Radiolabeled PET/MRI Nanoparticles for Tumor Imaging
Abstract
1. Introduction
1.1. MRI Contrast Agents
1.2. Nanoparticles
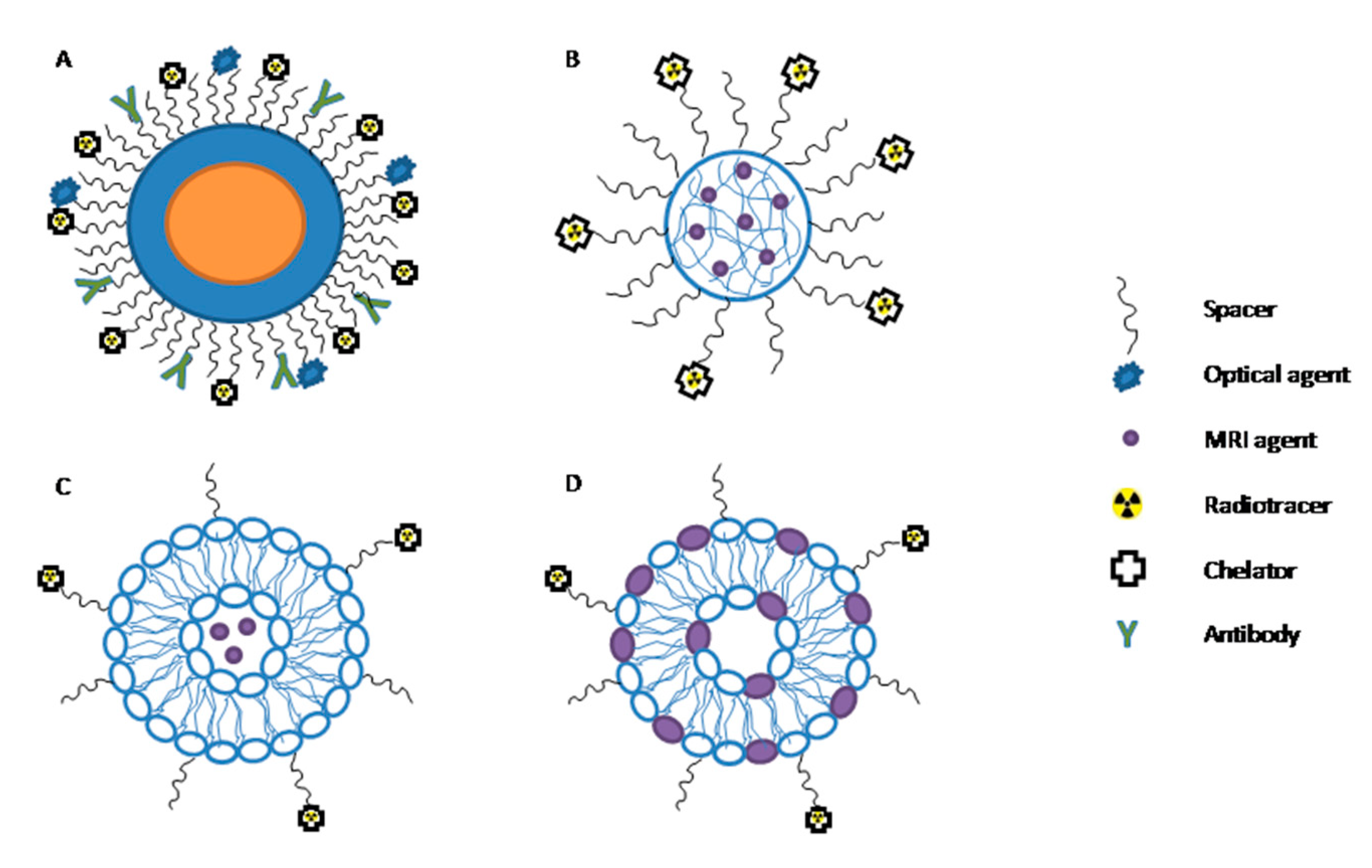
1.3. Radiolabeled Nanoparticles
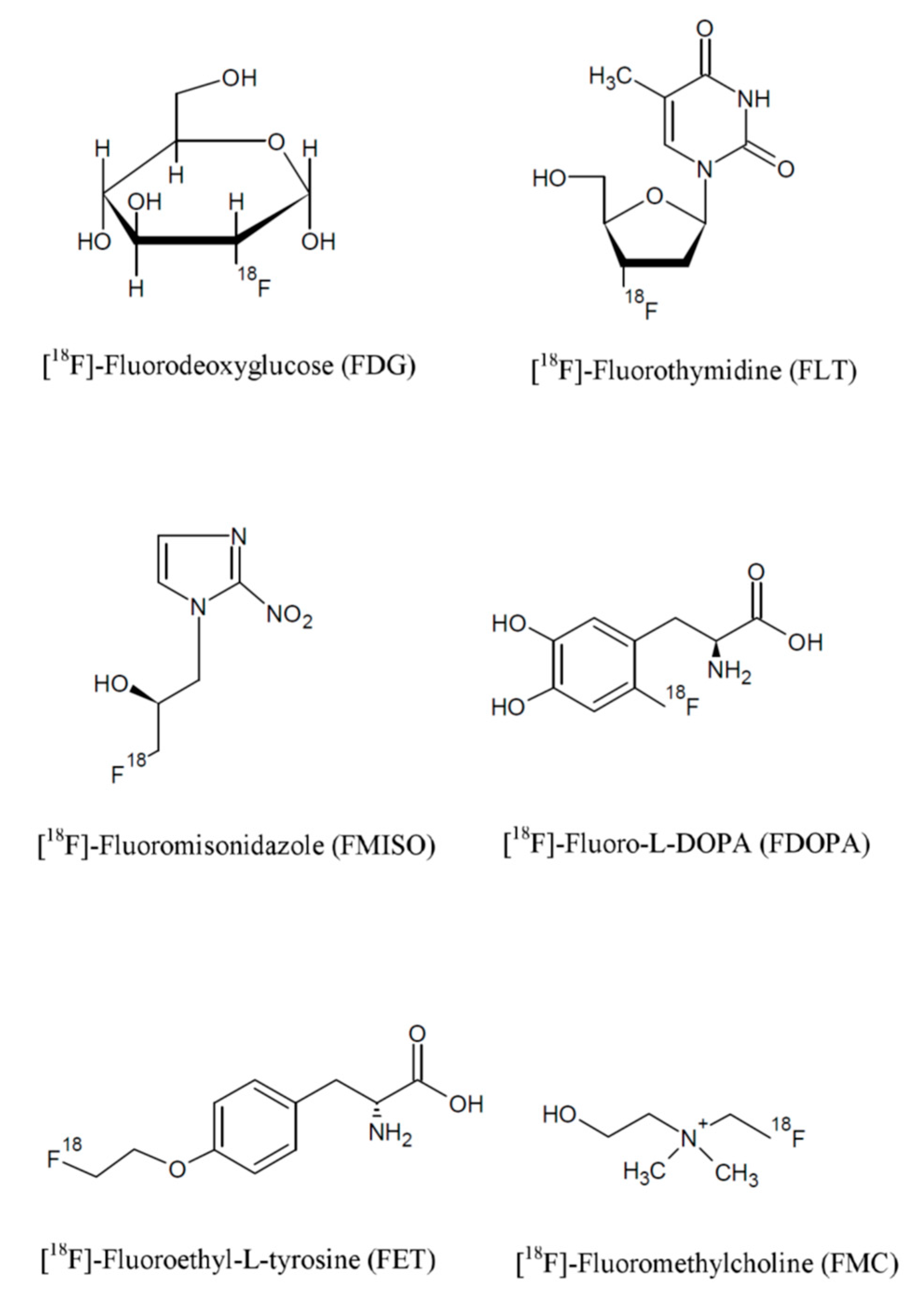
- Radiotracers for the evaluation of glucose metabolism, such as fluorodeoxyglucose (FDG);
- Radiotracers for cell proliferation, such as 18F-fluorothymidine (FLT);
- Radiotracers for the evaluation of vascular perfusion, which include 15O-water and 13N-ammonia;
- Radiotracers for the evaluation of hypoxia, such as 18F-fluoromisonidazole (FMISO).
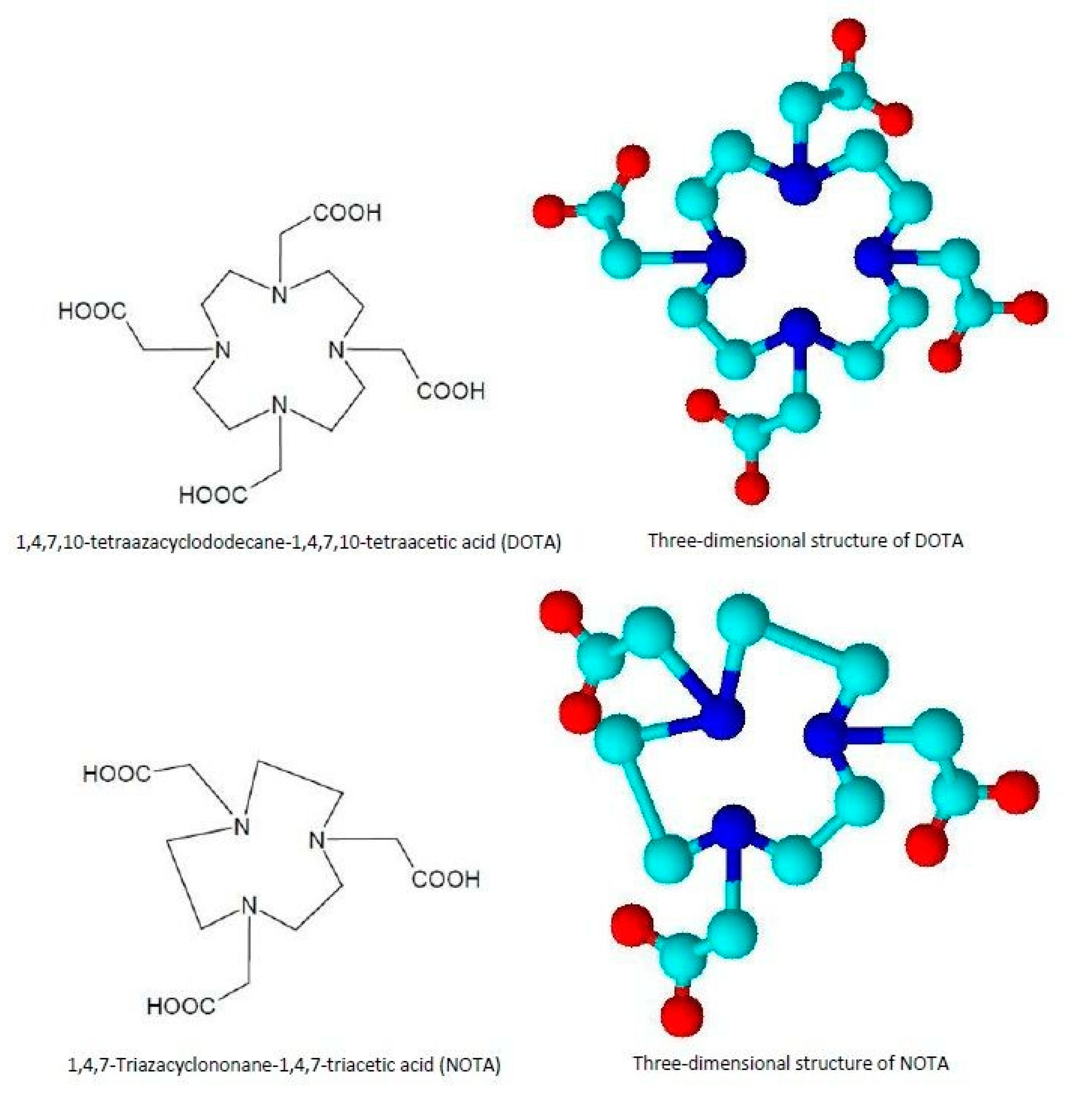
- Complexation reactions of metallic radioisotope ions through coordination chemistry with the use of chelators;
- Direct NP bombardment;
- NP synthesis from radioactive and non-radioactive precursors;
- Post-synthesis NP radiolabeling without the use of chelators.
1.4. PET/MRI Nanoparticles and Preclinical Applications
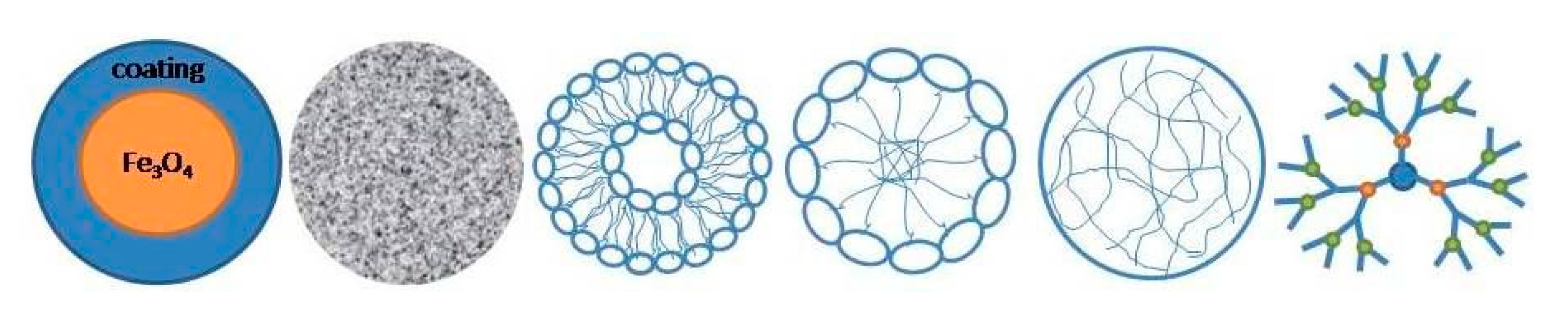
1.4.1. Iron-Oxide Nanoparticles
1.4.2. Silica-Based Nanoparticles
1.4.3. Organic Nanoparticles
2. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Orlacchio, A.; Ciarrapico, A.M.; Schillaci, O.; Chegai, F.; Tosti, D.; D’Alba, F.; Guazzaroni, M.; Simonetti, G. PET-CT in oncological patients: Analysis of informal care costs in cost-benefit assessment. Radiol. Med. 2014, 119, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Scimeca, M.; Toschi, N.; Bonfiglio, R.; Urbano, N.; Bonanno, E. Combining Diagnostic Imaging and Pathology for Improving Diagnosis and Prognosis of Cancer. Contrast Media Mol. Imaging 2019. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.J.; Kinahan, P. Positron Emission Tomography: Current Challenges and Opportunities for Technological Advances in Clinical and Preclinical Imaging Systems. Annu. Rev. Biomed. Eng. 2015, 17, 385–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Wu, S.; Song, F.H.; Zhang, H.; Tian, M. Molecular imaging using PET and SPECT for identification of breast cancer subtypes. Nucl. Med. Commun. 2016, 37, 1116–1124. [Google Scholar] [CrossRef]
- Grueneisen, J.; Nagarajah, J.; Buchbender, C.; Hoffmann, O.; Schaarschmidt, B.M.; Poeppel, T.; Forsting, M.; Quick, H.; Umutlu, L.; Kinner, S. Positron Emission Tomography/Magnetic Resonance Imaging for Local Tumor Staging in Patients With Primary Breast Cancer A Comparison With Positron Emission Tomography/Computed Tomography and Magnetic Resonance Imaging. Investig. Radiol. 2015, 50, 505–513. [Google Scholar] [CrossRef]
- Rampinelli, C.; de Marco, P.; Origgi, D.; Maisonneuve, P.; Casiraghi, M.; Veronesi, G.; Spaggiari, L.; Bellomi, M. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: Secondary analysis of trial data and risk-benefit analysis. BMJ Br. Med. J. 2017, 356. [Google Scholar] [CrossRef]
- Vannier, M. CT Clinical Perspective: Challenges and the Impact of Future Technology Developments. Med. Phys. 2009, 36. [Google Scholar] [CrossRef]
- Garcia-Figueiras, R.; Baleato-Gonzalez, S.; Padhani, A.R.; Luna-Alcala, A.; Vallejo-Casas, J.A.; Sala, E.; Vilanova, J.; Koh, D.; Herranz-Carnero, M.; Vargas, H. How clinical imaging can assess cancer biology. Insights Into Imaging 2019, 10, 28. [Google Scholar] [CrossRef]
- Vandenberghe, S.; Marsden, P.K. PET-MRI: A review of challenges and solutions in the development of integrated multimodality imaging. Phys. Med. Biol. 2015, 60, R115–R154. [Google Scholar] [CrossRef]
- Bercovich, E.; Javitt, M.C. Medical Imaging: From Roentgen to the Digital Revolution, and Beyond. Rambam Maimonides Med. J. 2018, 9. [Google Scholar] [CrossRef]
- Catana, C.; Procissi, D.; Wu, Y.; Judenhofer, M.S.; Qi, J.; Pichler, B.J.; Jacobs, R.E.; Cherry, S.R. Simultaneous in vivo positron emission tomography and magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 3705–3710. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.; Townsend, D.W.; Brun, T.; Kinahan, P.E.; Charron, M.; Roddy, R.; Jerin, J.; Young, J.; Byars, L.; Nutt, R. A combined PET/CT scanner for clinical oncology. J. Nucl. Med. 2000, 41, 1369–1379. [Google Scholar] [PubMed]
- Alessio, A.M.; Kinahan, P.E.; Cheng, P.M.; Vesselle, H.; Karp, J.S. PET/CT scanner instrumentation, challenges, and solutions. Radiol. Clin. N. Am. 2004, 42, 1017. [Google Scholar] [CrossRef]
- Townsend, D.W.; Beyer, T.; Blodgett, T.M. PET/CT scanners: A hardware approach to image fusion. Semin. Nucl. Med. 2003, 33, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Catana, C.; Wu, Y.; Judenhofer, M.S.; Qi, J.; Pichler, B.J.; Cherry, S.R. Simultaneous acquisition of multislice PET and MR images: Initial results with a MR-compatible PET scanner. J. Nucl. Med. 2006, 47, 1968–1976. [Google Scholar] [PubMed]
- Delso, G.; Ziegler, S. PET/MRI system design. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 86–92. [Google Scholar] [CrossRef]
- Garcia, J.; Tang, T.; Louie, A.Y. Nanoparticle-based multimodal PET/MRI probes. Nanomedicine 2015, 10, 1343–1359. [Google Scholar] [CrossRef]
- Nensa, F.; Beiderwellen, K.; Heusch, P.; Wetter, A. Clinical applications of PET/MRI: Current status and future perspectives. Diagn. Interv. Radiol. 2014, 20, 438–447. [Google Scholar] [CrossRef]
- Aiello, M.; Salvatore, E.; Cachia, A.; Pappata, S.; Cavaliere, C.; Prinster, A.; Nicolai, E.; Salvatore, M.; Baron, J.-C.; Quarantelli, M. Relationship between simultaneously acquired resting-state regional cerebral glucose metabolism and functional MRI: A PET/MR hybrid scanner study. Neuroimage 2015, 113, 111–121. [Google Scholar] [CrossRef]
- Covello, M.; Cavaliere, C.; Aiello, M.; Cianelli, M.S.; Mesolella, M.; Iorio, B.; Rossi, A.; Nicolai, E. Simultaneous PET/MR head-neck cancer imaging: Preliminary clinical experience and multiparametric evaluation. Eur. J. Radiol. 2015, 84, 1269–1276. [Google Scholar] [CrossRef]
- Pace, L.; Nicolai, E.; Aiello, M.; Catalano, O.A.; Salvatore, M. Whole-body PET/MRI in oncology: Current status and clinical applications. Clin. Transl. Imaging 2013, 1, 31–44. [Google Scholar] [CrossRef]
- Nappi, C.; Altiero, M.; Imbriaco, M.; Nicolai, E.; Giudice, C.A.; Aiello, M.; Diomiaiuti, C.; Pisani, A.; Spinelli, L.; Cuocolo, A. First experience of simultaneous PET/MRI for the early detection of cardiac involvement in patients with Anderson-Fabry disease. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Mahmood, U. Molecular imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, X. Design and Development of Molecular Imaging Probes. Curr. Top. Med. Chem. 2010, 10, 1227–1236. [Google Scholar] [CrossRef]
- Cai, W.; Chen, X. Multimodality molecular imaging of tumor angiogenesis. J. Nuclear Med. 2008, 49, 113S–128S. [Google Scholar] [CrossRef]
- Chen, K.; Conti, P.S. Target-specific delivery of peptide-based probes for PET imaging. Adv. Drug Deliv. Rev. 2010, 62, 1005–1022. [Google Scholar] [CrossRef]
- Bouziotis, P.; Psimadas, D.; Tsotakos, T.; Stamopoulos, D.; Tsoukalas, C. Radiolabeled Iron Oxide Nanoparticles As Dual-Modality SPECT/MRI and PET/MRI Agents. Curr. Top. Med. Chem. 2012, 12, 2694–2702. [Google Scholar] [CrossRef]
- Tweedle, M.F.; Wedeking, P.; Kumar, K. Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate, and gadodiamide in mice and rats. Investig. Radiol. 1995, 30, 372–380. [Google Scholar] [CrossRef]
- Kanda, T.; Oba, H.; Toyoda, K.; Kitajima, K.; Furui, S. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn. J. Radiol. 2016, 34, 3–9. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Paolini, M.A.; Murray, D.L.; Williamson, E.E.; Eckel, L.J. Gadolinium Deposition in Human Brain Tissues after Contrast-enhanced MR Imaging in Adult Patients without Intracranial Abnormalities. Radiology 2017, 285, 546–554. [Google Scholar] [CrossRef]
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.S.; Zhou, S.K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, M.; Pretorius, P.H. SPECT/CT: An update on technological developments and clinical applications. Br. J. Radiol. 2018, 91. [Google Scholar] [CrossRef] [PubMed]
- Volterrani, D.; Erba, P.A.; Carrio, I.; Strauss, H.W.; Mariani, G. Nuclear Medicine Textbook Methodology and Clinical Application; Springer Nature Switzerland AG: Basel, Switzerland, 2019; Volume 2, ISBN 978-3-319-95563-6. [Google Scholar]
- Lusic, H.; Grinstaff, M.W. X-ray-Computed Tomography Contrast Agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.B.; Bandi, V.; Wei, M.Y.; Pei, Y.B.; D’Souza, F.; Nguyen, K.T.; Hong, Y.; Yuan, B. High-Resolution Ultrasound-Switchable Fluorescence Imaging in Centimeter-Deep Tissue Phantoms with High Signal-To-Noise Ratio and High Sensitivity via Novel Contrast Agents. PLoS ONE 2016, 11, e0165963. [Google Scholar] [CrossRef] [PubMed]
- Kampschulte, M.; Langheinirch, A.C.; Sender, J.; Litzlbauer, H.D.; Althohn, U.; Schwab, J.D.; Alejandre-Lafont, E.; Martels, G.; Krombach, G.A. Nano-Computed Tomography: Technique and Applications. RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren 2016, 188, 146–154. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Jeong, J.H.; Oh, J.M.; Ahn, B.C. Drug Discovery by Molecular Imaging and Monitoring Therapy Response in Lymphoma. Int. J. Mol. Sci. 2017, 18, 1639. [Google Scholar] [CrossRef]
- Rosales, R. Potential clinical applications of bimodal PET-MRI or SPECT-MRI agents. J. Label. Compd. Radiopharm. 2014, 57, 298–303. [Google Scholar] [CrossRef]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Chen, G.Y.; Roy, I.; Yang, C.H.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef]
- Aime, S.; Frullano, L.; Crich, S.G. Compartmentalization of a gadolinium complex in the apoferritin cavity: A route to obtain high relaxivity contrast agents for magnetic resonance imaging. Angew. Chem. Int. Ed. 2002, 41, 1017. [Google Scholar] [CrossRef]
- Crich, S.G.; Lanzardo, S.; Alberti, D.; Belfiore, S.; Ciampa, A.; Giovenzanaz, G.B.; Lovazzano, C.; Pagliarin, R.; Aime, S. Magnetic resonance imaging detection of tumor cells by targeting low-density lipoprotein receptors with Gd-loaded low-density lipoprotein particles. Neoplasia 2007, 9, 1046–1056. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; FaroKhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.; Scheinberg, D.A. Will Nanotechnology Influence Targeted Cancer Therapy? Semin. Radiat. Oncol. 2011, 21, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef]
- Qiao, R.; Yang, C.; Gao, M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Berry, C.C.; Curtis, A.S.G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R198–R206. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Neuberger, T.; Schopf, B.; Hofmann, H.; Hofmann, M.; von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- de Rosales, R.T.M.; Tavaré, R.; Paul, R.L.; Jauregui-Osoro, M.; Protti, A.; Glaria, A.; Varma, G.; Szanda, I.; Blower, P.J. Synthesis of 64CuII–bis (dithiocarbamatebisphosphonate) and its conjugation with superparamagnetic iron oxide nanoparticles: In vivo evaluation as dual-modality PET–MRI agent. Angew. Chem. Int. Ed. 2011, 50, 5509–5513. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Belo, S.; Krueger, D.; Yan, Y.; de Rosales, R.T.M.; Jauregui-Osoro, M.; Ye, H.; Su, S.; Mathe, D.; Kovács, N.; et al. Aluminium hydroxide stabilised MnFe2O4 and Fe3O4 nanoparticles as dual-modality contrasts agent for MRI and PET imaging. Biomaterials 2014, 35, 5840–5846. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, M.; Kim, D.K.; Bobrysheva, N.; Osmolowsky, M.; Semenov, V.; Tsakalakos, T.; Muhammed, M. Superparamagnetism of magnetite nanoparticles: Dependence on surface modification. Langmuir 2004, 20, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- LaConte, L.E.W.; Nitin, N.; Zurkiya, O.; Caruntu, D.; O’Connor, C.J.; Hu, X.; Bao, G. Coating thickness of magnetic iron oxide nanoparticles affects R-2 relaxivity. J. Magn. Reson. Imaging 2007, 26, 1634–1641. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Jain, R.K. Design considerations for nanotherapeutics in oncology. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1893–1907. [Google Scholar] [CrossRef]
- Dolle, F. Fluorine-18-labelled fluoropyridines: Advances in radiopharmaceutical design. Curr. Pharm. Des. 2005, 11, 3221–3235. [Google Scholar] [CrossRef]
- Jacobson, O.; Chen, X.Y. PET Designated Flouride-18 Production and Chemistry. Curr. Top. Med. Chem. 2010, 10, 1048–1059. [Google Scholar] [CrossRef]
- Ismail, F.M.D. Important fluorinated drugs in experimental and clinical use. J. Fluor. Chem. 2002, 118, 27–33. [Google Scholar] [CrossRef]
- Sumer, B.; Gao, J. Theranostic nanomedicine for cancer. Nanomedicine 2008, 3, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.I. Molecular and cellular radiobiological effects of auger emitting radionuclides. Radiat. Prot. Dosim. 2011, 143, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Odonoghue, J.A.; Wheldon, T.E. Targeted radiotherapy using Auger electron emitters. Phys. Med. Biol. 1996, 41, 1973–1992. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cai, W.; Chen, X. Positron Emission Tomography Imaging Using Radio labeled Inorganic Nanomaterials. Acc. Chem. Res. 2015, 48, 286–294. [Google Scholar] [CrossRef]
- Ni, D.L.; Jiang, D.W.; Ehlerding, E.B.; Huang, P.; Cai, W.B. Radiolabeling Silica-Based Nanoparticles via Coordination Chemistry: Basic Principles, Strategies, and Applications. Acc. Chem. Res. 2018, 51, 778–788. [Google Scholar] [CrossRef]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef]
- Laverman, P.; McBride, W.J.; Sharkey, R.M.; Eek, A.; Joosten, L.; Oyen, W.J.G.; Goldenberg, D.M.; Boerman, O.C. A Novel Facile Method of Labeling Octreotide with F-18-Fluorine. J. Nucl. Med. 2010, 51, 454–461. [Google Scholar] [CrossRef]
- Perez-Campana, C.; Gomez-Vallejo, V.; Puigivila, M.; Martin, A.; Calvo-Fernandez, T.; Moya, S.E.; Ziolo, R.F.; Reese, T.; Llop, J. Biodistribution of Different Sized Nanoparticles Assessed by Positron Emission Tomography: A General Strategy for Direct Activation of Metal Oxide Particles. ACS Nano 2013, 7, 3498–3505. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, R.; Huang, M.; Lu, W.; Song, S.; Melancon, M.P.; Tian, M.; Liang, D.; Li, C. A Chelator-Free Multifunctional Cu-64 CuS Nanoparticle Platform for Simultaneous Micro-PET/CT Imaging and Photothermal Ablation Therapy. J. Am. Chem. Soc. 2010, 132, 15351–15358. [Google Scholar] [CrossRef]
- Wong, R.M.; Gilbert, D.A.; Liu, K.; Louie, A.Y. Rapid Size-Controlled Synthesis of Dextran-Coated, Cu-64-Doped Iron Oxide Nanoparticles. Acs Nano 2012, 6, 3461–3467. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Gangangari, K.; Kalidindi, T.M.; Punzalan, B.; Larson, S.M.; Pillarsetty, N.V.K. Copper-64 labeled liposomes for imaging bone marrow. Nucl. Med. Biol. 2016, 43, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Valdovinos, H.F.; Chen, F.; Lewis, C.M.; Ellison, P.A.; Luo, H.; Meyerand, M.E.; Nickles, R.J.; Cai, W. Intrinsically Germanium-69-Labeled Iron Oxide Nanoparticles: Synthesis and In-Vivo Dual-Modality PET/MR Imaging. Adv. Mater. 2014, 26, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, G.; Schoultz, B.W.; Michaelsen, T.E.; Bruland, O.S.; Larsen, R.H. Sterically stabilized liposomes as a carrier for alpha-emitting radium and actinium radionuclides. Nucl. Med. Biol. 2004, 31, 441–449. [Google Scholar] [CrossRef]
- Sofou, S.; Thomas, J.L.; Lin, H.Y.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Engineered Liposomes for potential alpha-particle therapy of metastatic cancer. J. Nucl. Med. 2004, 45, 253–260. [Google Scholar]
- Sofou, S.; Kappel, B.J.; Jaggi, J.S.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Enhanced retention of the alpha-particle-emitting daughters of actinium-225 by liposome carriers. Bioconjugate Chem. 2007, 18, 2061–2067. [Google Scholar] [CrossRef]
- Xing, Y.; Zhao, J.; Conti, P.S.; Chen, K. Radiolabeled Nanoparticles for Multimodality Tumor Imaging. Theranostics 2014, 4, 290–306. [Google Scholar] [CrossRef]
- Bulte, J.W.M.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499. [Google Scholar] [CrossRef]
- Thorek, D.; Czupryna, J.; Chen, A.; Tsourkas, A. Molecular Imaging of Cancer with Superparamagnetic Iron Oxide Particles. Cancer Imaging Instrum. Appl. 2008, 2, 85–95. [Google Scholar]
- Jung, C.W.; Jacobs, P. Physical and chemical-properties of superparamagnetic iron-oxide mr contrast agents—Ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging 1995, 13, 661–674. [Google Scholar] [CrossRef]
- Wang, Y.X.J.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Wunderbaldinger, P.; Josephson, L.; Weissleder, R. Crosslinked iron oxides (CLIO): A new platform for the development of targeted MR contrast agents. Acad. Radiol. 2002, 9, S304–S306. [Google Scholar] [CrossRef]
- Mornet, S.; Vasseur, S.; Grasset, F.; Duguet, E. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem. 2004, 14, 2161–2175. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.M.; Singh, N.; Jenkins, G.J.; Williams, P.M.; Orbaek, A.W.; Barron, A.R.; Wright, C.J.; Doak, S.H. Dextran coated ultrafine superparamagnetic iron oxide nanoparticles: Compatibility with common fluorometric and colorimetric dyes. Anal. Chem. 2011, 83, 3778–3785. [Google Scholar] [CrossRef] [PubMed]
- Babic, M.; Horák, D.; Trchová, M.; Jendelová, P.; Glogarová, K.; Lesný, P.; Herynek, V.; Hájek, M.; Syková, E. Poly (L-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjugate Chem. 2008, 19, 740–750. [Google Scholar] [CrossRef]
- Maeng, J.H.; Lee, D.-H.; Jung, K.H.; Bae, Y.-H.; Park, I.-S.; Jeong, S.; Jeon, Y.-S.; Shim, C.-K.; Kim, W.; Kim, J.; et al. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials 2010, 31, 4995–5006. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, H.; Lv, R.; Zhao, P.; Sun, X.; Wang, S.; Su, W.; Niu, R.; Chang, J. Polymeric liposomes-coated superparamagnetic iron oxide nanoparticles as contrast agent for targeted magnetic resonance imaging of cancer cells. Langmuir 2011, 27, 3100–3105. [Google Scholar] [CrossRef]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.-W.; Seo, J.-W.; Jang, J.-T.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.; Suh, J.-S.; et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar] [CrossRef]
- Lawaczeck, R.; Menzel, M.; Pietsch, H. Superparamagnetic iron oxide particles: Contrast media for magnetic resonance imaging. Appl. Organomet. Chem. 2004, 18, 506–513. [Google Scholar] [CrossRef]
- Blasiak, B.; van Veggel, F.C.; Tomanek, B. Applications of nanoparticles for MRI cancer diagnosis and therapy. J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Thorek, D.L.J.; Ulmert, D.; Diop, N.-F.M.; Lupu, M.E.; Doran, M.G.; Huang, R.; Abou, D.S.; Larson, S.M.; Grimm, J. Non-invasive mapping of deep-tissue lymph nodes in live animals using a multimodal PET/MRI nanoparticle. Nat. Commun. 2014, 5, 3097. [Google Scholar] [CrossRef] [PubMed]
- Madru, R.; Budassi, M.; Benveniste, H.; Lee, H.; Smith, S.D.; Schlyer, D.J.; Vaska, P.; Knutsson, L.; Strand, S.-E. Simultaneous Preclinical Positron Emission Tomography-Magnetic Resonance Imaging Study of Lymphatic Drainage of Chelator-Free Cu-64-Labeled Nanoparticles. Cancer Biother. Radiopharm. 2018, 33, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Li, Z.; Chen, K.; Hsu, A.R.; Xu, C.; Xie, J.; Sun, S.; Chen, X. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)—Conjugated radiolabeled iron oxide nanoparticles. J. Nucl. Med. 2008, 49, 1371–1379. [Google Scholar] [CrossRef]
- Kim, S.-M.; Chae, M.K.; Yim, M.S.; Jeong, I.H.; Cho, J.; Lee, C.; Ryu, E.K. Hybrid PET/MR imaging of tumors using an oleanolic acid-conjugated nanoparticle. Biomaterials 2013, 34, 8114–8121. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Huxford-Phillips, R.C.; Lin, W. Silica-based nanoprobes for biomedical imaging and theranostic applications. Chem. Soc. Rev. 2012, 41, 2673–2685. [Google Scholar] [CrossRef]
- Nakamura, T.; Sugihara, F.; Matsushita, H.; Yoshioka, Y.; Mizukami, S.; Kikuchi, K. Mesoporous silica nanoparticles for 19 F magnetic resonance imaging, fluorescence imaging, and drug delivery. Chem. Sci. 2015, 6, 1986–1990. [Google Scholar] [CrossRef]
- Patel, D.; Kell, A.; Simard, B.; Deng, J.; Xiang, B.; Lin, H.-Y.; Gruwel, M.; Tian, G. Cu2+-labeled, SPION loaded porous silica nanoparticles for cell labeling and multifunctional imaging probes. Biomaterials 2010, 31, 2866–2873. [Google Scholar] [CrossRef]
- Burke, B.P.; Baghdadi, N.; Kownacka, A.E.; Nigam, S.; Clemente, G.S.; Al-Yassiry, M.M.; Domarkas, J.; Lorch, M.; Pickles, M.; Gibbs, P.; et al. Chelator free gallium-68 radiolabelling of silica coated iron oxide nanorods via surface interactions. Nanoscale 2015, 7, 14889–14896. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Lee, S.; Swierczewska, M.; Kiesewetter, D.O.; Lang, L.; Zhang, G.; Zhu, L.; Gao, H.; Choi, H.S.; et al. Long-term multimodal imaging of tumor draining sentinel lymph nodes using mesoporous silica-based nanoprobes. Biomaterials 2012, 33, 4370–4378. [Google Scholar] [CrossRef] [PubMed]
- Elbayoumi, T.A.; Torchilin, V.P. Current trends in liposome research. Methods Mol. Biol. 2010, 605, 1–27. [Google Scholar] [PubMed]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-Based Nanoparticles as Pharmaceutical Drug Carriers: From Concepts to Clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Goins, B.; Zhang, L.; Bao, A. Novel Multifunctional Theranostic Liposome Drug Delivery System: Construction, Characterization, and Multimodality MR, Near-Infrared Fluorescent, and Nuclear Imaging. Bioconjugate Chem. 2012, 23, 1322–1332. [Google Scholar] [CrossRef]
- Mitchell, N.; Kalber, T.L.; Cooper, M.S.; Sunassee, K.; Chalker, S.L.; Shaw, K.P.; Ordidge, K.L.; Badar, A.; Janes, S.M.; Blower, P.J.; et al. Incorporation of paramagnetic, fluorescent and PET/SPECT contrast agents into liposomes for multimodal imaging. Biomaterials 2013, 34, 1179–1192. [Google Scholar] [CrossRef]
- Abou, D.S.; Thorek, D.L.J.; Ramos, N.N.; Pinkse, M.W.H.; Wolterbeek, H.T.; Carlin, S.D.; Beattie, B.J.; Lewis, J.S. Zr-89-Labeled Paramagnetic Octreotide-Liposomes for PET-MR Imaging of Cancer. Pharm. Res. 2013, 30, 878–888. [Google Scholar] [CrossRef]
- Savic, R.; Luo, L.B.; Eisenberg, A.; Maysinger, D. Micellar nanocontainers distribute to defined cytoplasmic organelles. Science 2003, 300, 615–618. [Google Scholar] [CrossRef]
- Trubetskoy, V.S. Polymeric micelles as carriers of diagnostic agents. Adv. Drug Deliv. Rev. 1999, 37, 81–88. [Google Scholar] [CrossRef]
- Enoch, H.G.; Strittmatter, P. Formation and properties of 1000-A-diameter, single-bilayer phospholipid vesicles. Proc. Natl. Acad. Sci. USA 1979, 76, 145–149. [Google Scholar] [CrossRef]
- Trubetskoy, V.S.; FrankKamenetsky, M.D.; Whiteman, K.R.; Wolf, G.L.; Torchilin, V.P. Stable polymeric micelles: Lymphangiographic contrast media for gamma scintigraphy and magnetic resonance imaging. Acad. Radiol. 1996, 3, 232–238. [Google Scholar] [CrossRef]
- Starmans, L.W.; Hummelink, M.A.; Rossin, R.; Kneepkens, E.; Lamerichs, R.; Donato, K.; Nicolay, K.; Grüll, H. 89Zr-and Fe-Labeled Polymeric Micelles for Dual Modality PET and T1-Weighted MR Imaging. Adv. Healthc. Mater. 2015, 4, 2137–2145. [Google Scholar] [CrossRef]
- Lee, D.-E.; Koo, H.; Sun, I.-C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Xu, H.; Regino, C.A.; Koyama, Y.; Hama, Y.; Gunn, A.J.; Bernardo, M.; Kobayashi, H.; Choyke, P.L.; Brechbiel, M.W. Preparation and preliminary evaluation of a biotin-targeted, lectin-targeted dendrimer-based probe for dual-modality magnetic resonance and fluorescence imaging. Bioconjugate Chem. 2007, 18, 1474–1482. [Google Scholar] [CrossRef]
- Koyama, Y.; Talanov, V.S.; Bernardo, M.; Hama, Y.; Regino, C.A.; Brechbiel, M.W.; Choyke, P.L.; Kobayashi, H. A dendrimer-based nanosized contrast agent dual-labeled for magnetic resonance and optical fluorescence imaging to localize the sentinel lymph node in mice. J. Magn. Reson. Imaging 2007, 25, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Koyama, Y.; Barrett, T.; Hama, Y.; Regino, C.A.; Shin, I.S.; Jang, B.-S.; Le, N.; Paik, C.H.; Choyke, P.L.; et al. Multimodal nanoprobes for radionuclide and five-color near-infrared optical lymphatic imaging. ACS Nano 2007, 1, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Li, K.; Cai, H.; Chen, Q.; Shen, M.; Huang, Y.; Peng, C.; Hou, W.; Zhu, M.; Zhang, G.; et al. Multifunctional dendrimer-entrapped gold nanoparticles for dual mode CT/MR imaging applications. Biomaterials 2013, 34, 1570–1580. [Google Scholar] [CrossRef]
- Almutairi, A.; Rossin, R.; Shokeen, M.; Hagooly, A.; Ananth, A.; Capoccia, B.; Guillaudeu, S.; Abendschein, D.; Anderson, C.J.; Welch, M.J.; et al. Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Wu, C.; Kim, M.-K.; Paik, C.H.; Carrasquillo, J.A.; Brechbiel, M.W. Evaluation of the in vivo biodistribution of indium-111 and yttrium-88 labeled dendrimer-1B4M-DTPA and its conjugation with anti-Tac monoclonal antibody. Bioconjugate Chem. 1999, 10, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhang, S.R.; Hao, G.Y.; Hassan, G.; Ramezani, S.; Sagiyama, K.; Lo, S.-T.; Takahashi, M.; Sherry, A.D.; Öz, O.K.; et al. Molecular Platform for Design and Synthesis of Targeted Dual Modality Imaging Probes. Bioconjugate Chem. 2015, 26, 549–558. [Google Scholar] [CrossRef] [PubMed]
- MaHam, A.; Tang, Z.; Wu, H.; Wang, J.; Lin, Y. Protein-Based Nanomedicine Platforms for Drug Delivery. Small 2009, 5, 1706–1721. [Google Scholar] [CrossRef]
- Vecchione, D.; Aiello, M.; Cavaliere, C.; Nicolai, E.; Netti, P.A.; Torino, E. Hybrid core shell nanoparticles entrapping Gd-DTPA and F-18-FDG for simultaneous PET/MRI acquisitions. Nanomedicine 2017, 12, 2223–2231. [Google Scholar] [CrossRef]
- Fan, Q.; Cheng, K.; Hu, X.; Ma, X.; Zhang, R.; Yang, M.; Lu, X.; Xing, L.; Huang, W.; Gambhir, S.S.; et al. Transferring Biomarker into Molecular Probe: Melanin Nanoparticle as a Naturally Active Platform for Multimodality Imaging. J. Am. Chem. Soc. 2014, 136, 15185–15194. [Google Scholar] [CrossRef]
- Shukla, S.; Steinmetz, N.F. Virus-based nanomaterials as positron emission tomography and magnetic resonance contrast agents: From technology development to translational medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 708–721. [Google Scholar] [CrossRef]
- Sharma, R.; Xu, Y.; Kim, S.W.; Schueller, M.J.; Alexoff, D.; Smith, S.D.; Wang, W.; Schlyer, D. Carbon-11 radiolabeling of iron-oxide nanoparticles for dual-modality PET/MR imaging. Nanoscale 2013, 5, 7476–7483. [Google Scholar] [CrossRef]
- Glaus, C.; Rossin, R.; Welch, M.J.; Bao, G. In vivo evaluation of 64Cu-labeled magnetic nanoparticles as a dual-modality PET/MR imaging agent. Bioconjugate Chem. 2010, 21, 715–722. [Google Scholar] [CrossRef]
- Yang, B.Y.; Moon, S.-H.; Seelam, S.R.; Jeon, M.J.; Lee, Y.-S.; Lee, D.S.; Chung, J.-K.; Kim, Y.; Jeong, J.M. Development of a multimodal imaging probe by encapsulating iron oxide nanoparticles with functionalized amphiphiles for lymph node imaging. Nanomedicine 2015, 10, 1899–1910. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, K.; Qi, S.; Liu, H.; Jiang, Y.; Jiang, H.; Li, J.; Chen, K.; Zhang, H.; Cheng, Z. Affibody modified and radiolabeled gold-Iron oxide hetero-nanostructures for tumor PET, optical and MR imaging. Biomaterials 2013, 34, 2796–2806. [Google Scholar] [CrossRef]
- Aryal, S.; Key, J.; Stigliano, C.; Landis, M.D.; Lee, D.Y.; Decuzzi, P. Positron Emitting Magnetic Nanoconstructs for PET/MR Imaging. Small 2014, 10, 2688–2696. [Google Scholar] [CrossRef]
- Malinge, J.; Geraudie, B.; Savel, P.; Nataf, V.; Prignon, A.; Provost, C.; Zhang, Y.; Ou, P.; Kerrou, K.; Talbot, J.-N.; et al. Liposomes for PET and MR Imaging and for Dual Targeting (Magnetic Field/Glucose Moiety): Synthesis, Properties, and in Vivo Studies. Mol. Pharm. 2017, 14, 406–414. [Google Scholar] [CrossRef]
- Choi, J.-S.; Park, J.C.; Nah, H.; Woo, S.; Oh, J.; Kim, K.M.; Cheon, G.J.; Chang, Y.; Yoo, J.; Cheon, J. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew. Chem. Int. Ed. 2008, 47, 6259–6262. [Google Scholar] [CrossRef]
- Truillet, C.; Bouziotis, P.; Tsoukalas, C.; Brugiere, J.; Martini, M.; Sancey, L.; Brichart, T.; Denat, F.; Boschetti, F.; Darbost, U.; et al. Ultrasmall particles for Gd-MRI and Ga-68-PET dual imaging. Contrast Media Mol. Imaging 2015, 10, 309–319. [Google Scholar] [CrossRef]
| Imaging Technique | Source of Imaging | Spatial Resolution | Tissue Penetration Depth | Sensitivity | Agent | Ref. |
|---|---|---|---|---|---|---|
| Magnetic resonance imaging (MRI) | Radio wave | 25–100 µm | No limit | mM to µM (low) | Para-(Gd3+) or superparamagnetic (Fe3O4) materials | [31] |
| Single-photon emission computed tomography (SPECT) | γ-ray | 6–7 mm | No limit | pM (high) | Radionuclides (99mTc,201Tl,111In,131I, 123I, 67Ga) | [32] |
| Positron emission tomography (PET) | γ-ray | 1–2 mm | No limit | pM (high) | Radionuclides (18F,11C,13N,15O,124I,64Cu, 68Ga) | [33] |
| Computed tomography (CT) | X-ray | 50–200 µm | No limit | n.c. | High-atomic-number atoms (iodine, barium sulfate) | [34] |
| Ultrasonography (US) | Ultrasounds | 50–500 µm | mm to cm | n.c. | Microbubbles | [35] |
| Optical fluorescence imaging | Visible or near-infrared light | In vivo 2–3 mm in vitro µm | <1 cm | nM to pM (medium) | Fluorescent dyes, quantum dots | [36,37] |
| Brand Name | Active Substance | Chemical Name | Molecular Structure | Company | Current Status |
|---|---|---|---|---|---|
| Omniscan | Gadodiamide | Gd-DTPA-BMA | Linear, non-ionic | GE Healthcare | Suspended |
| OptiMARK | Gadoversetamide | Gd-DTPA-BMEA | Linear, non-ionic | Mallinckrodt | Suspended |
| Magnevist | Gadopentetic acid | Gd-DTPA | Linear, ionic | Bayer | Suspended |
| MultiHance | Gadobenic acid | Gd-BOPTA | Linear, ionic | Bracco | Only for liver scans |
| Primovist | Gadoxetic acid | Gd-EOB-DTPA | Linear, ionic | Bayer | In use |
| ProHance | Gadoteridol | Gd-HP-DO3A | Cyclic, non-ionic | Bracco | In use |
| Gadovist | Gadobutrol | Gd-BT-Do3A | Cyclic, non-ionic | Bayer | In use |
| Dotarem | Gadoteric acid | Gd-DOTA | Cyclic, ionic | Guerbet | In use |
| Radionuclide | Half-Life Time * | Electronic Emission Energy β+ | Production |
|---|---|---|---|
| 11C | 20.385 min | 386 keV | Cyclotron |
| 13N | 9.965 min | 492 keV | Cyclotron |
| 15O | 122.24 s | 735 keV | Cyclotron |
| 18F | 109.77 min | 250 keV | Cyclotron |
| 64Cu | 12.701 h | 655 keV | Cyclotron or reactor |
| 68Ga | 67.629 min | 836 and 353 keV ** | Generator |
| Nanostructure | MRI Component | PET Component | Chelator | Other | Biological Target | Ref. |
|---|---|---|---|---|---|---|
| Bacteriophages/plant viruses | Iron oxide/Gd3+ | 18F | Passive targeting | [124] | ||
| Hyaluronic acid + chitosan | Gd-DTPA | 18F-FDG | No chelator | Passive targeting | [122] | |
| Iron oxide + ligands (–NH2 –COOH) | Iron oxide | 11C | No chelator | Passive targeting | [125] | |
| Iron oxide + micelle + PEG | Iron oxide | 64Cu | DOTA | Passive targeting | [126] | |
| Iron oxide + dextran | Iron oxide | 64Cu | DTCBP | Passive targeting | [53] | |
| Iron oxide + HSA | Iron oxide | 64Cu | DOTA | Cy5.5 | Passive targeting | [95] |
| Iron oxide + mannose | Iron oxide | 68Ga | NOTA | Passive targeting | [127] | |
| Iron oxide + micelle + PEG | Iron oxide | 68Ga | NOTA | Oleanolic acid | [97] | |
| Iron oxide + PASP | Iron oxide | 64Cu | DOTA | RGD * | [96] | |
| Iron oxide + PEG | Iron oxide | 64Cu | NOTA | Au | Anti EGFR affibody | [128] |
| Iron oxide + PLGA + lipids + PEG | Iron oxide | 64Cu | DOTA | Passive targeting | [129] | |
| Iron oxide + polyglucose | Iron oxide | 89Zr | Desferrioxamine | Passive targeting | [93] | |
| Iron oxide + PEG | Iron oxide | 64Cu | No chelator | Passive targeting | [94] | |
| Iron oxide + silica + PEG | Iron oxide | 68Ga | DO3A | Passive targeting | [101] | |
| Liposome | Iron oxide | 68Ga | NODA | Glucose | External magnetic field + Warburg effect | [130] |
| Liposome | Gd-DTPA | 89Zr | no chelator | Octreotide | [107] | |
| Liposome | Gd 3+ | 64Cu | DOTA | IRDye–doxorubici n–99mTc | Passive targeting | [105] |
| Liposome + nEG spacer | Gd 3+ | 64Cu | DOTA | 111I–fluorescein | Passive targeting | [106] |
| Melanine NP + PEG | Fe3+ | 64Cu | No chelator | RGD * | [123] | |
| Mesoporous silica NP | Gd3+ | 64Cu | DOTA | ZW800 | Passive targeting | [102] |
| Micelle | Fe3+ | 89Zr | Desferrioxamine | Passive targeting | [112] | |
| MnMEIO/iron oxide + Al(OH)3 | MnMEIO/iron oxide | 18F/64Cu | No chelator/DTCBP | Passive targeting | [54] | |
| MnMEIO + SA | MnMEIO | 124I | No chelator | Passive targeting | [131] | |
| Silica NP | Gd3+ | 68Ga | DOTAGA/NODAGA | Passive targeting | [132] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forte, E.; Fiorenza, D.; Torino, E.; Costagliola di Polidoro, A.; Cavaliere, C.; Netti, P.A.; Salvatore, M.; Aiello, M. Radiolabeled PET/MRI Nanoparticles for Tumor Imaging. J. Clin. Med. 2020, 9, 89. https://doi.org/10.3390/jcm9010089
Forte E, Fiorenza D, Torino E, Costagliola di Polidoro A, Cavaliere C, Netti PA, Salvatore M, Aiello M. Radiolabeled PET/MRI Nanoparticles for Tumor Imaging. Journal of Clinical Medicine. 2020; 9(1):89. https://doi.org/10.3390/jcm9010089
Chicago/Turabian StyleForte, Ernesto, Dario Fiorenza, Enza Torino, Angela Costagliola di Polidoro, Carlo Cavaliere, Paolo A. Netti, Marco Salvatore, and Marco Aiello. 2020. "Radiolabeled PET/MRI Nanoparticles for Tumor Imaging" Journal of Clinical Medicine 9, no. 1: 89. https://doi.org/10.3390/jcm9010089
APA StyleForte, E., Fiorenza, D., Torino, E., Costagliola di Polidoro, A., Cavaliere, C., Netti, P. A., Salvatore, M., & Aiello, M. (2020). Radiolabeled PET/MRI Nanoparticles for Tumor Imaging. Journal of Clinical Medicine, 9(1), 89. https://doi.org/10.3390/jcm9010089





