Clinical Applications, Pitfalls, and Uncertainties of Thrombin Generation in the Presence of Platelets
Abstract
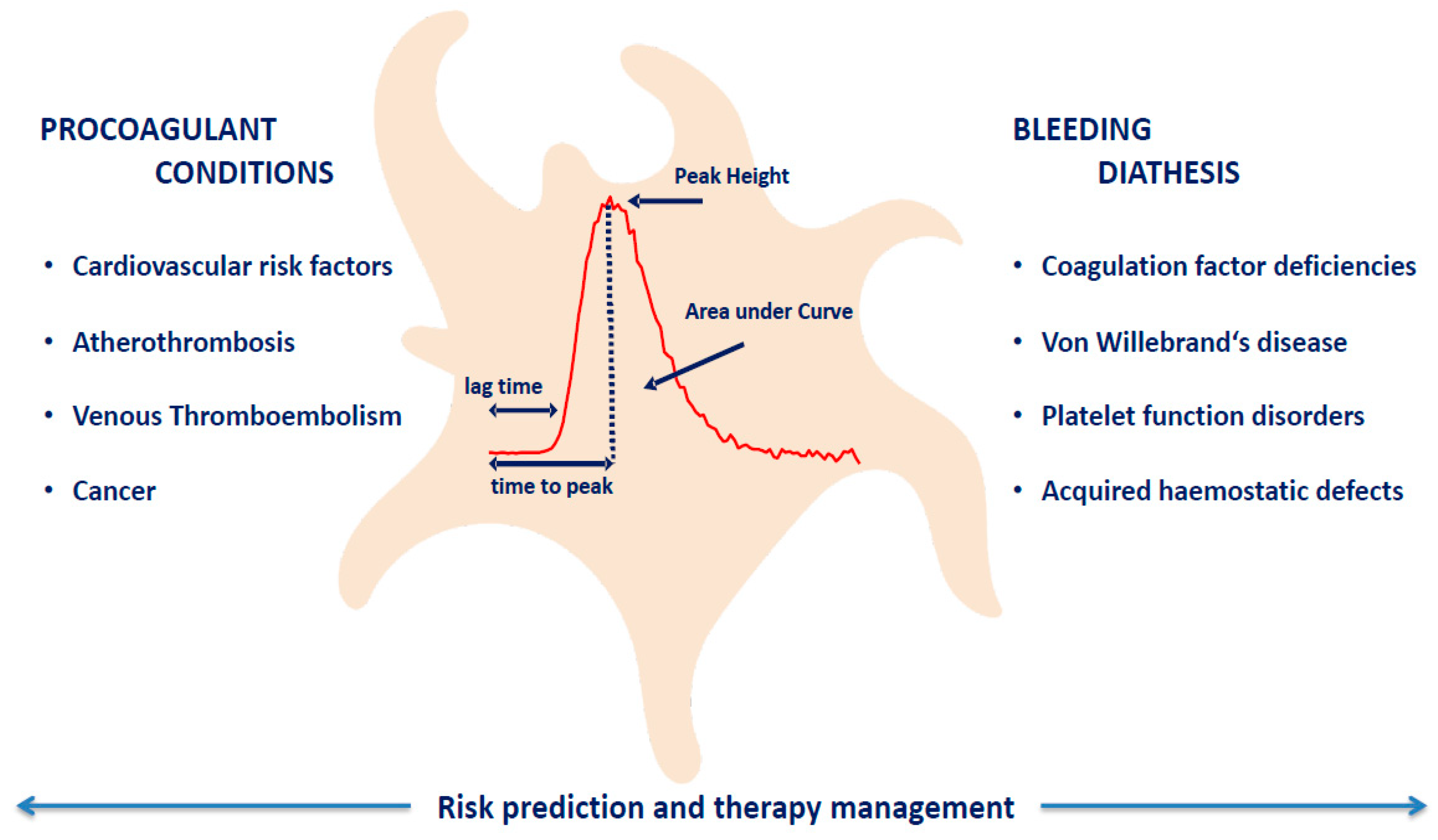
1. Introduction
2. Platelet-Dependent Thrombin Generation in Prothrombotic Conditions
3. Platelet-Dependent Thrombin Generation in Bleeding Diathesis
4. Uncertainties and Limitations of Thrombin Generation in the Presence of Platelets
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tritschler, T.; Kraaijpoel, N.; Le Gal, G.; Wells, P.S. Venous Thromboembolism: Advances in Diagnosis and Treatment. JAMA 2018, 320, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Boender, J.; Kruip, M.J.; Leebeek, F.W. A diagnostic approach to mild bleeding disorders. J. Thromb. Haemost. 2016, 14, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Hemker, H.C.; Giesen, P.; AlDieri, R.; Regnault, V.; de Smed, E.; Wagenvoord, R.; Lecompte, T.; Beguin, S. The calibrated automated thrombogram (CAT): A universal routine test for hyper- and hypocoagulability. Pathophysiol. Haemost. Thromb. 2002, 32, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Hemker, H.C.; Giesen, P.L.; Ramjee, M.; Wagenvoord, R.; Beguin, S. The thrombogram: Monitoring thrombin generation in platelet-rich plasma. Thromb. Haemost. 2000, 83, 589–591. [Google Scholar]
- Dargaud, Y.; Wolberg, A.S.; Luddington, R.; Regnault, V.; Spronk, H.; Baglin, T.; Lecompte, T.; Ten Cate, H.; Negrier, C. Evaluation of a standardized protocol for thrombin generation measurement using the calibrated automated thrombogram: An international multicentre study. Thromb. Res. 2012, 130, 929–934. [Google Scholar] [CrossRef]
- Hemker, H.C.; Giesen, P.; Al Dieri, R.; Regnault, V.; de Smedt, E.; Wagenvoord, R.; Lecompte, T.; Beguin, S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol. Haemost. Thromb. 2003, 33, 4–15. [Google Scholar] [CrossRef]
- Loeffen, R.; Kleinegris, M.C.; Loubele, S.T.; Pluijmen, P.H.; Fens, D.; van Oerle, R.; ten Cate, H.; Spronk, H.M. Preanalytic variables of thrombin generation: Towards a standard procedure and validation of the method. J. Thromb. Haemost. 2012, 10, 2544–2554. [Google Scholar] [CrossRef]
- Kossmann, S.; Lagrange, J.; Jackel, S.; Jurk, K.; Ehlken, M.; Schonfelder, T.; Weihert, Y.; Knorr, M.; Brandt, M.; Xia, N.; et al. Platelet-localized FXI promotes a vascular coagulation-inflammatory circuit in arterial hypertension. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Panova-Noeva, M.; Schulz, A.; Spronk, H.M.; Beicht, A.; Laubert-Reh, D.; van Oerle, R.; Arnold, N.; Prochaska, J.H.; Blettner, M.; Beutel, M.; et al. Clinical Determinants of Thrombin Generation Measured in Presence and Absence of Platelets-Results from the Gutenberg Health Study. Thromb. Haemost. 2018, 118, 873–882. [Google Scholar] [CrossRef]
- Panova-Noeva, M.; Neu, M.A.; Eckerle, S.; Spix, C.; Schneider, A.; Schmidtmann, I.; Spronk, H.M.; Pfeiffer, N.; Beutel, M.; Lackner, K.J.; et al. Cardiovascular risk factors are important determinants of platelet-dependent thrombin generation in adult survivors of childhood cancer. Clin. Res. Cardiol. 2018. [Google Scholar] [CrossRef]
- Faber, J.; Wingerter, A.; Neu, M.A.; Henninger, N.; Eckerle, S.; Munzel, T.; Lackner, K.J.; Beutel, M.E.; Blettner, M.; Rathmann, W.; et al. Burden of cardiovascular risk factors and cardiovascular disease in childhood cancer survivors: Data from the German CVSS-study. Eur. Heart J. 2018, 39, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Vazzana, N.; Liani, R.; Guagnano, M.T.; Davi, G. Platelet activation in obesity and metabolic syndrome. Obes. Rev. 2012, 13, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Horigome, H.; Tanaka, K.; Nakata, Y.; Ohkawara, K.; Katayama, Y.; Matsui, A. Impact of weight reduction on production of platelet-derived microparticles and fibrinolytic parameters in obesity. Thromb. Res. 2007, 119, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sonnevi, K.; Tchaikovski, S.N.; Holmstrom, M.; Antovic, J.P.; Bremme, K.; Rosing, J.; Larfars, G. Obesity and thrombin-generation profiles in women with venous thromboembolism. Blood Coagul. Fibrinolysis 2013, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Zabeo, E.; Radu, C.M.; Spiezia, L.; Gavasso, S.; Fadin, M.; Woodhams, B.; Vettor, R.; Simioni, P. Hypercoagulability in overweight and obese subjects who are asymptomatic for thrombotic events. Thromb. Haemost. 2015, 113, 85–96. [Google Scholar] [CrossRef]
- Tripodi, A.; Primignani, M.; Badiali, S.; de Ruberto, F.; Granelli, P.; Tosetti, G.; Clerici, M.; Padovan, L.; Chantarangkul, V.; Scalambrino, E.; et al. Body mass index reduction improves the baseline procoagulant imbalance of obese subjects. J. Thromb. Thrombolysis 2019, 48, 52–60. [Google Scholar] [CrossRef]
- Schneider, D.J.; Hardison, R.M.; Lopes, N.; Sobel, B.E.; Brooks, M.M.; Pro-Thrombosis Ancillary Study Group. Association between increased platelet P-selectin expression and obesity in patients with type 2 diabetes: A BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) substudy. Diabetes Care 2009, 32, 944–949. [Google Scholar] [CrossRef]
- Davi, G.; Patrono, C. Platelet activation and atherothrombosis. N. Engl. J. Med. 2007, 357, 2482–2494. [Google Scholar] [CrossRef]
- Ten Cate, H.; Hemker, H.C. Thrombin Generation and Atherothrombosis: What Does the Evidence Indicate? J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Faber, C.G.; Lodder, J.; Kessels, F.; Troost, J. Thrombin generation in platelet-rich plasma as a tool for the detection of hypercoagulability in young stroke patients. Pathophysiol. Haemost. Thromb. 2003, 33, 52–58. [Google Scholar] [CrossRef]
- Favaretto, E.; Sartori, M.; Legnani, C.; Rondelli, F.; Cini, M.; Guarino, M.; Cosmi, B. Thrombin generation and intracranial atherosclerotic disease in patients with a transient ischaemic attack. Thromb. Res. 2017, 155, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Chantarangkul, V.; Clerici, M.; Bressi, C.; Giesen, P.L.; Tripodi, A. Thrombin generation assessed as endogenous thrombin potential in patients with hyper- or hypo-coagulability. Haematologica 2003, 88, 547–554. [Google Scholar] [PubMed]
- Besser, M.; Baglin, C.; Luddington, R.; van Hylckama Vlieg, A.; Baglin, T. High rate of unprovoked recurrent venous thrombosis is associated with high thrombin-generating potential in a prospective cohort study. J. Thromb. Haemost. 2008, 6, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Ten Cate-Hoek, A.J.; Dielis, A.W.; Spronk, H.M.; van Oerle, R.; Hamulyak, K.; Prins, M.H.; ten Cate, H. Thrombin generation in patients after acute deep-vein thrombosis. Thromb. Haemost. 2008, 100, 240–245. [Google Scholar]
- Hron, G.; Kollars, M.; Binder, B.R.; Eichinger, S.; Kyrle, P.A. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA 2006, 296, 397–402. [Google Scholar] [CrossRef]
- Tappenden, K.A.; Gallimore, M.J.; Evans, G.; Mackie, I.J.; Jones, D.W. Thrombin generation: A comparison of assays using platelet-poor and -rich plasma and whole blood samples from healthy controls and patients with a history of venous thromboembolism. Br. J. Haematol. 2007, 139, 106–112. [Google Scholar] [CrossRef]
- Panova-Noeva, M.; Marchetti, M.; Spronk, H.M.; Russo, L.; Diani, E.; Finazzi, G.; Salmoiraghi, S.; Rambaldi, A.; Barbui, T.; Ten Cate, H.; et al. Platelet-induced thrombin generation by the calibrated automated thrombogram assay is increased in patients with essential thrombocythemia and polycythemia vera. Am. J. Hematol. 2011, 86, 337–342. [Google Scholar] [CrossRef]
- Panova-Noeva, M.; Marchetti, M.; Russo, L.; Tartari, C.J.; Leuzzi, A.; Finazzi, G.; Rambaldi, A.; ten Cate, H.; Falanga, A. ADP-induced platelet aggregation and thrombin generation are increased in Essential Thrombocythemia and Polycythemia Vera. Thromb. Res. 2013, 132, 88–93. [Google Scholar] [CrossRef]
- Altman, R.; Scazziota, A.S.; Herrera Mde, L.; Gonzalez, C. Thrombin generation by activated factor VII on platelet activated by different agonists. Extending the cell-based model of hemostasis. Thromb. J. 2006, 4, 5. [Google Scholar] [CrossRef][Green Version]
- Altman, R.; Scazziota, A.; De Lourdes Herrera, M.; Gonzalez, C. Recombinant factor VIIa reverses the inhibitory effect of aspirin or aspirin plus clopidogrel on in vitro thrombin generation. J. Thromb. Haemost. 2006, 4, 2022–2027. [Google Scholar] [CrossRef]
- Van der Meijden, P.E.; Feijge, M.A.; Giesen, P.L.; Huijberts, M.; van Raak, L.P.; Heemskerk, J.W. Platelet P2Y12 receptors enhance signalling towards procoagulant activity and thrombin generation. A study with healthy subjects and patients at thrombotic risk. Thromb. Haemost. 2005, 93, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Berezovskaya, G.; Smirnova, O.; Malev, E.; Khromov-Borisov, N.; Klokova, E.; Karpenko, M.; Papayan, L.; Petrishchev, N. Thrombin generation test for evaluation of antiplatelet treatment in patients with coronary artery disease after percutaneous coronary intervention. Platelets 2018, 29, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Consolo, F.; Pozzi, L.; Pieri, M.; Valle, P.D.; Redaelli, A.; D’Angelo, A.; Pappalardo, F. Influence of Different Antithrombotic Regimens on Platelet-Mediated Thrombin Generation in Patients with Left Ventricular Assist Devices. ASAIO J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Membre, A.; Wahl, D.; Latger-Cannard, V.; Max, J.P.; Lacolley, P.; Lecompte, T.; Regnault, V. The effect of platelet activation on the hypercoagulability induced by murine monoclonal antiphospholipid antibodies. Haematologica 2008, 93, 566–573. [Google Scholar] [CrossRef]
- Stopa, J.D.; Neuberg, D.; Puligandla, M.; Furie, B.; Flaumenhaft, R.; Zwicker, J.I. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI Insight 2017, 2, e89373. [Google Scholar] [CrossRef]
- Zwicker, J.I.; Schlechter, B.L.; Stopa, J.D.; Liebman, H.A.; Aggarwal, A.; Puligandla, M.; Caughey, T.; Bauer, K.A.; Kuemmerle, N.; Wong, E.; et al. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Makhoul, S.; Panova-Noeva, M.; Regnault, V.; Ruf, W.; Wenzel, P.; Lagrange, J. Rivaroxaban Effects Illustrate the Underestimated Importance of Activated Platelets in Thrombin Generation Assessed by Calibrated Automated Thrombography. J. Clin. Med. 2019, 8, 1990. [Google Scholar] [CrossRef]
- Wartiovaara-Kautto, U.; Joutsi-Korhonen, L.; Ilveskero, S.; Armstrong, E.; Lassila, R. Platelets significantly modify procoagulant activities in haemophilia A. Haemophilia 2011, 17, 743–751. [Google Scholar] [CrossRef]
- Santagostino, E.; Mancuso, M.E.; Tripodi, A.; Chantarangkul, V.; Clerici, M.; Garagiola, I.; Mannucci, P.M. Severe hemophilia with mild bleeding phenotype: Molecular characterization and global coagulation profile. J. Thromb. Haemost. 2010, 8, 737–743. [Google Scholar] [CrossRef]
- Siegemund, T.; Petros, S.; Siegemund, A.; Scholz, U.; Engelmann, L. Thrombin generation in severe haemophilia A and B: The endogenous thrombin potential in platelet-rich plasma. Thromb. Haemost. 2003, 90, 781–786. [Google Scholar] [CrossRef]
- Rugeri, L.; Beguin, S.; Hemker, C.; Bordet, J.C.; Fleury, R.; Chatard, B.; Negrier, C.; Dargaud, Y. Thrombin-generating capacity in patients with von Willebrand’s disease. Haematologica 2007, 92, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Keularts, I.M.; Hamulyak, K.; Hemker, H.C.; Beguin, S. The effect of DDAVP infusion on thrombin generation in platelet-rich plasma of von Willebrand type 1 and in mild haemophilia A patients. Thromb. Haemost. 2000, 84, 638–642. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szanto, T.; Nummi, V.; Jouppila, A.; Brinkman, H.J.M.; Lassila, R. Platelets compensate for poor thrombin generation in type 3 von Willebrand disease. Platelets 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Duckers, C.; Simioni, P.; Spiezia, L.; Radu, C.; Dabrilli, P.; Gavasso, S.; Rosing, J.; Castoldi, E. Residual platelet factor V ensures thrombin generation in patients with severe congenital factor V deficiency and mild bleeding symptoms. Blood 2010, 115, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, E.; Duckers, C.; Radu, C.; Spiezia, L.; Rossetto, V.; Tagariello, G.; Rosing, J.; Simioni, P. Homozygous F5 deep-intronic splicing mutation resulting in severe factor V deficiency and undetectable thrombin generation in platelet-rich plasma. J. Thromb. Haemost. 2011, 9, 959–968. [Google Scholar] [CrossRef]
- Rugeri, L.; Quelin, F.; Chatard, B.; De Mazancourt, P.; Negrier, C.; Dargaud, Y. Thrombin generation in patients with factor XI deficiency and clinical bleeding risk. Haemophilia 2010, 16, 771–777. [Google Scholar] [CrossRef]
- Gueguen, P.; Galinat, H.; Blouch, M.T.; Bridey, F.; Duchemin, J.; Le Gal, G.; Abgrall, J.F.; Pan-Petesch, B. Biological determinants of bleeding in patients with heterozygous factor XI deficiency. Br. J. Haematol. 2012, 156, 245–251. [Google Scholar] [CrossRef]
- Pike, G.N.; Cumming, A.M.; Hay, C.R.; Bolton-Maggs, P.H.; Burthem, J. Sample conditions determine the ability of thrombin generation parameters to identify bleeding phenotype in FXI deficiency. Blood 2015, 126, 397–405. [Google Scholar] [CrossRef]
- Lewis, S.J.; Stephens, E.; Florou, G.; Macartney, N.J.; Hathaway, L.S.; Knipping, J.; Collins, P.W. Measurement of global haemostasis in severe haemophilia A following factor VIII infusion. Br. J. Haematol. 2007, 138, 775–782. [Google Scholar] [CrossRef]
- Shenkman, B.; Livnat, T.; Misgav, M.; Budnik, I.; Einav, Y.; Martinowitz, U. The in vivo effect of fibrinogen and factor XIII on clot formation and fibrinolysis in Glanzmann’s thrombasthenia. Platelets 2012, 23, 604–610. [Google Scholar] [CrossRef]
- Levy-Mendelovich, S.; Levy, T.; Budnik, I.; Barg, A.A.; Rosenberg, N.; Seligsohn, U.; Kenet, G.; Livnat, T. Low Concentrations of Recombinant Factor VIIa May Improve the Impaired Thrombin Generation of Glanzmann Thrombasthenia Patients. Thromb. Haemost. 2019, 119, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Ninivaggi, M.; Feijge, M.A.; Baaten, C.C.; Kuiper, G.J.; Marcus, M.A.; Ten Cate, H.; Lance, M.D.; Heemskerk, J.W.; van der Meijden, P.E. Additive roles of platelets and fibrinogen in whole-blood fibrin clot formation upon dilution as assessed by thromboelastometry. Thromb. Haemost. 2014, 111, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Schols, S.E.; van der Meijden, P.E.; van Oerle, R.; Curvers, J.; Heemskerk, J.W.; van Pampus, E.C. Increased thrombin generation and fibrinogen level after therapeutic plasma transfusion: Relation to bleeding. Thromb. Haemost. 2008, 99, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Bosch, Y.P.; Al Dieri, R.; ten Cate, H.; Nelemans, P.J.; Bloemen, S.; de Laat, B.; Hemker, C.; Weerwind, P.W.; Maessen, J.G.; Mochtar, B. Measurement of thrombin generation intra-operatively and its association with bleeding tendency after cardiac surgery. Thromb. Res. 2014, 133, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Regnault, V.; Beguin, S.; Lecompte, T. Calibrated automated thrombin generation in frozen-thawed platelet-rich plasma to detect hypercoagulability. Pathophysiol. Haemost. Thromb. 2003, 33, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ljungkvist, M.; Lovdahl, S.; Zetterberg, E.; Berntorp, E. Low agreement between fresh and frozen-thawed platelet-rich plasma in the calibrated automated thrombogram assay. Haemophilia 2017, 23, e214–e218. [Google Scholar] [CrossRef]
- Baglin, T.; Besser, M.; Cattaneo, M.; Dargaud, Y.; Gray, E.; Key, N.; Wolberg, A. Towards a recommendation for the standardization of the measurement of platelet-dependent thrombin generation. J. Thromb. Haemost. 2011, 9, 1859–1861. [Google Scholar] [CrossRef]
- Gerotziafas, G.T.; Depasse, F.; Busson, J.; Leflem, L.; Elalamy, I.; Samama, M.M. Towards a standardization of thrombin generation assessment: The influence of tissue factor, platelets and phospholipids concentration on the normal values of Thrombogram-Thrombinoscope assay. Thromb. J. 2005, 3, 16. [Google Scholar] [CrossRef]
- Dargaud, Y.; Spronk, H.M.; Leenders, P.; Hemker, H.C.; Ten Cate, H. Monitoring platelet dependent thrombin generation in mice. Thromb. Res. 2010, 126, 436–441. [Google Scholar] [CrossRef]
- Van Der Meijden, P.E.; Van Schilfgaarde, M.; Van Oerle, R.; Renne, T.; ten Cate, H.; Spronk, H.M. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J. Thromb. Haemost. 2012, 10, 1355–1362. [Google Scholar] [CrossRef]
- Dargaud, Y.; Luddington, R.; Baglin, T.P. Elimination of contact factor activation improves measurement of platelet-dependent thrombin generation by calibrated automated thrombography at low-concentration tissue factor. J. Thromb. Haemost. 2006, 4, 1160–1161. [Google Scholar] [CrossRef] [PubMed]
- Calzavarini, S.; Brodard, J.; Quarroz, C.; Maire, L.; Nutzi, R.; Jankovic, J.; Rotondo, L.C.; Giabbani, E.; Fiedler, G.M.; Nagler, M.; et al. Thrombin generation measurement using the ST Genesia Thrombin Generation System in a cohort of healthy adults: Normal values and variability. Res. Pract. Thromb. Haemost. 2019, 3, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Douxfils, J.; Morimont, L.; Bouvy, C.; de Saint-Hubert, M.; Devalet, B.; Devroye, C.; Dincq, A.S.; Dogne, J.M.; Guldenpfennig, M.; Baudar, J.; et al. Assessment of the analytical performances and sample stability on ST Genesia system using the STG-DrugScreen application. J. Thromb. Haemost. 2019, 17, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panova-Noeva, M.; van der Meijden, P.E.J.; ten Cate, H. Clinical Applications, Pitfalls, and Uncertainties of Thrombin Generation in the Presence of Platelets. J. Clin. Med. 2020, 9, 92. https://doi.org/10.3390/jcm9010092
Panova-Noeva M, van der Meijden PEJ, ten Cate H. Clinical Applications, Pitfalls, and Uncertainties of Thrombin Generation in the Presence of Platelets. Journal of Clinical Medicine. 2020; 9(1):92. https://doi.org/10.3390/jcm9010092
Chicago/Turabian StylePanova-Noeva, Marina, Paola E.J. van der Meijden, and Hugo ten Cate. 2020. "Clinical Applications, Pitfalls, and Uncertainties of Thrombin Generation in the Presence of Platelets" Journal of Clinical Medicine 9, no. 1: 92. https://doi.org/10.3390/jcm9010092
APA StylePanova-Noeva, M., van der Meijden, P. E. J., & ten Cate, H. (2020). Clinical Applications, Pitfalls, and Uncertainties of Thrombin Generation in the Presence of Platelets. Journal of Clinical Medicine, 9(1), 92. https://doi.org/10.3390/jcm9010092




