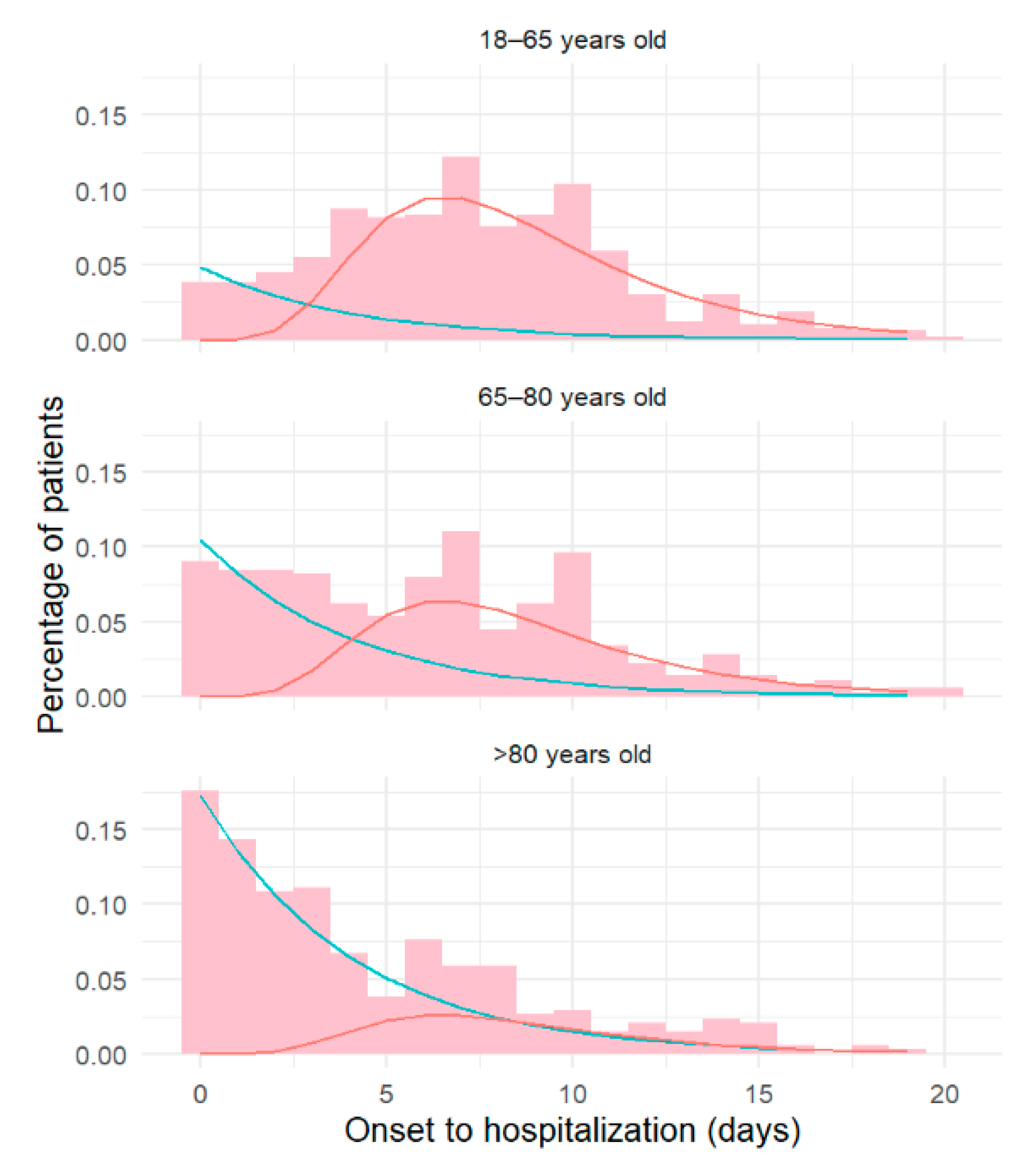
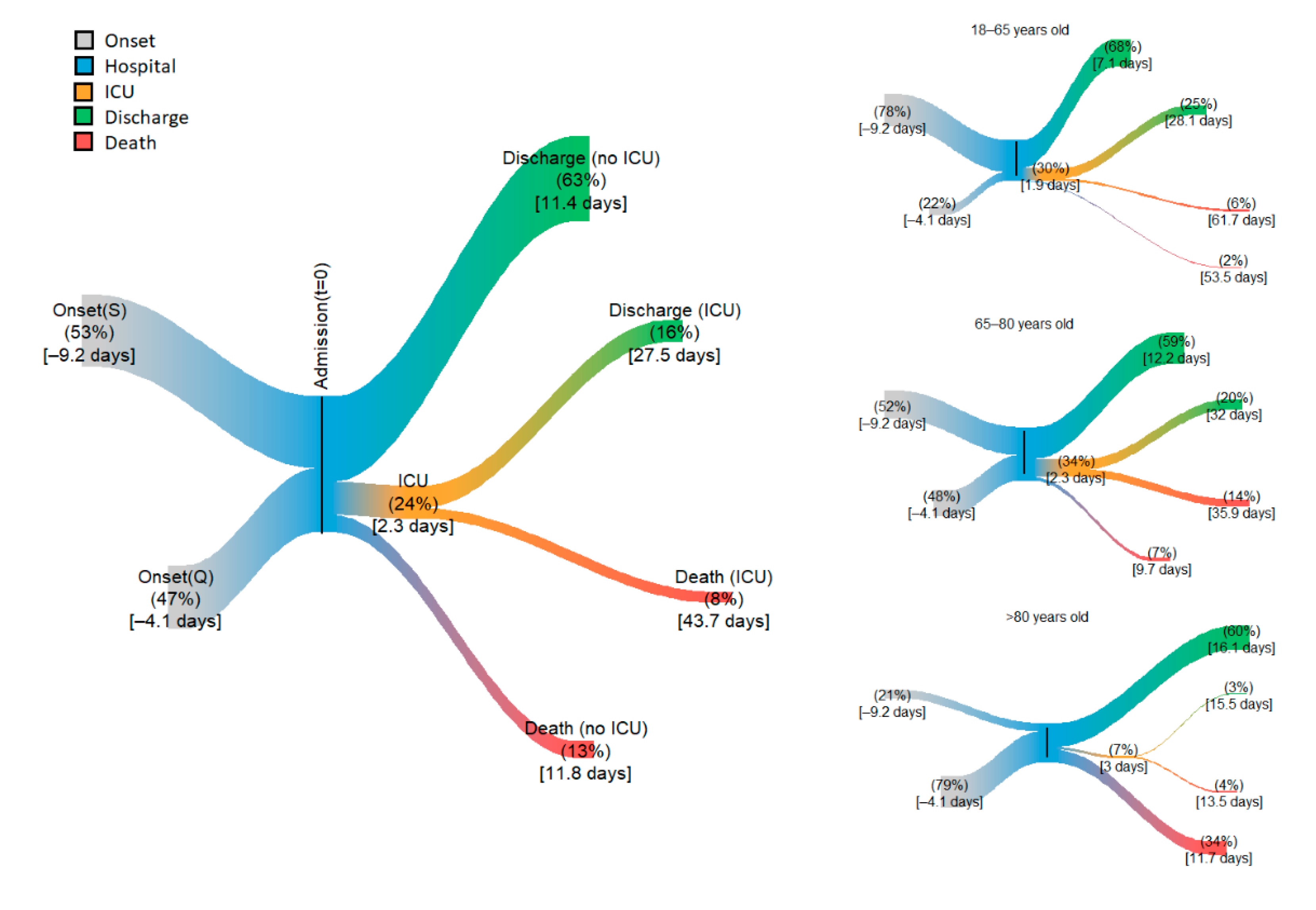
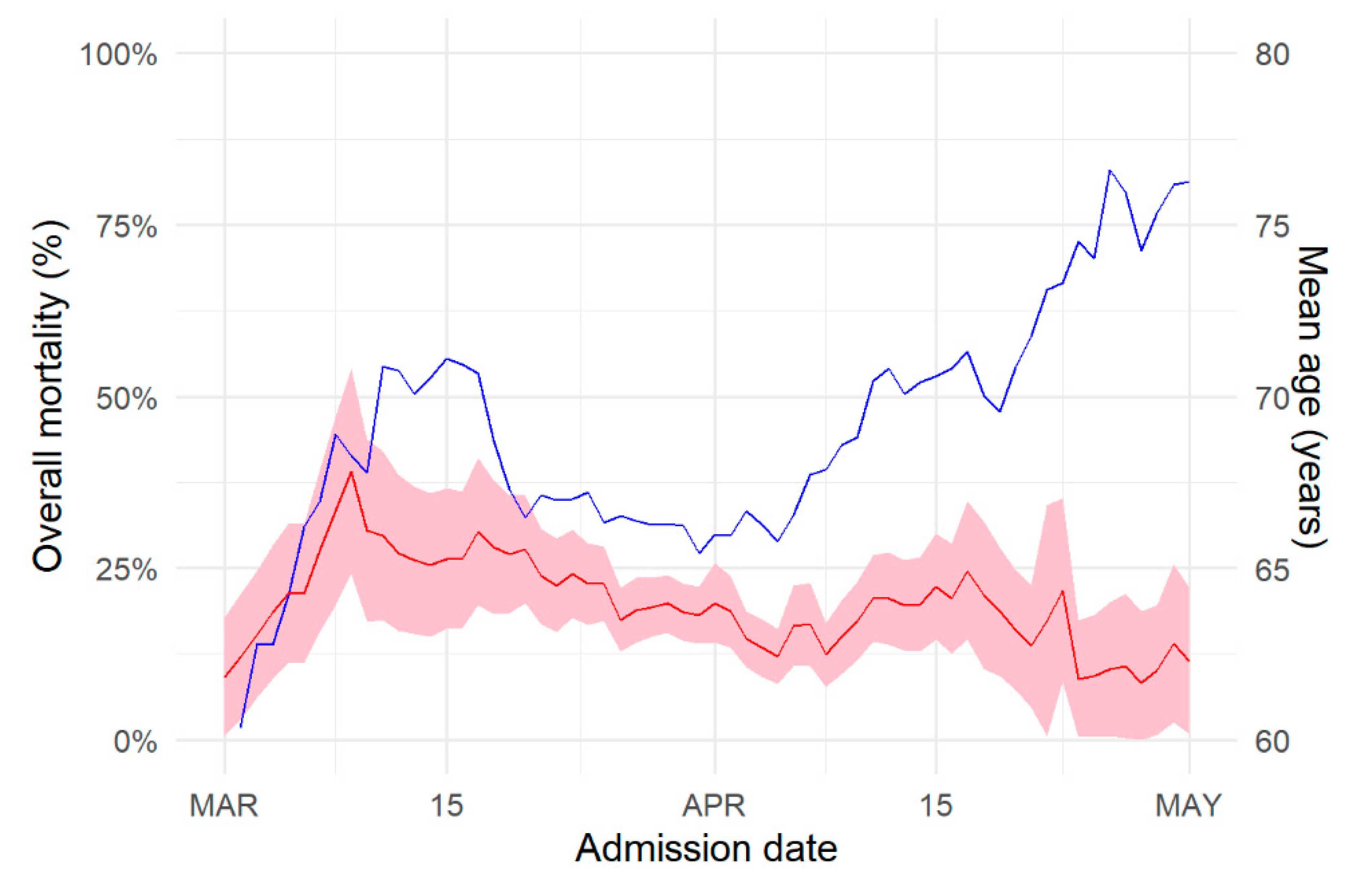
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stoecklin, S.B.; Rolland, P.; Silue, Y.; Mailles, A.; Campese, C.; Simondon, A.; Mechain, M.; Meurice, L.; Nguyen, M.; Bassi, C.; et al. First cases of coronavirus disease 2019 (COVID-19) in France: Surveillance, investigations and control measures, January 2020. Eurosurveillance 2020, 25, 2000094. [Google Scholar]
- Boelle, P.-Y.; Souty, C.; Launay, T.; Guerrisi, C.; Turbelin, C.; Behillil, S.; Enouf, V.; Poletto, C.; Lina, B.; Van Der Werf, S.; et al. Excess cases of influenza-like illnesses synchronous with coronavirus disease (COVID-19) epidemic, France, March 2020. Eurosurveillance 2020, 25, 2000326. [Google Scholar] [CrossRef]
- Schoenfeld, D. Survival methods, including those using competing risk analysis, are not appropriate for intensive care unit outcome studies. Crit. Care 2005, 10, 103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lescure, F.-X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.-H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef]
- Argenziano, M.G.; Bruce, S.L.; Slater, C.L.; Tiao, J.R.; Baldwin, M.R.; Barr, R.G.; Chang, B.P.; Chau, K.H.; Choi, J.J.; Gavin, N.; et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ 2020, 369, m1996. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Riquelme, R.; Torres, A.; El-Ebiary, M.; Mensa, J.; Estruch, R.; Ruiz, M.; Angrill, J.; Soler, N. Community-acquired Pneumonia in the Elderly: Clinical and nutritional aspects. Am. J. Respir. Crit. Care Med. 1997, 156, 1908–1914. [Google Scholar] [CrossRef]
- Poissy, J.; Goutay, J.; Caplan, M.; Parmentier, E.; Duburcq, T.; Lassalle, F.; Jeanpierre, E.; Rauch, A.; Labreuche, J.; Susen, S.; et al. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation 2020, 142, 184–186. [Google Scholar] [CrossRef]
- Rauch, A.; Labreuche, J.; Lassalle, F.; Goutay, J.; Caplan, M.; Charbonnier, L.; Rohn, A.; Jeanpierre, E.; Dupont, A.; Duhamel, A.; et al. Coagulation biomarkers are independent predictors of increased oxygen requirements in COVID-19. J. Thromb. Haemost. 2020. ahead of print. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2020. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Mortensen, E.M.; Rello, J.; Brody, J.; Anzueto, A. Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest 2010, 137, 552–557. [Google Scholar] [CrossRef]
- Flaatten, H.; Van Heerden, V.; Jung, C.; Beil, M.; Leaver, S.; Rhodes, A.; Guidet, B.; Delange, D.W. The good, the bad and the ugly: Pandemic priority decisions and triage. J. Med. Ethics 2020. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, C.; Bonmarin, I.; Bitar, D.; Cardoso, T.; Duport, N.; Herida, M.; Isnard, H.; Guidet, B.; Mimoz, O.; Richard, J.C.M.; et al. Adult intensive-care patients with 2009 pandemic influenza A(H1N1) infection. Epidemiol. Infect. 2010, 139, 1202–1209. [Google Scholar] [CrossRef]
- Rees, E.M.; Nightingale, E.S.; Jafari, Y.; Waterlow, N.R.; Clifford, S.; Pearson, C.A.B.; CMMID Working Group; Jombart, T.; Procter, S.R.; Knight, G.M. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Med. 2020, 18, 270. [Google Scholar] [CrossRef]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators; Cavalcanti, A.B.; Suzumura, E.A.; Laranjeira, L.N.; Paisani, D.D.M.; Damiani, L.P.; Guimarães, H.P.; Romano, E.R.; Regenga, M.D.M.; Taniguchi, L.N.T.; et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017, 318, 1335–1345. [Google Scholar]
- Gupta, S.; Hayek, S.S.; Wang, W.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern. Med. 2020. ahead of print. [Google Scholar] [CrossRef]
- Wood, R.M.; McWilliams, C.J.; Thomas, M.J.; Bourdeaux, C.P.; Vasilakis, C. COVID-19 scenario modelling for the mitigation of capacity-dependent deaths in intensive care. Health Care Manag. Sci. 2020, 23, 315–324. [Google Scholar] [CrossRef]
- Iannaccone, S.; Alemanno, F.; Houdayer, E.; Brugliera, L.; Castellazzi, P.; Cianflone, D.; Meloni, C.; Ambrosio, A.; Mortini, P.; Spina, A.; et al. COVID-19 rehabilitation units are twice as expensive as regular rehabilitation units. J. Rehabil. Med. 2020, 52, jrm00073. [Google Scholar] [CrossRef]
- Biased and Unbiased Estimation of the Average Lengths of Stay in Intensive Care Units in the COVID-19 Pandemic. Available online: https://www.medrxiv.org/content/10.1101/2020.04.21.20073916v2 (accessed on 14 September 2020).
- McLachlan, G.J.; McGiffin, D.C. On the role of finite mixture models in survival analysis. Stat. Methods Med. Res. 1994, 3, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Liu, V.X.; Jackson, M.L.; Schmidt, M.A.; Jewell, B.L.; Flores, J.P.; Jentz, C.; Northrup, G.R.; Mahmud, A.; Reingold, A.L.; et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: Prospective cohort study. BMJ 2020, 369, m1923. [Google Scholar] [CrossRef] [PubMed]
- Imam, Z.; Odish, F.; Gill, I.; O’Connor, D.; Armstrong, J.; Vanood, A.; Ibironke, O.; Hanna, A.; Ranski, A.; Halalau, A. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J. Intern. Med. 2020, 288, 469–476. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Leaf, R.S.K.; Dzik, W.H.; Carlson, J.C.T.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.K.; Bornikova, L.; Gupta, S.; et al. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020, 136, 489–500. [Google Scholar] [CrossRef]
- Patel, M.; Gangemi, A.; Marron, R.; Chowdhury, J.; Yousef, I.; Zheng, M.; Mills, N.; Tragesser, L.; Giurintano, J.; Gupta, R.; et al. Retrospective analysis of high flow nasal therapy in COVID-19-related moderate-to-severe hypoxaemic respiratory failure. BMJ Open Respir. Res. 2020, 7, e000650. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Gemelli against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients after Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar]
| Characteristics | Age Group (n) | Overall (n = 1321) | ||
|---|---|---|---|---|
| 18–65 (n = 523) | 66–80 (n = 400) | >80 (n = 398) | ||
| Male (% (n)) | 57% (300) | 60% (232) | 44% (168) | 55% (700) |
| Age (years) (median [IQR]) | 52.0 [43.9, 59.0] | 72.0 [68.0, 75.8] | 86.0 [83.0, 90.0] | 69.0 [55.8, 82.0] |
| Time from onset to hospitalization (days) (median [IQR]) | 8 [5, 11] | 7 [3, 10] | 4 [2, 8] | 7 [3, 10] |
| ICU < 24 h after admission (% (n)) | 17% (91) | 20% (78) | 6% (23) | 14% (192) |
| Age | ||||
|---|---|---|---|---|
| Trajectory Characteristics | 18–65 | 66–80 | >80 | Overall |
| (n = 523) | (n = 400) | (n = 398) | (n = 1321) | |
| Overall length of stay | 10 [6, 16] | 14 [9, 24] | 13 [8, 21] | 12 [7, 20] |
| Death (%) | 9 | 20 | 38 | 20 |
| Time to death (days) | 53 [31, 91] | 16 [9, 28] | 10 [6, 17] | 14 [8, 25] |
| Time to discharge (days) | 8 [5, 13] | 14 [9, 22] | 14 [9, 23] | 12 [7, 19] |
| ICU (%) | 30 | 34 | 7 | 24 |
| Time to ICU (days) | 1 [0, 2] | 1 [0, 3] | 1 [0, 3] | 1 [0, 2] |
| Time in the ICU (days) | 20 [10, 39] | 22 [11, 42] | 9 [5, 18] | 20 [10, 39] |
| Death in the ICU (%) | 18 | 42 | 62 | 33 |
| Time to death (days) | 40 [22, 75] | 23 [13, 44] | 9 [5, 16] | 27 [14, 52] |
| Discharge from the ICU (%) | 82 | 58 | 38 | 67 |
| Time to discharge (days) | 17 [9, 34] | 20.0 [10, 38] | 10 [5, 19] | 17 [9, 33] |
| No ICU (%) | 70 | 66 | 93 | 75 |
| Time in the hospital (days) | 6 [4, 10] | 9 [6, 15] | 11 [7, 17] | 9 [6, 14] |
| Death in hospital (%) | 3 | 11 | 36 | 13 |
| Time to death (days) | 41 [25, 67] | 8 [5, 12] | 9 [6, 15] | 9 [6, 15] |
| Discharge from hospital (%) | 97 | 89 | 64 | 63 |
| Time to discharge (days) | 6 [4, 9] | 10 [7, 15] | 13 [9, 20] | 9 [6, 14] |
| Period (n) | ||||
|---|---|---|---|---|
| >15 March (n = 158) | 15–30 March (n = 646) | 1–15 April (n = 370) | 1–15 April (n = 147) | |
| Time from onset to hospitalization (days) (median [IQR]) | 5 [2, 9] | 6 [3, 9] | 6 [2, 10] | 4 [2, 8] |
| ICU < 24 h after admission (% (n)) | 20 (31) | 25 (159) | 24 (89) | 13 (19) |
| Length of stay (median [IQR]) | 13 [8, 21] | 11 [7, 18] | 12 [7, 20] | 17 [10, 27] |
| Death (%) | 22% | 21% | 17% | 12% |
| Time to discharge (median [IQR]) | 13 [8, 21] | 10 [6, 17] | 12 [7, 19] | 19 [12, 32] |
| Time to death (median [IQR]) | 13 [8, 21] | 14 [8, 24] | 13 [8, 22] | 8 [5, 12] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boëlle, P.-Y.; Delory, T.; Maynadier, X.; Janssen, C.; Piarroux, R.; Pichenot, M.; Lemaire, X.; Baclet, N.; Weyrich, P.; Melliez, H.; et al. Trajectories of Hospitalization in COVID-19 Patients: An Observational Study in France. J. Clin. Med. 2020, 9, 3148. https://doi.org/10.3390/jcm9103148
Boëlle P-Y, Delory T, Maynadier X, Janssen C, Piarroux R, Pichenot M, Lemaire X, Baclet N, Weyrich P, Melliez H, et al. Trajectories of Hospitalization in COVID-19 Patients: An Observational Study in France. Journal of Clinical Medicine. 2020; 9(10):3148. https://doi.org/10.3390/jcm9103148
Chicago/Turabian StyleBoëlle, Pierre-Yves, Tristan Delory, Xavier Maynadier, Cécile Janssen, Renaud Piarroux, Marie Pichenot, Xavier Lemaire, Nicolas Baclet, Pierre Weyrich, Hugues Melliez, and et al. 2020. "Trajectories of Hospitalization in COVID-19 Patients: An Observational Study in France" Journal of Clinical Medicine 9, no. 10: 3148. https://doi.org/10.3390/jcm9103148
APA StyleBoëlle, P.-Y., Delory, T., Maynadier, X., Janssen, C., Piarroux, R., Pichenot, M., Lemaire, X., Baclet, N., Weyrich, P., Melliez, H., Meybeck, A., Lanoix, J.-P., & Robineau, O. (2020). Trajectories of Hospitalization in COVID-19 Patients: An Observational Study in France. Journal of Clinical Medicine, 9(10), 3148. https://doi.org/10.3390/jcm9103148






