Impact of Sustained Virological Response for Gastroesophageal Varices in Hepatitis-C-Virus-Related Liver Cirrhosis
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. GEVs and Our Study Endpoint
2.3. Definition of Follow-Up
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Treatment for SVR
3.3. GEVs Progression
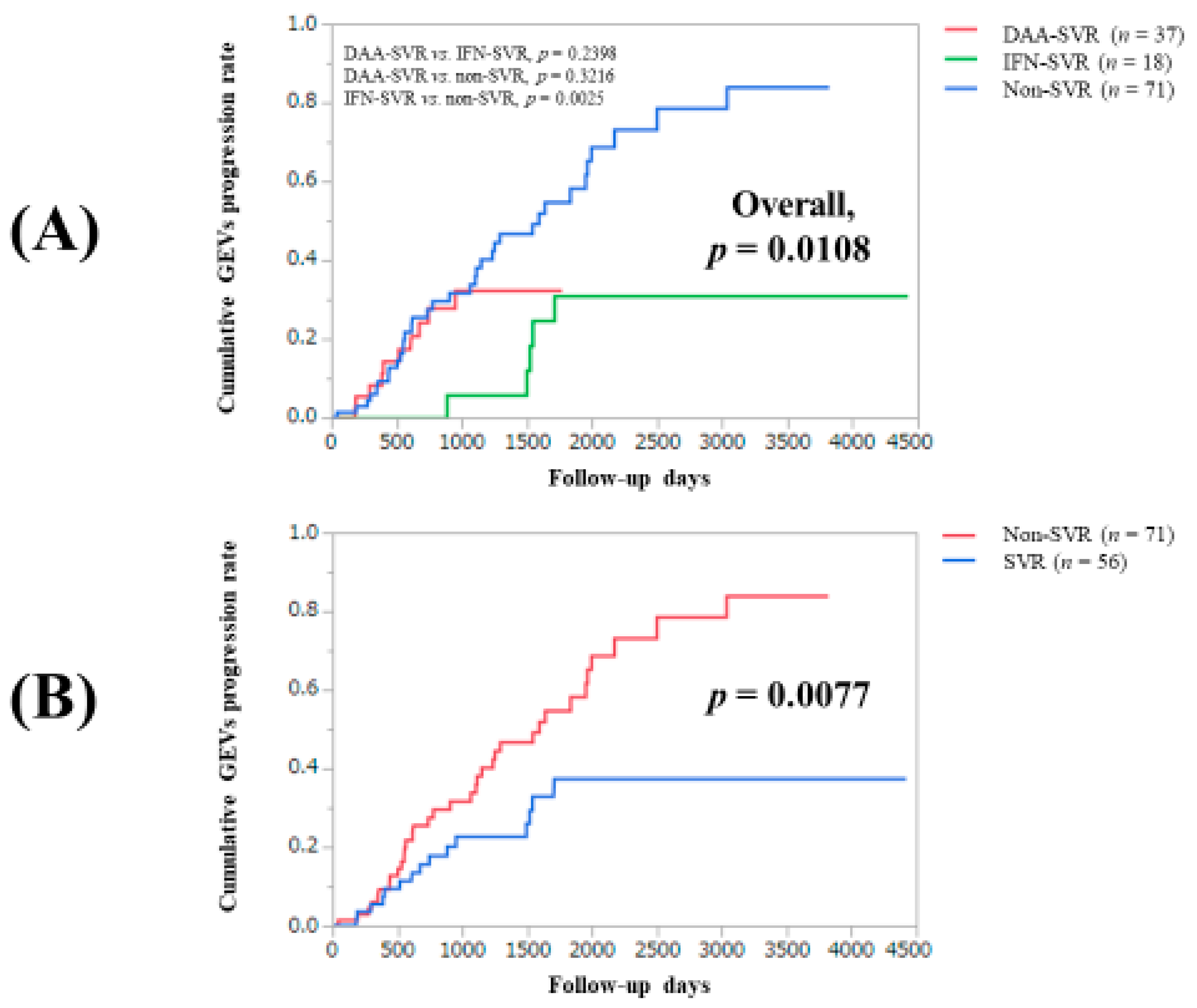
3.4. Comparison of Baseline Characteristics among IFN-SVR, DAA-SVR, and Non-SVR Groups
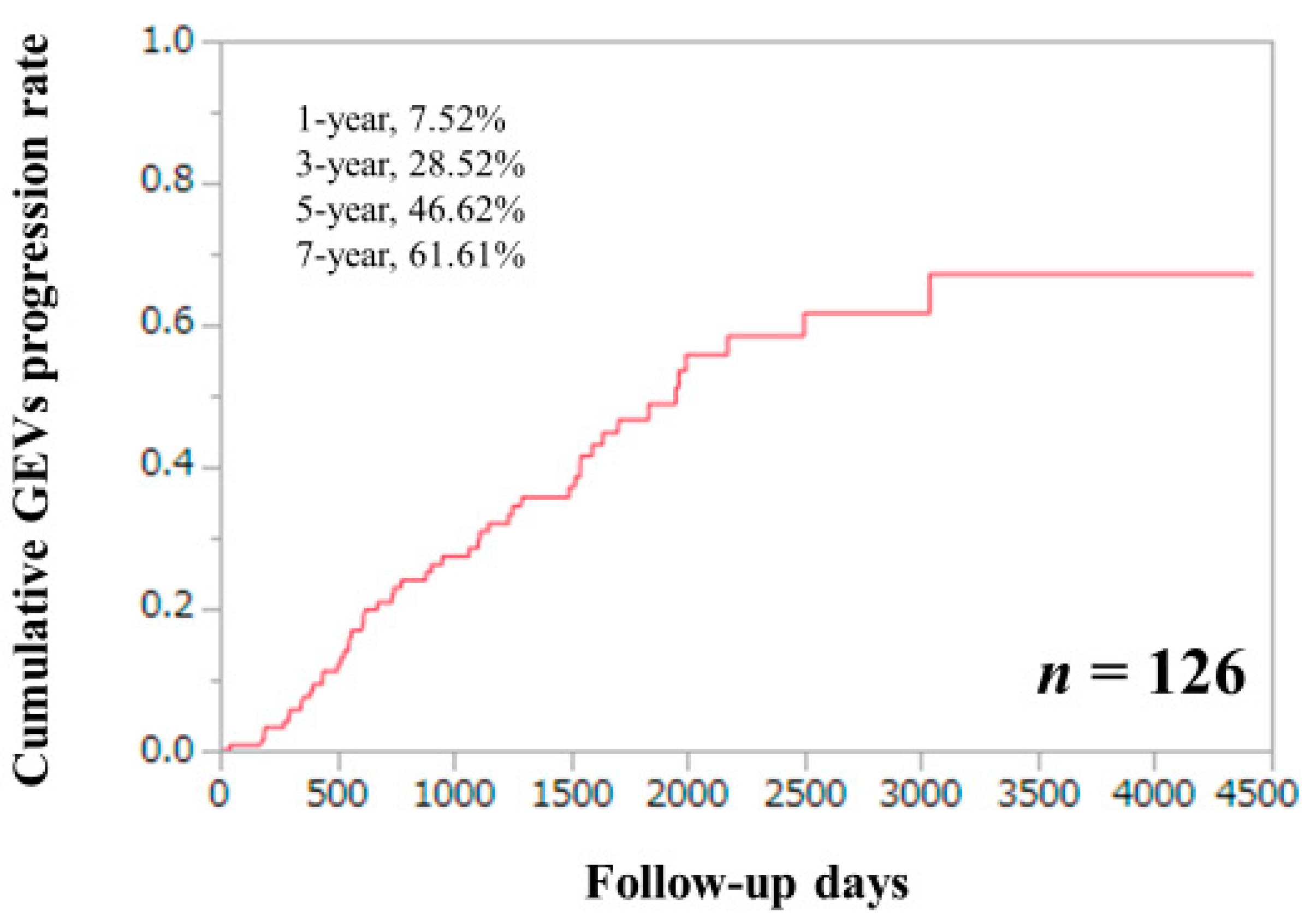
3.5. Cumulative GEVs Progression Rates for the Entire Cohort
3.6. Univariate and Multivariate Analyses of Parameters Contributing to GEVs Progression
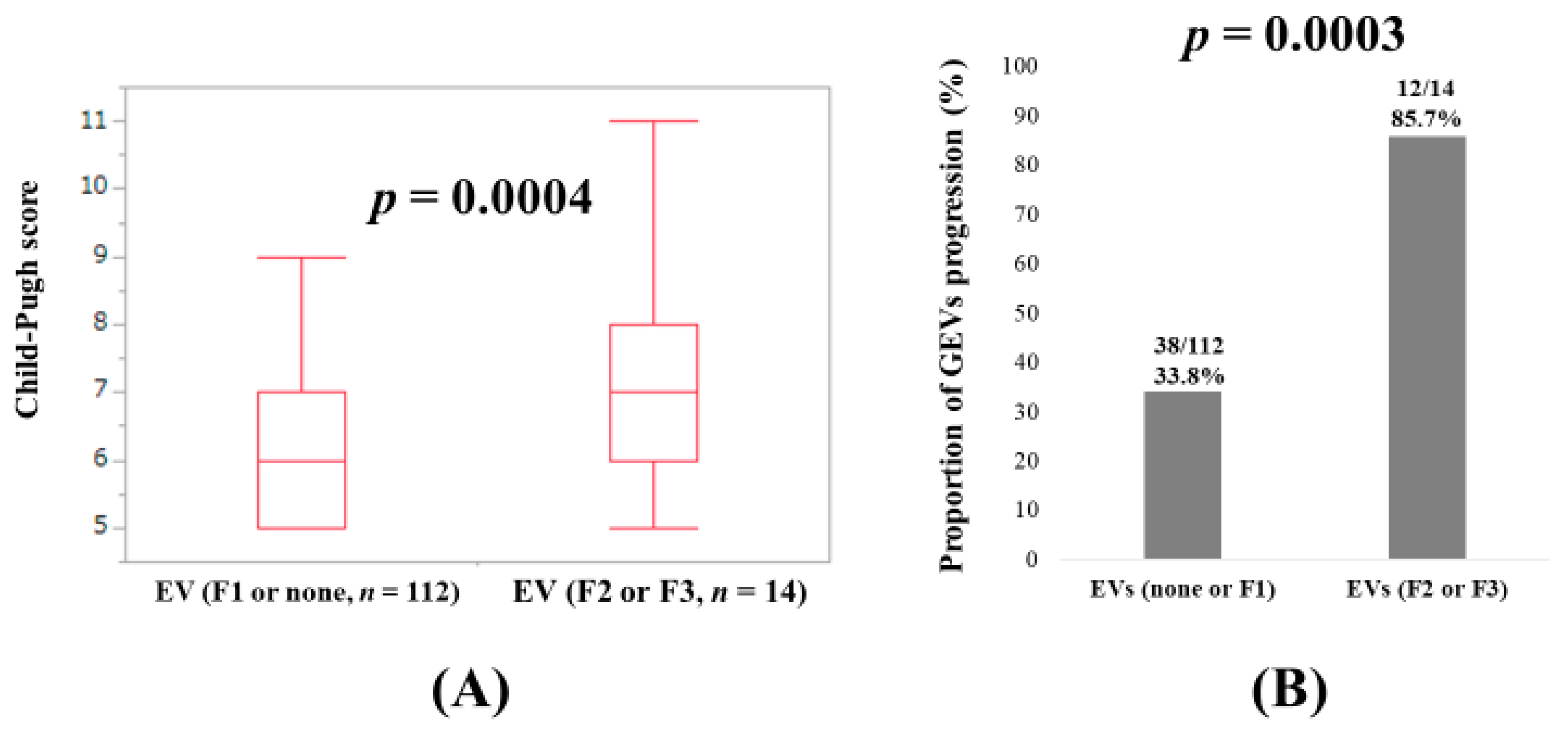
3.7. Child–Pugh Score and GEVs Progression Rate between Patients with EVs F1 or None and Those with EVs F2 or F3
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HCV | hepatitis C virus |
| LC | liver cirrhosis |
| PH | portal hypertension |
| HCC | hepatocellular carcinoma |
| SVR | sustained virological response |
| IFN | interferon |
| GEVs | gastroesophageal varices |
| DAAs | direct-acting antivirals |
| HVPG | hepatic venous pressure gradient |
| CSPH | clinically significant portal hypertension |
| EGD | esophagogastroduodenoscopy |
| PCR | polymerase chain reaction |
| IQR | interquartile range |
| PEG | pegylated |
| HR | hazard ratio |
| CI | confidence interval |
| EVs | esophageal varices |
| GVs | gastric varices |
References
- Webster, D.P.; Klenerman, P.; Dusheiko, G.M. Hepatitis C. Lancet 2015, 385, 1124–1135. [Google Scholar] [CrossRef]
- Emmanuel, B.; Wilson, E.M.; O’Brien, T.R.; Kottilil, S.; Lau, G. Shortening the duration of therapy for chronic hepatitis C infection. Lancet Gastroenterol. Hepatol. 2017, 2, 832–836. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Desbois, A.C.; Comarmond, C.; Saadoun, D. Impact of sustained virological response on the extrahepatic manifestations of chronic hepatitis C: A meta-analysis. Gut 2018, 67, 2025–2034. [Google Scholar] [CrossRef]
- Van der Meer, A.J.; Feld, J.J.; Hofer, H.; Almasio, P.L.; Calvaruso, V.; Fernández-Rodríguez, C.M.; Aleman, S.; Ganne-Carrié, N.; D’Ambrosio, R.; Pol, S.; et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J. Hepatol. 2017, 66, 485–493. [Google Scholar] [CrossRef]
- Van der Meer, A.J.; Berenguer, M. Reversion of disease manifestations after HCV eradication. J. Hepatol. 2016, 65, S95–S108. [Google Scholar] [CrossRef]
- Smith-Palmer, J.; Cerri, K.; Valentine, W. Achieving sustained virologic response in hepatitis C: A systematic review of the clinical, economic and quality of life benefits. BMC Infect. Dis. 2015, 15, 19. [Google Scholar] [CrossRef]
- Di Marco, V.; Calvaruso, V.; Ferraro, D.; Bavetta, M.G.; Cabibbo, G.; Conte, E.; Cammà, C.; Grimaudo, S.; Pipitone, R.M.; Simone, F.; et al. Effects of Eradicating Hepatitis C Virus Infection in Patients With Cirrhosis Differ With Stage of Portal Hypertension. Gastroenterology 2016, 151, 130–139.e2. [Google Scholar] [CrossRef]
- Yoh, K.; Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Hasegawa, K.; Nakano, C.; et al. Predictors Associated with Increase in Skeletal Muscle Mass after Sustained Virological Response in Chronic Hepatitis C Treated with Direct Acting Antivirals. Nutrients 2017, 9, 1135. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Hézode, C.; Fontaine, H.; Dorival, C.; Zoulim, F.; Larrey, D.; Canva, V.; De Ledinghen, V.; Poynard, T.; Samuel, D.; Bourliere, M.; et al. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology 2014, 147, 132–142.e4. [Google Scholar] [CrossRef]
- Aizawa, N.; Enomoto, H.; Takashima, T.; Sakai, Y.; Iwata, K.; Ikeda, N.; Tanaka, H.; Iwata, Y.; Saito, M.; Imanishi, H.; et al. Thrombocytopenia in pegylated interferon and ribavirin combination therapy for chronic hepatitis C. J. Gastroenterol. 2014, 49, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Tamai, H.; Mori, Y.; Shingaki, N.; Shimizu, R.; Nuta, J.; Moribata, K.; Maeda, Y.; Muraki, Y.; Deguchi, H.; Inoue, I.; et al. Prognostic effect of response to interferon therapy after laparoscopic splenectomy among patients with marked thrombocytopenia and hepatitis C virus-related cirrhosis. Hepatol. Int. 2015, 9, 67–75. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address: Easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [PubMed]
- Mangia, A.; Cenderello, G.; Copetti, M.; Verucchi, G.; Piazzolla, V.; Lorusso, C.; Santoro, R.; Squillante, M.M.; Orlandini, A.; Minisini, R.; et al. SVR12 Higher than 97% in GT3 Cirrhotic Patients with Evidence of Portal Hypertension Treated with SOF/VEL without Ribavirin: A Nation-Wide Cohort Study. Cells 2019, 8, 313. [Google Scholar] [CrossRef]
- Modi, A.A.; Nazario, H.E.; Gonzales, G.R.; Gonzalez, S.A. Safety and efficacy of ledipasvir/sofosbuvir with or without ribavirin in hepatitis C genotype 1 patients including those with decompensated cirrhosis who failed prior treatment with simeprevir/sofosbuvir. Aliment. Pharmacol. Ther. 2018, 47, 1409–1415. [Google Scholar] [CrossRef]
- Persico, M.; Rosato, V.; Aglitti, A.; Precone, D.; Corrado, M.; De Luna, A.; Morisco, F.; Camera, S.; Federico, A.; Dallio, M.; et al. Sustained virological response by direct antiviral agents in HCV leads to an early and significant improvement of liver fibrosis. Antivir. Ther. 2018, 23, 129–138. [Google Scholar] [CrossRef]
- Mangia, A.; Susser, S.; Piazzolla, V.; Agostinacchio, E.; De Stefano, G.; Palmieri, V.; Spinzi, G.; Carraturo, I.; Potenza, D.; Losappio, R.; et al. Sofosbuvir and ribavirin for genotype 2 HCV infected patients with cirrhosis: A real life experience. J. Hepatol. 2017, 66, 711–717. [Google Scholar] [CrossRef]
- Villani, R.; Monami, M.; Di Cosimo, F.; Fioravanti, G.; Mannucci, E.; Vendemiale, G.; Serviddio, G. Direct-acting antivirals for HCV treatment in older patients: A systematic review and meta-analysis. J. Viral Hepat. 2019, 26, 1249–1256. [Google Scholar] [CrossRef]
- Afdhal, N.; Everson, G.T.; Calleja, J.L.; McCaughan, G.W.; Bosch, J.; Brainard, D.M.; McHutchison, J.G.; De-Oertel, S.; An, D.; Charlton, M.; et al. Effect of viral suppression on hepatic venous pressure gradient in hepatitis C with cirrhosis and portal hypertension. J. Viral Hepat. 2017, 24, 823–831. [Google Scholar] [CrossRef]
- Thabut, D.; Bureau, C.; Layese, R.; Bourcier, V.; Hammouche, M.; Cagnot, C.; Marcellin, P.; Guyader, D.; Pol, S.; Larrey, D.; et al. Validation of Baveno VI Criteria for Screening and Surveillance of Esophageal Varices in Patients with Compensated Cirrhosis and a Sustained Response to Antiviral Therapy. Gastroenterology 2019, 156, 997–1009.e5. [Google Scholar] [CrossRef] [PubMed]
- Puigvehí, M.; Londoño, M.C.; Torras, X.; Lorente, S.; Vergara, M.; Morillas, R.M.; Masnou, H.; Serrano, T.; Miquel, M.; Gallego, A.; et al. Impact of sustained virological response with DAAs on gastroesophageal varices and Baveno criteria in HCV-cirrhotic patients. J. Gastroenterol. 2019. [Google Scholar] [CrossRef]
- Lens, S.; Alvarado-Tapias, E.; Mariño, Z.; Londoño, M.C.; Llop, E.; Martinez, J.; Fortea, J.I.; Ibañez, L.; Ariza, X.; Baiges, A.; et al. Effects of All-Oral Anti-Viral Therapy on HVPG and Systemic Hemodynamics in Patients With Hepatitis C Virus-Associated Cirrhosis. Gastroenterology 2017, 153, 1273–1283.e1. [Google Scholar] [CrossRef]
- Libânio, D.; Marinho, R.T. Impact of hepatitis C oral therapy in portal hypertension. World J. Gastroenterol. 2017, 23, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health Consensus Development Conference Statement. Management of hepatitis C: June 10–12, 2002. Hepatology 2002, 36 (Suppl. 1), S3–S20. [CrossRef]
- Fukui, H.; Saito, H.; Ueno, Y.; Uto, H.; Obara, K.; Sakaida, I.; Shibuya, A.; Seike, M.; Nagoshi, S.; Segawa, M.; et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J. Gastroenterol. 2016, 51, 629–650. [Google Scholar]
- European Association for the Study of the Liver. Electronic address: Easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Feld, J.J. What Are the Benefits of a Sustained Virologic Response to Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection? Gastroenterology 2019, 156, 446–460.e2. [Google Scholar] [CrossRef]
- Pradat, P.; Virlogeux, V.; Trépo, E. Epidemiology and Elimination of HCV-Related Liver Disease. Viruses 2018, 10, 545. [Google Scholar] [CrossRef]
- Bradshaw, D.; Mbisa, J.L.; Geretti, A.M.; Healy, B.J.; Cooke, G.S.; Foster, G.R.; Thomson, E.C.; McLauchlan, J.; Agarwal, K.; Sabin, C.; et al. Consensus recommendations for resistance testing in the management of chronic hepatitis C virus infection: Public Health England HCV Resistance Group. J. Infect. 2019, 79, 503–512. [Google Scholar] [CrossRef]
- Bruno, S.; Crosignani, A.; Facciotto, C.; Rossi, S.; Roffi, L.; Redaelli, A.; de Franchis, R.; Almasio, P.L.; Maisonneuve, P. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology 2010, 51, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Mandorfer, M.; Kozbial, K.; Schwabl, P.; Freissmuth, C.; Schwarzer, R.; Stern, R.; Chromy, D.; Stättermayer, A.F.; Reiberger, T.; Beinhardt, S.; et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J. Hepatol. 2016, 65, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Ziada, D.; Salama, M.; Hamisa, M.; Badawi, R.; Hawash, N.; Selim, A.; Abd-Elsalam, S. Predictors for Fibrosis Regression in Chronic HCV Patients after Treatment with DAAS: Results of a Real-world Cohort Study. Endocr. Metab. Immune Disord. Drug Targets 2019. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Tada, T.; Yasuda, S.; Mizuno, K.; Ito, T.; Kumada, T. Dynamic Evaluation of Liver Fibrosis to Assess the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis C Who Achieved Sustained Virologic Response. Clin. Infect. Dis. 2019, ciz359. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Cases (n = 126) |
|---|---|
| Age (years) | 66 (61.75, 70.25) |
| Sex, male/female | 70/56 |
| HCV genotype, 1/2/others/not tested | 97/19/6/4 |
| HCV viral load, high/low | 101/25 |
| Child–Pugh classification, A/B/C | 82/43/1 |
| Our type classification | 18/37/71 |
| IFN-SVR/DAA-SVR/non-SVR | |
| Presence of ascites, yes/no | 28/98 |
| Presence of encephalopathy, yes/no | 8/118 |
| Total bilirubin (mg/dL) | 1.0 (0.7, 1.4) |
| Serum albumin (g/dL) | 3.7 (3.3, 4.0) |
| Prothrombin time (%) | 76.2 (69.175, 84.3) |
| Platelet count (×104/mm3) | 7.7 (6.0, 9.925) |
| AST (IU/L) | 60.5 (41.75, 87.25) |
| ALT (IU/L) | 49 (30, 82.25) |
| Endoscopic findings | |
| Esophageal varices, F3/F2/F1/not detected | 0/14/107/5 |
| Gastric varices, F3/F2/F1/not detected | 1/10/34/81 |
| Variables | IFN-SVR (n = 18) | DAA-SVR (n = 37) | Non-SVR (n = 71) | p Value IFN vs. DAA | p Value IFN vs. Non | p Value DAA vs. Non | Overall p Value |
|---|---|---|---|---|---|---|---|
| Age (years) | 66.5 (57.75, 70) | 68 (63, 70) | 66 (62, 71) | 0.2996 | 0.5088 | 0.5218 | 0.5648 |
| Sex, male/female | 13/5 | 16/21 | 41/30 | 0.0510 | 0.2944 | 0.1622 | 0.1089 |
| Child–Pugh A/B/C | 14/4/0 | 22/15/0 | 46/24/1 | 0.2343 | 0.5420 | 0.6261 | 0.6274 |
| Ascites, yes/no | 4/14 | 6/31 | 18/53 | 0.7128 | 0.9999 | 0.3358 | 0.5558 |
| Encephalopathy, yes/no | 0/18 | 2/35 | 6/65 | 0.9999 | 0.3409 | 0.7126 | 0.4059 |
| Total bilirubin (mg/dL) | 0.9 (0.7, 1.325) | 1.1 (0.8, 1.35) | 1.0 (0.7, 1.5) | 0.1130 | 0.3126 | 0.5245 | 0.3545 |
| Serum albumin (g/dL) | 3.8 (3.375, 4.125) | 3.5 (3.3, 3.9) | 3.8 (3.3, 4.0) | 0.2161 | 0.4688 | 0.6302 | 0.5706 |
| Prothrombin time (%) | 78.15 (66.175, 86.225) | 71.4 (66.05, 76.45) | 78.8 (71.2, 87.0) | 0.0455 | 0.6464 | 0.0004 | 0.0018 |
| Platelet (×104/mm3) | 10.45 (8.8, 14.075) | 7.7 (5.6, 10.55) | 7.2 (5.8, 9.2) | 0.0151 | <0.0001 | 0.1682 | 0.0002 |
| AST (IU/L) | 52 (33, 79) | 53 (41.5, 79.5) | 63 (43, 93) | 0.6996 | 0.9956 | 0.1931 | 0.2961 |
| ALT (IU/L) | 48 (26.25, 99.75) | 40 (28.5, 76) | 54 (32, 85) | 0.5300 | 0.8142 | 0.0993 | 0.1346 |
| EVs, F3/F2/F1/ND | 0/1/16/1 | 0/4/32/1 | 0/9/59/3 | 0.7236 | 0.6833 | 0.8800 | 0.9088 |
| GVs, F3/F2/ F1/ND | 0/3/7/8 | 0/2/11/24 | 1/5/16/49 | 0.2384 | 0.2059 | 0.7561 | 0.4466 |
| Variables | Number of Each Category | Univariate |
|---|---|---|
| p Value | ||
| Age (years) 66 or more, yes/no | 69/57 | 0.5812 |
| Sex, male/female | 70/56 | 0.0253 |
| Presence of ascites, yes/no | 28/98 | 0.2331 |
| Presence of encephalopathy, yes/no | 8/118 | 0.1877 |
| Child–Pugh A, yes/no | 82/44 | 0.2607 |
| Our type classification, IFN-SVR/DAA-SVR/non-SVR | 18/37/71 | 0.0108 |
| Esophageal varices F2 or more, yes/no | 14/112 | <0.0001 |
| Gastric varices F2 or more, yes/no | 11/115 | 0.5475 |
| Serum albumin 3.7 g/dL or more, yes/no | 66/60 | 0.4992 |
| Total bilirubin 1.0 mg/dL or more, yes/no | 71/55 | 0.7782 |
| Prothrombin time 76.2% or more, yes/no | 63/63 | 0.0821 |
| Platelet count 7.7 ×104/mm3 or more, yes/no | 64/62 | 0.3308 |
| AST 60.5 IU/L or more, yes/no | 63/63 | 0.1204 |
| ALT 49 IU/L or more, yes/no | 64/62 | 0.5389 |
| Variables | Multivariate Analysis | ||
|---|---|---|---|
| Hazard Ratio | 95% CI | p Value | |
| Sex | |||
| Male | Reference | ||
| Female | 0.524 | 0.280–0.980 | 0.0430 |
| Esophageal varices F2 or more | |||
| No | Reference | ||
| Yes | 5.898 | 2.900–11.995 | <0.0001 |
| Our type classification | |||
| IFN-SVR | Reference | ||
| DAA-SVR | 4.496 | 1.380–14.655 | 0.0126 |
| Non-SVR | 5.126 | 1.910–13.756 | 0.0012 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuri, Y.; Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; et al. Impact of Sustained Virological Response for Gastroesophageal Varices in Hepatitis-C-Virus-Related Liver Cirrhosis. J. Clin. Med. 2020, 9, 95. https://doi.org/10.3390/jcm9010095
Yuri Y, Nishikawa H, Enomoto H, Yoh K, Iwata Y, Sakai Y, Kishino K, Ikeda N, Takashima T, Aizawa N, et al. Impact of Sustained Virological Response for Gastroesophageal Varices in Hepatitis-C-Virus-Related Liver Cirrhosis. Journal of Clinical Medicine. 2020; 9(1):95. https://doi.org/10.3390/jcm9010095
Chicago/Turabian StyleYuri, Yukihisa, Hiroki Nishikawa, Hirayuki Enomoto, Kazunori Yoh, Yoshinori Iwata, Yoshiyuki Sakai, Kyohei Kishino, Naoto Ikeda, Tomoyuki Takashima, Nobuhiro Aizawa, and et al. 2020. "Impact of Sustained Virological Response for Gastroesophageal Varices in Hepatitis-C-Virus-Related Liver Cirrhosis" Journal of Clinical Medicine 9, no. 1: 95. https://doi.org/10.3390/jcm9010095
APA StyleYuri, Y., Nishikawa, H., Enomoto, H., Yoh, K., Iwata, Y., Sakai, Y., Kishino, K., Ikeda, N., Takashima, T., Aizawa, N., Takata, R., Hasegawa, K., Ishii, N., Nishimura, T., Iijima, H., & Nishiguchi, S. (2020). Impact of Sustained Virological Response for Gastroesophageal Varices in Hepatitis-C-Virus-Related Liver Cirrhosis. Journal of Clinical Medicine, 9(1), 95. https://doi.org/10.3390/jcm9010095




