Abstract
Immune reconstitution inflammatory syndrome (IRIS) is an immune reaction that occurs along with the recovery of the patient’s immunity. Tuberculosis-related IRIS (TB-IRIS) upon tumor necrosis factor (TNF)-α inhibitor treatment has been reported in non-human immunodeficiency virus (HIV) patients. However, the importance of biological treatment, as a risk factor of IRIS, has not yet been established. In this study, we examined TB-IRIS in non-HIV patients to explore the role of TNF-α inhibitor treatment. Out of 188 patients with pulmonary TB, seven patients had IRIS. We examined univariate logistic and multivariate analysis to elucidate risk factors of TB-IRIS. Univariate analysis indicated that usage of immunosuppressive drugs, TNF-α inhibitors, and history of food or drug allergy were significantly related with TB-IRIS. On initial treatment, the values of serological markers such as serum albumin and serum calcium were significantly related with TB-IRIS. There was a higher mortality rate in patients with TB-IRIS. Furthermore, multivariate analysis revealed that usage of TNF-α inhibitors, history of allergy, and serum hypercalcemia were related to TB-IRIS. Usage of TNF-α inhibitors, history of allergy, and serum hypercalcemia may be independent predictors of TB-IRIS in non-HIV patients. Since higher mortality has been reported for TB-IRIS, we should pay attention to TB patients with these risk factors.
1. Introduction
Immune reconstitution inflammatory syndrome (IRIS) is well-known as an initial exacerbation after tuberculosis (TB) treatment. It is defined as the deterioration of disease despite appropriate treatment against sensitive Mycobacterium tuberculosis and is an immune reaction that occurs with the recovery of the patient’s immunity [1]. In addition to TB, IRIS occurs in infection with cytomegalovirus or cryptococcus [2]. Tuberculosis-related IRIS (TB-IRIS) is reported to occur in 2–25% of human immunodeficiency virus (HIV)-negative pulmonary TB patients [1,3,4,5], and it often occurs during highly active antiretroviral therapy in human HIV-positive patients [6,7]. The development of IRIS is related to mortality rate within 48 weeks after TB treatment [8].
Risk factors of IRIS in a patient undergoing treatment with tumor necrosis factor (TNF)-α inhibitors (TNFIs) are disseminated TB, history of TB, and use of steroids at diagnosis [9,10]. In the TB patient without HIV infection it has been reported that IRIS is not related to the immunosuppressed state [11]. While neutropenic patients or organ transplant recipients have increased risk of IRIS [2], the immunosuppressed state poses a lower risk to IRIS [12] in non-HIV patients. However, whether the use of TNFIs is significantly related to the IRIS development as compared to the patients without TNFI treatment has not yet been elucidated. In this study, we examined the cases of pulmonary TB and examined the frequency and the risk factors of IRIS, and the effect of IRIS on the mortality in non-HIV patients.
2. Methods
2.1. Study Population
A total of 201 patients were enrolled in this study from amongst the pulmonary TB patients without HIV infection consecutively treated with anti-tuberculosis therapy in our hospital from January 2005 to December 2016. Pulmonary TB was diagnosed by the appearance of infiltrates or consolidates in the radiological examination and the presence of tubercle bacilli in the sputum. This study was conducted with the approval of the Ethics Review Committee of Gunma University Hospital, No. 2017-026.
2.2. Diagnosis of IRIS
Immune reconstitution inflammatory syndrome was defined as the deterioration of the existing lesion or appearance of a new lesion in the chest radiological examination despite appropriate anti-tuberculosis therapy performed for more than two weeks [3]. We defined the IRIS-positive group after confirming the IRIS condition according to strict criteria as shown in Table 1 [11] and excluding the complications of other disease, worsening pulmonary shadows, non-sensitivity to initial treatment, and the poor compliance with anti-tuberculosis therapy. We evaluated various factors related to the development of IRIS and examined the association of IRIS on the total mortality during TB treatment. Past TB infection was included in the latent tuberculosis infection (LTBI). Corticosteroids, biological drugs, anti-metabolites, and calcineurin inhibitors were included as immunosuppressive drugs.

Table 1.
Diagnosis of immune reconstitution inflammatory syndrome (IRIS) with fulfilment of the four following criteria. TB: Tuberculosis.
2.3. Statistical Analysis
For each factor of the IRIS-positive group and the IRIS negative-group, the number of cases and the ratio were calculated on the nominal and average scale, and the standard deviation was calculated on the order scale. Using a logistic regression model for each factor in the presence or absence of IRIS as a dependent variable, univariate analysis was performed to calculate the odds ratio (OR) and 95% confidence interval (CI). Multivariate analysis was performed for the factors with significant differences in univariate analysis.
3. Results
Of the consecutive 201 patients with pulmonary TB without HIV infection, 188 patients were enrolled in this study. Ten patients died within two weeks after TB treatment and three patients skipped follow-up for more than two weeks. In this study, seven patients (3.7%) had IRIS (Figure 1).
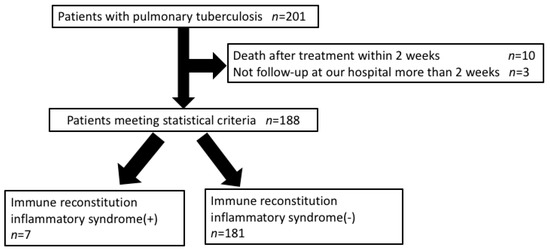
Figure 1.
Study population.
3.1. Association of Patient Background with IRIS Development
As shown in Table 2, there was no significant difference in the age, sex, body mass index (BMI), preference of alcohol, race, history of M. tuberculosis infection, and smoking history between IRIS-positive and negative patients. Immunosuppressive drugs were used in 57.1% (four patients) IRIS-positive and 9.4% (17 patients) IRIS-negative patients, and the usage of immunosuppressive drug was found to be significantly associated with the onset of IRIS (p = 0.002, (OR) (95% CI) 12.90 (2.65–62.30)). The usage of TNF-α inhibitors (TNFIs) as a biological drug was found to be significantly associated with the onset of IRIS in 28.6% (two patients) IRIS-positive and 1.1% (two patients) IRIS-negative patients (p = 0.001, OR (95%CI) 35.80 (4.16–308.0)). Although immunosuppressive drugs, except biological drugs, were used in 28.6% (two patients) of IRIS-positive and 8.8% (16 patients) of IRIS-negative patients, they were not significantly related with the onset of IRIS (p = 0.106, OR (95% CI) 4.12 (0.74–23.00)). Diabetes mellitus (DM), renal dialysis, > 70 years of age at the initial treatment, and disseminated TB were not related to the development of IRIS. History of the malignant tumor was not found in the IRIS-positive patients and was not associated with the development of IRIS. There was a significant difference in the presence of food or drug allergy between IRIS-positive (three patients, 42.9%) and IRIS-negative (14 patients, 7.7%) patients (p = 0.007, OR (95%CI) 8.95 (1.82–44.00)), as shown in Table 2.

Table 2.
Background factors with or without immune reconstitution inflammatory syndrome (IRIS).
3.2. Association of Clinical Parameters with IRIS Development
As shown in Table 3 in the values of serological markers at initial TB treatment, serum albumin showed a significant difference, at 2.44 ± 0.72 g/dL in the IRIS-positive and 3.28 ± 0.80 g/dL in the IRIS-negative patients (p = 0.016, OR (95%CI) 0.26 (0.09–0.78)). The serum calcium value corrected by serum albumin value was 10.38 ± 0.86 mg/dL in the IRIS-positive and 9.72 ± 0.68 mg/dL in the IRIS-negative patients, and showed a significant difference (p = 0.039, OR (95%CI) 2.38 (1.04–5.44)). There was no significant difference in the other serological markers. Comparison of the periods until three consecutive negative conversions of sputum smears of M. tuberculosis showed values of 7.00 ± 4.24 weeks in the IRIS-positive group and 8.03 ± 6.31 weeks in the IRIS-negative group, and no significant difference was found (p = 0.746, OR (95% CI) 0.97 (0.80–1.17)). Of the 188 patients, three patients (42.9%) in the IRIS-positive group and 14 patients (7.7%) in the IRIS-negative group died, and the mortality was significantly related with the onset of IRIS (p = 0.007, OR (95%CI) 8.95 (1.82–44.00).

Table 3.
Laboratory findings with or without immune reconstitution inflammatory syndrome.
3.3. Assessment by Multivariate Analysis of IRIS Development
Multivariate analysis was additionally performed for values with significant differences in the univariate analysis. Spearman’s correlation coefficient revealed that serum albumin value correlated with the serum calcium value (correlation coefficients −0.472 and p value < 0.001). Fisher’s exact test also revealed that usage of immunosuppressive drugs correlated with history of allergy (p = 0.027) or usage of biological drugs (p < 0.001). A one-way analysis of variance (ANOVA) between the usage of immunosuppressive drugs and serum calcium value showed a correlation with IRIS onset (p = 0.001).
Excluding these factors to avoid correlation, stepwise forward multiple regression analysis of the relationship between IRIS and these factors revealed a significant correlation; the most important factor causing IRIS was the usage of TNF-α (p = 0.001) along with history of allergy (p = 0.035), and serum calcium value (p = 0.024) (Table 4).

Table 4.
Univariate or multivariate analysis for IRIS development.
Because a small number of IRIS events occurred in this study, we evaluated several models of multivariate analysis predicting IRIS development [13,14]. In analyzed multivariate models, use of TNFI, history of allergy, and hypercalcemia were also important for predicting IRIS development (Table 5).

Table 5.
Multivariate predictors of IRIS development.
In this study, four patients were treated with TNFI before TB diagnosis. The characteristics of the patients treated with TNFI are shown in Table 6. The TNFI treatment was terminated in two patients, while it was continued in two patients during TB treatment.

Table 6.
Patient characteristics treated with anti-tumor necrosis factor-α antibodies.
4. Discussion
In this study, the usage of TNF-α inhibitors and history of allergy were significantly associated with IRIS development, while the history of TB, tumors, renal dialysis, immunosuppressive drugs except for biological agents, DM, aging, or disseminated TB were not associated with IRIS development. To our knowledge, this is the first study showing the usage of biological drugs, history of allergy, and serum hypercalcemia as a risk factor of IRIS development in pulmonary TB without HIV infection.
In HIV-positive patients, high levels of D-dimer [15], a low number of CD4-positive T cells, high dose of HIV virus, low body mass index, and sputum smear-positive pulmonary TB [8] have been known as risk factors of IRIS. In non-HIV patients, infection, anemia, hypoalbuminemia, low serum lymphocytes [3], elevated eosinophil counts, and low total protein in the pleural effusion in tuberculous pleuritis [16] have also been reported as risk factors of IRIS. Although it has been known that HIV infection is involved with the onset of IRIS, Brown et al. reported that immunosuppressive condition decreased the odds ratio of IRIS onset in HIV-negative cases [12]. In recent years, case reports on TB-IRIS upon treatment with anti-TNF-α antibodies have been increased in HIV-negative cases [10,17,18,19]. In the analysis of patients using anti-TNF-α antibodies against underlying diseases such as rheumatoid arthritis (RA) or Crohn’s disease, disseminated TB, past TB infection, and corticosteroid use at the time of diagnosis have been reported as the risk factors of IRIS development [9]. However, there is no report describing the significance of TNFI treatment on IRIS development. In this study, TNFIs were associated with IRIS development, except the immunosuppressive drugs without TNFIs. Interestingly, RA and Crohn’s disease are Th1 cell-dominant diseases [20,21,22,23], and TNFI promotes the Th2 cytokine-dominant balance via inhibition of TNF-α. The Th2 cytokine-dominant balance caused by TNFI at the initial treatment of TB may be partly associated with IRIS development.
In this study, a significant relationship was found between IRIS development and history of allergy. Most of the food allergies and a part of drug allergies are thought to be an immunoglobulin (IgE)-mediated, Th2 cell-dominant immune response. Initiation of antiretroviral therapy (ART) for HIV patients is associated with a shift from the Th2 to Th1 cell balance, together with IRIS development [24,25]. Patients with a history of allergy or TNFI treatment and patients with the progression of HIV infection to acquired immune deficiency syndrome (AIDS) before ART [26] are in Th2 cytokine-dominant balance. Recently, it has been reported that TNFI treatment for RA deteriorated asthma symptoms, while benralizumab, an anti-IL-5 receptor antagonist, worsened arthritis in a patient with RA-complicated with asthma [27]. This case report indicates that the blockage of the Th1-dominant balance by TNFI treatment promotes the Th1 to Th2 shift and worsens the Th2-dominant disorder, and the inhibition of Th2-dominant balance by IL-5 antagonist initiates Th2 to Th1 shift and deteriorate Th1 dominant disease. There is a possibility that the shift from the Th2 cell cytokine-dominant balance to the Th1 cell cytokine-dominant balance caused by TB infection in a patient with a history of allergy may have influenced IRIS development (Figure 2). Further studies are required to elucidate the mechanism underlying the development of IRIS, which is caused by Th1 cell-dominant balance [2], from the Th2 cell-dominant balance in TB patients with TNFI treatment or history of allergy.
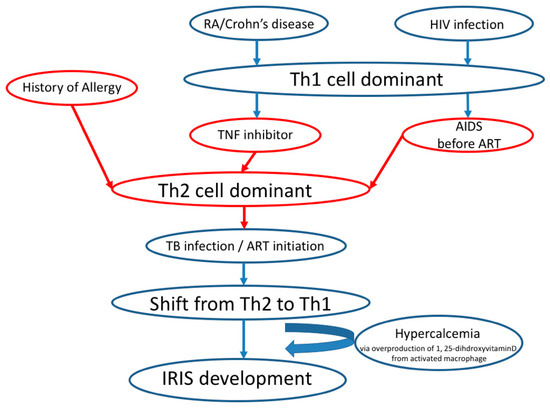
Figure 2.
Proposed mechanism from Th2 to Th1 cytokine-dominant balance in IRIS development.
High levels of serum calcium were a risk factor for IRIS development in this study. Consistently, hypercalcemia related to IRIS is known to be caused by overproduction of 1, 25-dihdroxyvitamin D3 (1, 25[OH2]D3) secreted from disease-activated macrophages together with Th1 cytokines, e.g., interferon-gamma (IFN-γ) and granuloma formation [28]. The Th1-driven immune responses are thought to be essential for IRIS development in HIV-infected and uninfected patients [2,28].
While the prognosis of TB is relatively good and ≥95% patients may have improved health condition [29], the TB mortality rate was reportedly 12.3% in the culture-positive group [30], and 10.5% in pulmonary TB group [31]. Diabetes mellitus, sputum smear-positive TB, anemia, smoking, and drug-induced hepatitis increased the mortality rate [31]. Erbes et al. reported 25.9% mortality for TB patients cared for in the intensive care unit [32]. Systemic steroid administration was found to be effective in severe IRIS cases [33]. In this study, the mortality rate was higher in the IRIS group (42.9%) than in the non-IRIS group (7.2%), consistent with the previous report [11,34], and patients who developed IRIS should be careful while undergoing treatment with steroid.
There are several limitations in this study. Only 188 patients in a single center were examined, which is a small number. The history of allergy, smoking history, and alcohol intake history was self-reported without any means of validation, and the extent of the history of allergy is unclear. In this study, the history of allergy was diagnosed either as a food or drug allergy by self-report. Therefore, the severity of the allergy remains unknown.
5. Conclusions
We demonstrated the possibility of the involvement of TNF-α inhibitors, history of allergy, and hypercalcemia in the development of IRIS in non-HIV pulmonary TB patients. Immune reconstitution inflammatory syndrome is also suggested to be strongly associated with mortality, and patients with these risk factors should be treated carefully.
Author Contributions
Y.H., Y.K. (Yasuhiko Koga), and K.K. conceptualized and designed the study; Y.H. performed data collection; Y.H., S.K., and H.T. performed statistical analysis; Y.H., Y.K. (Yasuhiko Koga), M.Y., H.A.-S., H.T., N.S., T.M., Y.K. (Yosuke Kamide), T.I., and T.H.; performed the literature search and data interpretation. Y.H. and Y.K. (Yasuhiko Koga) wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by JSPS KAKENHI (#18K08382).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheng, V.C.; Yam, W.C.; Woo, P.C.; Lau, S.K.; Hung, I.F.; Wong, S.P.; Cheung, W.C.; Yuen, K.Y. Risk factors for development of paradoxical response during antituberculosis therapy in HIV-negative patients. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Singh, N. Immune reconstitution inflammatory syndrome in non-HIV immunocompromised patients. Curr. Opin. Infect. Dis. 2009, 22, 394–402. [Google Scholar] [CrossRef]
- Cheng, S.L.; Wang, H.C.; Yang, P.C. Paradoxical response during anti-tuberculosis treatment in HIV-negative patients with pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2007, 11, 1290–1295. [Google Scholar] [PubMed]
- Breen, R.A.; Smith, C.J.; Bettinson, H.; Dart, S.; Bannister, B.; Johnson, M.A.; Lipman, M.C. Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax 2004, 59, 704–707. [Google Scholar] [CrossRef]
- Jung, J.W.; Shin, J.W.; Kim, J.Y.; Park, I.W.; Choi, B.W.; Seo, J.S.; Choi, J.C. Risk factors for development of paradoxical response during anti-tuberculosis treatment in HIV-negative patients with pleural tuberculosis. Tohoku J. Exp. Med. 2011, 223, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Ashkin, D.; Hollender, E.S.; Pitchenik, A.E. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am. J. Respir. Crit. Care Med. 1998, 158, 157–161. [Google Scholar] [CrossRef]
- Lipman, M.; Breen, R. Immune reconstitution inflammatory syndrome in HIV. Curr. Opin. Infect. Dis. 2006, 19, 20–25. [Google Scholar] [CrossRef]
- Bonnet, M.; Baudin, E.; Jani, I.V.; Nunes, E.; Verhoustraten, F.; Calmy, A.; Bastos, R.; Bhatt, N.B.; Michon, C. Incidence of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome and impact on patient outcome. PLoS ONE 2013, 8, e84585. [Google Scholar] [CrossRef]
- Rivoisy, C.; Tubach, F.; Roy, C.; Nicolas, N.; Mariette, X.; Salmon, D.; Lortholary, O.; Bourgarit, A. Paradoxical anti-TNF-associated TB worsening: Frequency and factors associated with IRIS. Jt. BoneSpine 2016, 83, 173–178. [Google Scholar] [CrossRef]
- Tanaka, T.; Sekine, A.; Tsunoda, Y.; Takoi, H.; Lin, S.Y.; Yatagai, Y.; Hayasihara, K.; Saito, T. Central nervous system manifestations of tuberculosis-associated immune reconstitution inflammatory syndrome during adalimumab therapy: A case report and review of the literature. Intern. Med. 2015, 54, 847–851. [Google Scholar] [CrossRef][Green Version]
- Geri, G.; Passeron, A.; Heym, B.; Arlet, J.B.; Pouchot, J.; Capron, L.; Ranque, B. Paradoxical reactions during treatment of tuberculosis with extrapulmonary manifestations in HIV-negative patients. Infection 2013, 41, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.S.; Smith, C.J.; Breen, R.A.; Ormerod, L.P.; Mittal, R.; Fisk, M.; Milburn, H.J.; Price, N.M.; Bothamley, G.H.; Lipman, M.C. Determinants of treatment-related paradoxical reactions during anti-tuberculosis therapy: A case control study. BMC Infect. Dis. 2016, 16, 479. [Google Scholar] [CrossRef] [PubMed]
- Kasama, S.; Toyama, T.; Sumino, H.; Nakazawa, M.; Matsumoto, N.; Sato, Y.; Kumakura, H.; Takayama, Y.; Ichikawa, S.; Suzuki, T.; et al. Prognostic value of serial cardiac 123I-MIBG imaging in patients with stabilized chronic heart failure and reduced left ventricular ejection fraction. J. Nucl. Med. 2008, 49, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Kasama, S.; Toyama, T.; Kurabayashi, M. Serial (1)(2)(3)I-metaiodobenzylguanidine imaging predicts the risk of sudden cardiac death in patients with chronic heart failure. Int. J. Cardiol. 2015, 179, 82–83. [Google Scholar] [CrossRef]
- Musselwhite, L.W.; Andrade, B.B.; Ellenberg, S.S.; Tierney, A.; Belaunzaran-Zamudio, P.F.; Rupert, A.; Lederman, M.M.; Sanne, I.; Sierra Madero, J.G.; Sereti, I. Vitamin D, D-dimer, Interferon gamma, and sCD14 Levels are Independently Associated with Immune Reconstitution Inflammatory Syndrome: A Prospective, International Study. EBioMedicine 2016, 4, 115–123. [Google Scholar] [CrossRef]
- Jeon, K.; Choi, W.I.; An, J.S.; Lim, S.Y.; Kim, W.J.; Park, G.M.; Park, S.S.; Choi, H.S.; Lee, B.H.; Choi, J.C.; et al. Paradoxical response in HIV-negative patients with pleural tuberculosis: A retrospective multicentre study. Int. J. Tuberc. Lung Dis. 2012, 16, 846–851. [Google Scholar] [CrossRef]
- Watanabe, S.; Kaneko, Y.; Kawamoto, H.; Maehara, T.; Baba, Y.; Fujisaki, I.; Saito, N.; Ryu, K.; Seki, A.; Horikiri, T.; et al. Paradoxical response with increased tumor necrosis factor-alpha levels to anti-tuberculosis treatment in a patient with disseminated tuberculosis. Respir. Med. Case Rep. 2017, 20, 201–204. [Google Scholar]
- Unlu, M.; Cimen, P.; Ayranci, A.; Akarca, T.; Karaman, O.; Dereli, M.S. Disseminated tuberculosis infection and paradoxical reaction during antimycobacterial treatment related to TNF-alpha blocker agent Infliximab. Respir. Med. Case Rep. 2014, 13, 43–47. [Google Scholar] [CrossRef]
- Melboucy-Belkhir, S.; Flexor, G.; Stirnemann, J.; Morin, A.S.; Boukari, L.; Polliand, C.; Cruaud, P.; Fain, O. Prolonged paradoxical response to anti-tuberculous treatment after infliximab. Int. J. Infect. Dis. 2010, 14, e333–e334. [Google Scholar] [CrossRef]
- Yamada, H. Current perspectives on the role of IL-17 in autoimmune disease. J. Inflamm. Res. 2010, 3, 33–44. [Google Scholar] [CrossRef]
- Ruschen, S.; Stellberg, W.; Warnatz, H. Kinetics of cytokine secretion by mononuclear cells of the blood from rheumatoid arthritis patients are different from those of healthy controls. Clin. Exp. Immunol. 1992, 89, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Van Roon, J.A.; Verhoef, C.M.; van Roy, J.L.; Gmelig-Meyling, F.H.; Huber-Bruning, O.; Lafeber, F.P.; Bijlsma, J.W. Decrease in peripheral type 1 over type 2 T cell cytokine production in patients with rheumatoid arthritis correlates with an increase in severity of disease. Ann. Rheum. Dis. 1997, 56, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Okamoto, S.; Hisamatsu, T.; Kamada, N.; Chinen, H.; Saito, R.; Kitazume, M.T.; Nakazawa, A.; Sugita, A.; Koganei, K.; et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut 2008, 57, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Imami, N.; Antonopoulos, C.; Hardy, G.A.; Gazzard, B.; Gotch, F.M. Assessment of type 1 and type 2 cytokines in HIV type 1-infected individuals: Impact of highly active antiretroviral therapy. AIDS Res. Hum. Retrovir. 1999, 15, 1499–1508. [Google Scholar] [CrossRef]
- Lanzafame, M.; Vento, S. Tuberculosis-immune reconstitution inflammatory syndrome. J. Clin. Tuberc. Other Mycobact. Dis. 2016, 3, 6–9. [Google Scholar] [CrossRef]
- Douglas, S.D.; Durako, S.; Sullivan, K.E.; Camarca, M.; Moscicki, A.B.; Wilson, C.M. TH1 and TH2 cytokine mRNA and protein levels in human immunodeficiency virus (HIV)-seropositive and HIV-seronegative youths. Clin. Diagn. Lab. Immunol. 2003, 10, 399–404. [Google Scholar] [CrossRef]
- Yamada, H.; Hida, N.; Kurashima, Y.; Satoh, H.; Saito, T.; Hizawa, N. A case of severe eosinophilic asthma and refractory rheumatoid arthritis well controlled by combination of IL-5Ralpha antibody and TNFalpha inhibitor. Allergol. Int. 2019, 68, 536–538. [Google Scholar] [CrossRef]
- Singh, N. Hypercalcemia related to immune reconstitution in organ transplant recipients with granulomatous opportunistic infections. Transplantation 2006, 82, 986. [Google Scholar] [CrossRef]
- Pornsuriyasak, P.; Suwatanapongched, T. Thoracic manifestations of paradoxical immune reconstitution inflammatory syndrome during or after antituberculous therapy in HIV-negative patients. Diagn. Interv. Radiol. 2015, 21, 134–139. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, C.J.; Kuo, Y.W.; Wang, J.Y.; Hsu, C.L.; Chen, J.M.; Cheng, W.C.; Lee, L.N. Tuberculosis mortality: Patient characteristics and causes. BMC Infect. Dis. 2014, 14, 5. [Google Scholar] [CrossRef]
- Alavi-Naini, R.; Moghtaderi, A.; Metanat, M.; Mohammadi, M.; Zabetian, M. Factors associated with mortality in tuberculosis patients. J. Res. Med. Sci. 2013, 18, 52–55. [Google Scholar] [PubMed]
- Erbes, R.; Oettel, K.; Raffenberg, M.; Mauch, H.; Schmidt-Ioanas, M.; Lode, H. Characteristics and outcome of patients with active pulmonary tuberculosis requiring intensive care. Eur. Respir. J. 2006, 27, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Meintjes, G.; Boulle, A. Immune reconstitution inflammatory syndrome in a large multicenter cohort study: Case definition and comparability. Expert. Rev. Anti. Infect. 2012, 10, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Sainz-de-la-Maza, S.; Casado, J.L.; Perez-Elias, M.J.; Moreno, A.; Quereda, C.; Moreno, S.; Corral, I. Incidence and prognosis of immune reconstitution inflammatory syndrome in HIV-associated progressive multifocal leucoencephalopathy. Eur. J. Neurol. 2016, 23, 919–925. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).