The Radical Scavenger NZ-419 Suppresses Intestinal Polyp Development in Apc-Mutant Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Animals
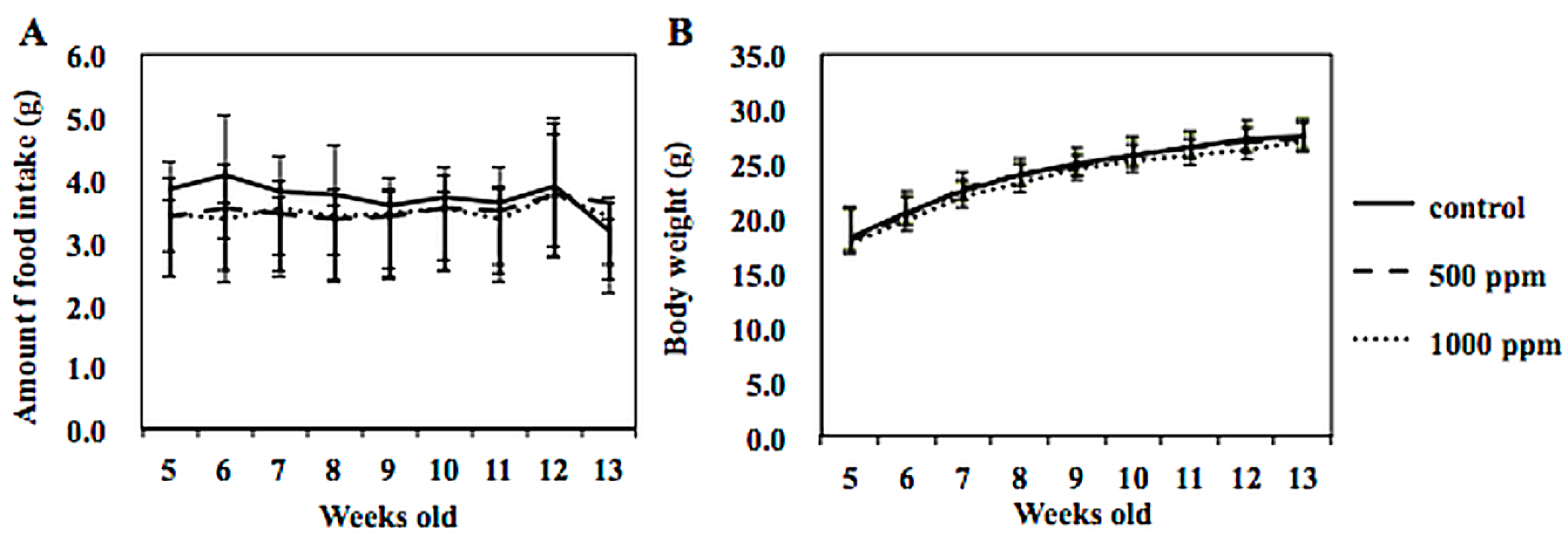
2.4. Animal Experimental Protocol
2.5. Measurement of Intracellular ROS
2.6. Luciferase Assays for Nrf2 Promoter Transcriptional Activity
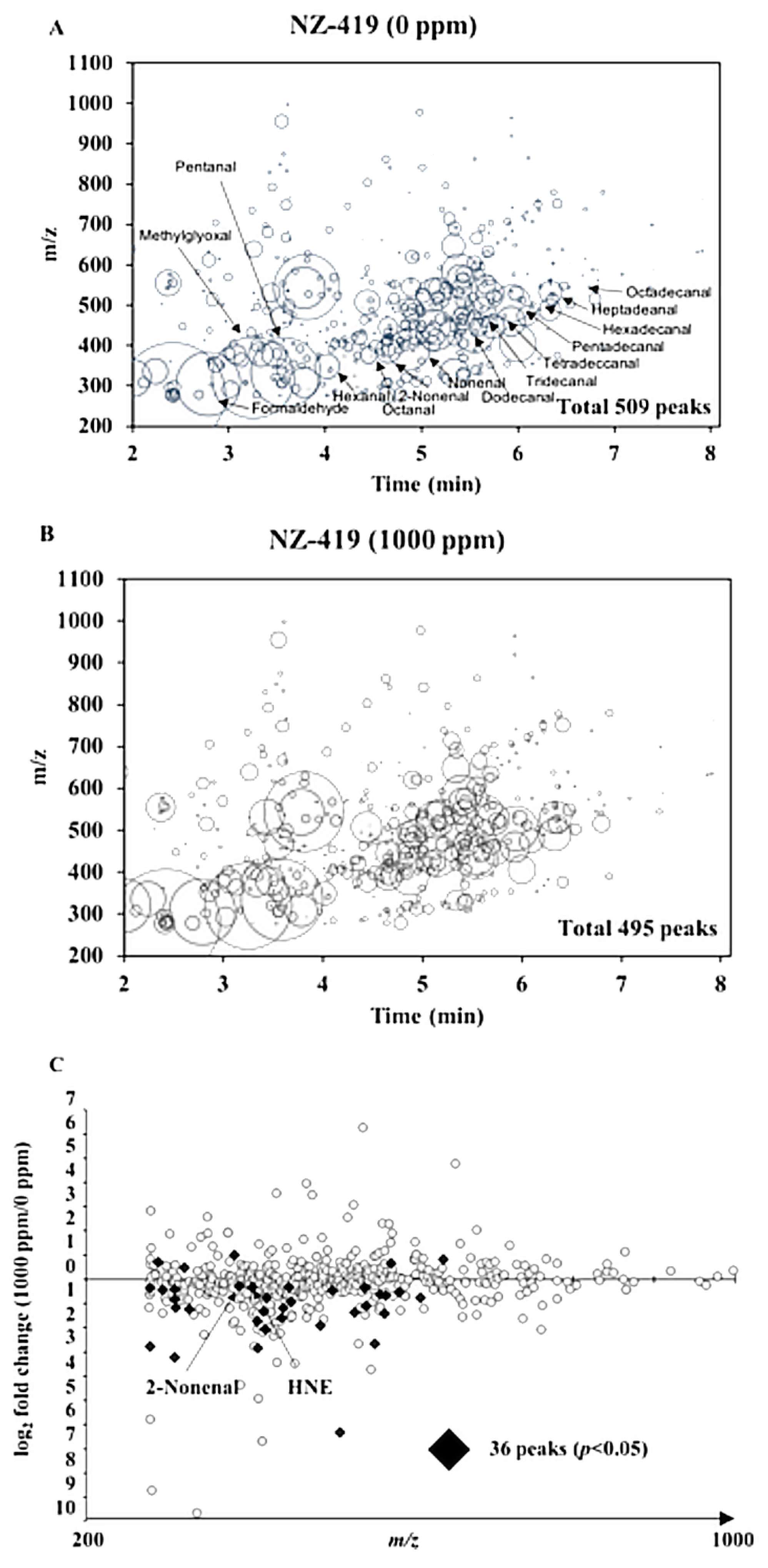
2.7. Extraction and Analysis of Reactive Carbonyl Species
2.8. Quantitative Real-Time Polymerase Chain Reaction (qPCR) Analyses
2.9. Immunohistochemical Staining
2.10. Statistical Analyses
3. Results
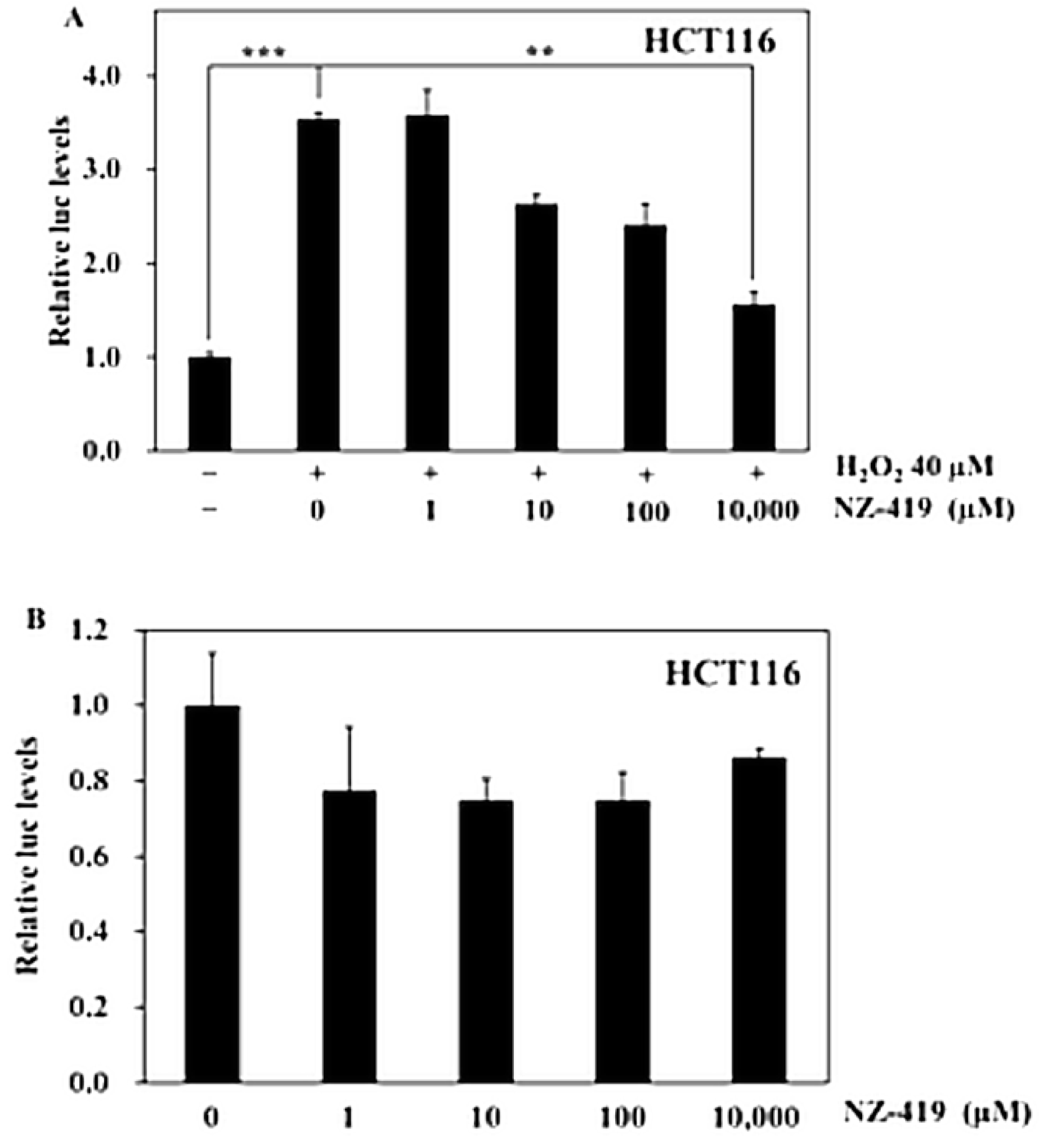
3.1. NZ-419 Eliminated ROS in HCT116 Cells
3.2. NZ-419 Suppressed H2O2-Induced Nrf2 Promoter Transcriptional Activity
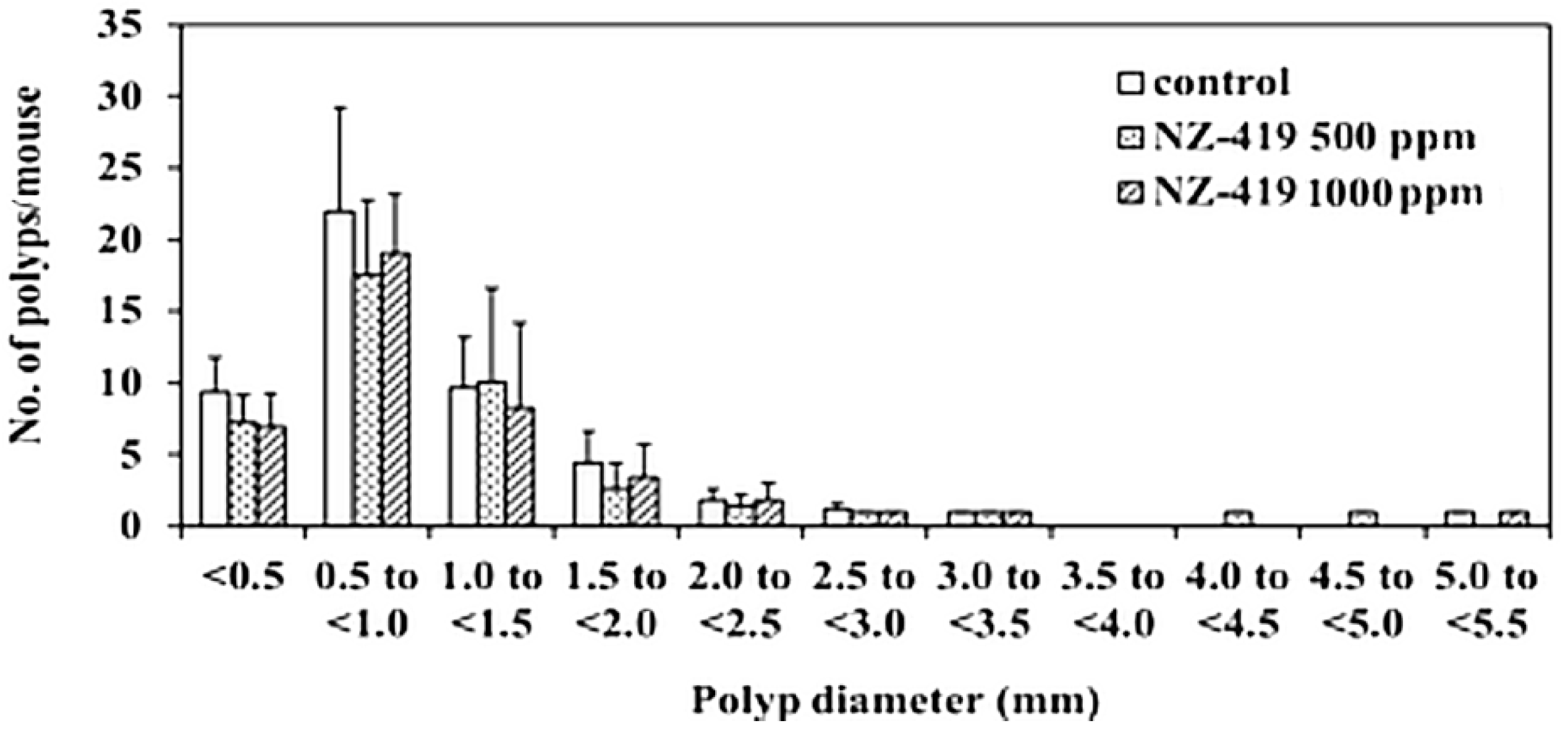
3.3. Suppression of Intestinal Polyp Formation in Min Mice by NZ-419
3.4. The Levels of Oxidative Stress-Related Markers in Min Mice by NZ-419
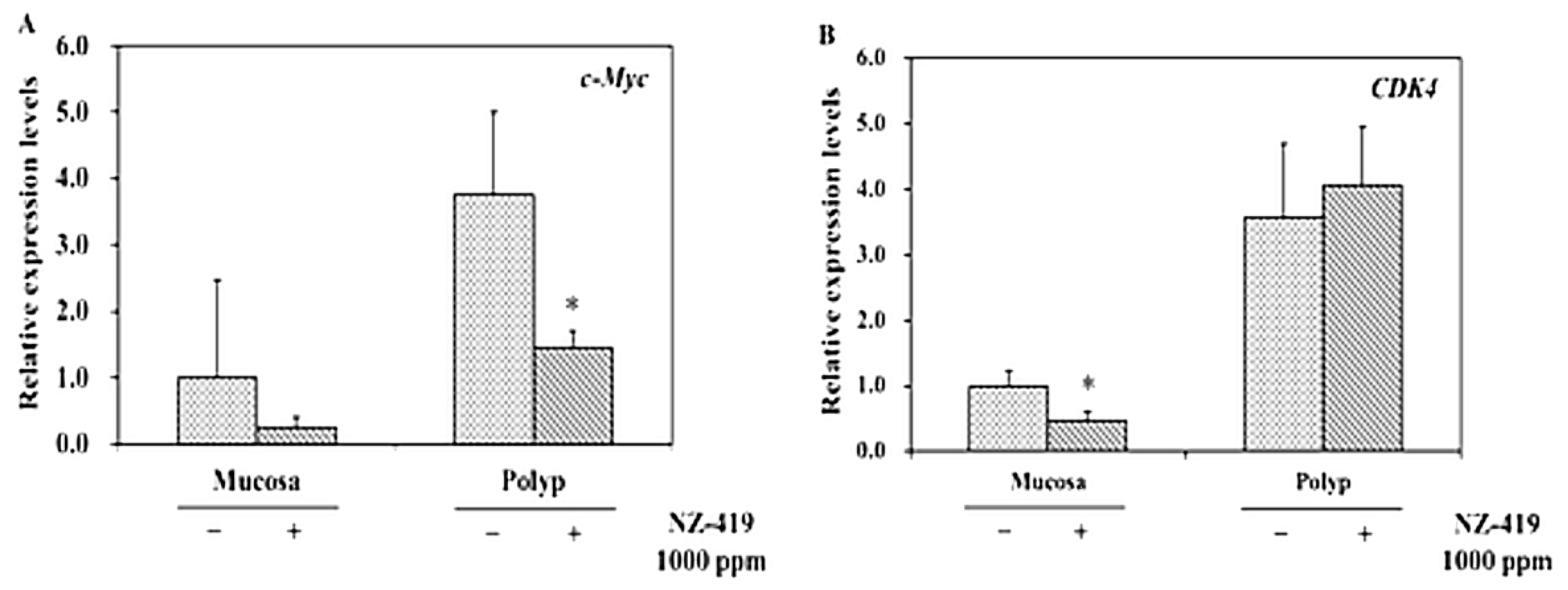
3.5. Suppression of mRNA Levels of Proliferation-Related Factor in Intestinal Polyp of Min Mice by NZ-419
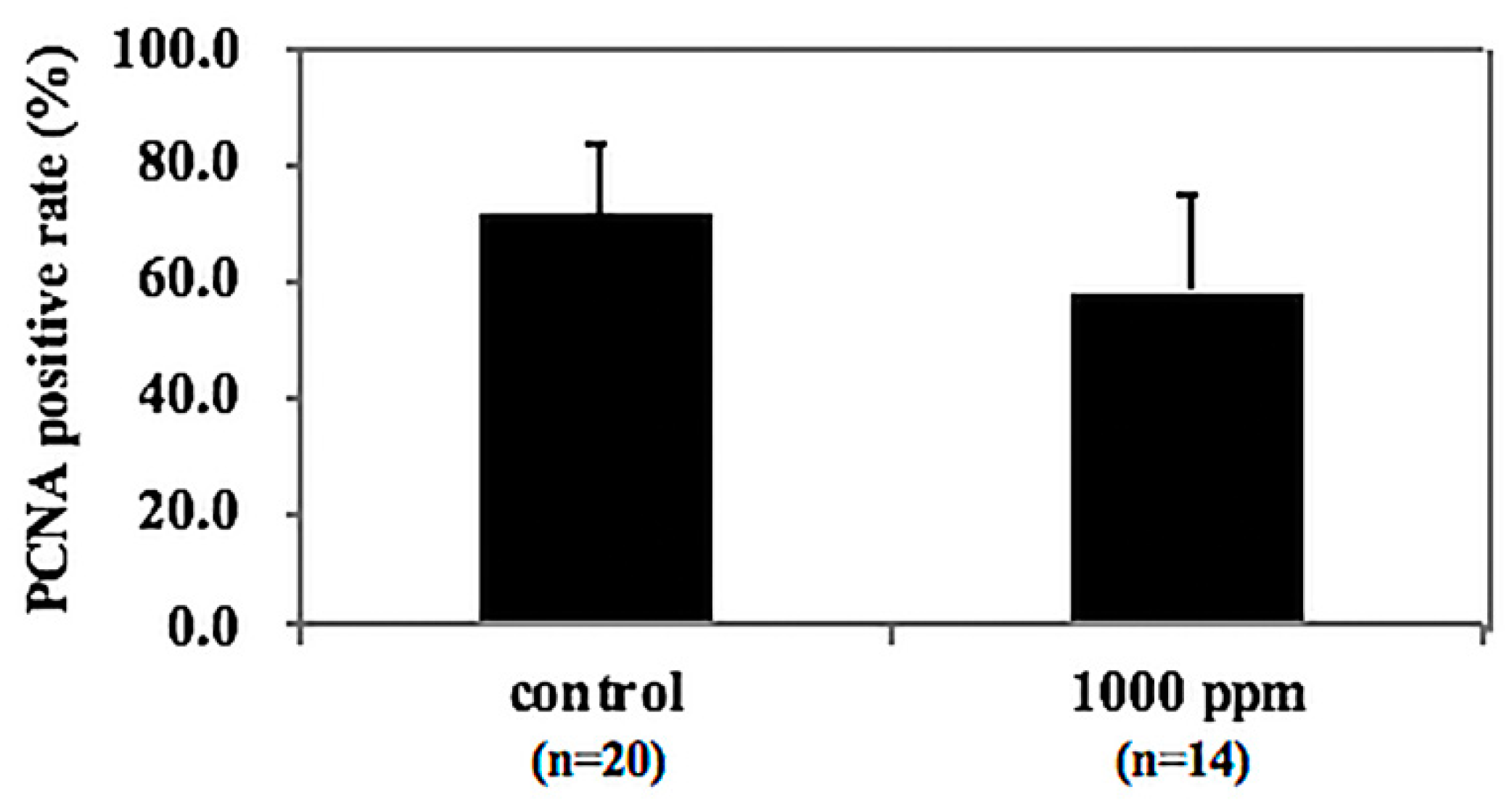
3.6. Tendency of NZ-419 to Decrease Cell Growth in Intestinal Polyps
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-ASA | 5-aminosalicylic acid |
| CRC | colorectal cancer |
| COX | cyclooxygenase |
| EPA | eicosapentaenoic acid |
| FAP | familial adenomatous polyposis |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GPCRs | G protein-coupled receptors |
| NAC | N-Acetyl-l-cysteine |
| Keap1 | Kelch-like ECH-associated protein 1 |
| Nrf2 | nuclear factor erythroid-2 related factor 2 |
| PCR | polymerase chain reaction |
| PUFAs | polyunsaturated fatty acids |
| PCNA | proliferating cell nuclear antigen |
| RCs | reactive carbonyl species |
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Cockbain, A.J.; Toogood, G.J.; Hull, M.A. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut 2012, 61, 135–149. [Google Scholar] [CrossRef] [PubMed]
- West, N.J.; Clark, S.K.; Phillips, R.K.; Hutchinson, J.M.; Leicester, R.J.; Belluzzi, A.; Hull, M.A. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut 2010, 59, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.M.; Kanakasabai, S.; Gokarn, S.V.; Krueger, E.G.; Bright, J.J. Dietary lutein modulates growth and survival genes in prostate cancer cells. J. Med. Food 2015, 18, 173–181. [Google Scholar] [CrossRef]
- Pei, Y.X.; Heng, Z.C.; Duan, G.C.; Wang, M.C. The mechanisms and effects of lutein on inducing the cell differentiation of human esophagus cancer EC9706. Sichuan Da Xue Xue Bao Yi Xue Ban 2007, 38, 629–632. [Google Scholar]
- Sumantran, V.N.; Zhang, R.; Lee, D.S.; Wicha, M.S. Differential regulation of apoptosis in normal versus transformed mammary epithelium by lutein and retinoic acid. Cancer Epidemiol. Biomark. Prev. 2000, 9, 257–263. [Google Scholar]
- Lorenzo, Y.; Azqueta, A.; Luna, L.; Bonilla, F.; Domínguez, G.; Collins, A.R. The carotenoid beta-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis 2009, 30, 308–314. [Google Scholar] [CrossRef]
- Uchiyama, S.; Yamaguchi, M. Beta-cryptoxanthin stimulates apoptotic cell death and suppresses cell function in osteoclastic cells: Change in their related gene expression. J. Cell Biochem. 2006, 98, 1185–1195. [Google Scholar] [CrossRef]
- Liu, C.; Bronson, R.T.; Russell, R.M.; Wang, X.D. β-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets. Cancer Prev. Res. 2011, 4, 1255–1266. [Google Scholar] [CrossRef]
- Terasaki, M.; Mutoh, M.; Fujii, G.; Takahashi, M.; Ishigamori, R.; Masuda, S. Potential ability of xanthophylls to prevent obesity-associated cancer. World J. Pharm. 2014, 3, 140–152. [Google Scholar] [CrossRef]
- Graham, P.M.; Li, J.Z.; Dou, X.; Zhu, H.; Misra, H.P.; Jia, Z.; Li, Y. Protection against peroxynitrite-induced DNA damage by mesalamine: Implications for anti-inflammation and anti-cancer activity. Mol. Cell Biochem. 2013, 378, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Ritland, S.R.; Leighton, J.A.; Hirsch, R.E.; Morrow, J.D.; Weaver, A.L.; Gendler, S.J. Evaluation of 5-aminosalicylic acid (5-ASA) for cancer chemoprevention: Lack of efficacy against nascent adenomatous polyps in the ApcMin mouse. Clin. Cancer Res. 1999, 5, 855–863. [Google Scholar] [PubMed]
- Ienaga, K.; Yokozawa, T. Treatment with NZ-419 (5-Hydroxy-1-methylimidazoline-2,4-dione), a novel intrinsic antioxidant, against the progression of chronic kidney disease at stages 3 and 4 in rats. Biol. Pharm. Bull. 2010, 33, 809–815. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ienaga, K.; Yokozawa, T. Creatinine and HMH (5-hydroxy-1-methylhydantoin, NZ-419) as intrinsic hydroxyl radical scavengers. Drug Discov. Ther. 2011, 5, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Akakabe, Y.; Nyuugaku, T. An efficient conversion of carboxylic acids to one-carbon degraded aldehydes via 2-hydroperoxy acids. Biosci. Biotechnol. Biochem. 2007, 71, 1370–1371. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, M.; Sanz, A.; Castresana, C. Alpha-oxidation of fatty acids in higher plants. Identification of a pathogen-inducible oxygenase (piox) as an alpha-dioxygenase and biosynthesis of 2-hydroperoxylinolenic acid. J. Biol. Chem. 1999, 274, 24503–24513. [Google Scholar] [CrossRef]
- Wentworth, P., Jr.; McDunn, J.E.; Wentworth, A.D.; Takeuchi, C.; Nieva, J.; Jones, T.; Bautista, C.; Ruedi, J.M.; Gutierrez, A.; Janda, K.D.; et al. Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science 2002, 298, 2195–2199. [Google Scholar] [CrossRef]
- Tomono, S.; Miyoshi, N.; Ohshima, H. Comprehensive analysis of the lipophilic reactive carbonyls present in biological specimens by LC/ESI-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 988, 149–156. [Google Scholar]
- Onuma, W.; Tomono, S.; Miyamoto, S.; Fujii, G.; Hamoya, T.; Fujimoto, K.; Miyoshi, N.; Fukai, F.; Wakabayashi, K.; Mutoh, M. Irsogladine maleate, a gastric mucosal protectant, suppresses intestinal polyp development in Apc-mutant mice. Oncotarget 2016, 7, 8640–8686. [Google Scholar] [CrossRef]
- Lyakhovich, A.; Gasche, C. Systematic review: Molecular chemoprevention of colorectal malignancy by mesalazine. Aliment Pharm. 2010, 31, 202–209. [Google Scholar] [CrossRef]
- Nosál’ová, V.; Cerná, S.; Bauer, V. Effect of N-acetylcysteine on colitis induced by acetic acid in rats. Gen Pharmacol. 2000, 35, 77–81. [Google Scholar] [CrossRef]
- Ahnfelt-Rønne, I.; Nielsen, O.H.; Christensen, A.; Langholz, E.; Binder, V.; Riis, P. Clinical evidence supporting the radical scavenger mechanism of 5-aminosalicylic acid. Gastroenterology 1990, 5, 1162–1169. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Hishikawa, A.; Hayashi, K.; Itoh, H. Transcription factors as therapeutic targets in chronic kidney disease. Molecules 2018, 9, 1123. [Google Scholar] [CrossRef]
- Niho, N.; Mutoh, M.; Komiya, M.; Ohta, T.; Sugimura, T.; Wakabayashi, K. Improvement of hyperlipidemia by indomethacin in Min mice. Int. J. Cancer 2007, 121, 1665–1669. [Google Scholar] [CrossRef]
- Nakatsugi, S.; Fukutake, M.; Takahashi, M.; Fukuda, K.; Isoi, T.; Taniguchi, Y.; Sugimura, T.; Wakabayashi, K. Suppression of intestinal polyp development by nimesulide, a selective cyclooxygenase-2 inhibitor, in Min mice. Jpn. J. Cancer Res. 1997, 88, 1117–1120. [Google Scholar] [CrossRef]
- Shimizu, S.; Fujii, G.; Takahashi, M.; Nakanishi, R.; Komiya, M.; Shimura, M.; Noma, N.; Onuma, W.; Terasaki, M.; Yano, T.; et al. Sesamol suppresses cyclooxygenase-2 transcriptional activity in colon cancer cells and modifies intestinal polyp development in ApcMin/+ mice. J. Clin. Biochem. Nutr. 2014, 54, 95–101. [Google Scholar] [CrossRef]
- Komiya, M.; Fujii, G.; Miyamoto, S.; Takahashi, M.; Ishigamori, R.; Onuma, W.; Ishino, K.; Totsuka, Y.; Fujimoto, K.; Mutoh, M. Suppressive effects of the NADPH oxidase inhibitor apocynin on intestinal tumorigenesis in obese KK-Ay and Apc mutant Min mice. Cancer Sci. 2015, 106, 1499–1505. [Google Scholar] [CrossRef]
- Niho, N.; Mutoh, M.; Takahashi, M.; Tsutsumi, K.; Sugimura, T.; Wakabayashi, K. Concurrent suppression of hyperlipidemia and intestinal polyp formation by NO-1886, increasing lipoprotein lipase activity in Min mice. Proc. Natl. Acad. Sci. USA 2005, 102, 2970–2974. [Google Scholar] [CrossRef]
- Hamoya, T.; Miyamoto, S.; Tomono, S.; Fujii, G.; Nakanishi, R.; Komiya, M.; Tamura, S.; Fujimoto, K.; Toshima, J.; Wakabayashi, K.; et al. Chemopreventive effects of a low-side-effect antibiotic drug, erythromycin, on mouse intestinal tumors. J. Clin. Biochem. Nutr. 2017, 60, 199–207. [Google Scholar] [CrossRef]
- Niho, N.; Takahashi, M.; Shoji, Y.; Takeuchi, Y.; Matsubara, S.; Sugimura, T.; Wakabayashi, K. Dose-dependent suppression of hyperlipidemia and intestinal polyp formation in Min mice by pioglitazone, a PPAR gamma ligand. Cancer Sci. 2003, 94, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Komiya, M.; Fujii, G.; Takahashi, M.; Iigo, M.; Mutoh, M. Prevention and intervention trials for colorectal cancer. Jpn. J. Clin. Oncol. 2013, 43, 685–694. [Google Scholar] [CrossRef] [PubMed]

| Dose | No. of Mice | Small Intestine | Colon | Total | ||
|---|---|---|---|---|---|---|
| (ppm) | Proximal | Middle | Distal | |||
| 0 | 9 | 5.8 ± 2.9 | 11.7 ± 3.4 | 29.3 ± 5.4 | 0.7 ± 1.0 | 47.4 ± 5.2 |
| 500 | 9 | 3.9 ± 1.6 | 7.3 ± 2.9 * | 27.0 ± 10.2 | 1.0 ± 0.9 | 39.2 ± 10.3 |
| 1000 | 8 | 5.1 ± 2.4 | 8.3 ± 2.8 | 25.6 ± 7.2 | 0.4 ± 0.7 | 39.4 ± 9.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurokawa, Y.; Fujii, G.; Tomono, S.; Miyamoto, S.; Hamoya, T.; Takahashi, M.; Narita, T.; Komiya, M.; Kobayashi, M.; Higami, Y.; et al. The Radical Scavenger NZ-419 Suppresses Intestinal Polyp Development in Apc-Mutant Mice. J. Clin. Med. 2020, 9, 270. https://doi.org/10.3390/jcm9010270
Kurokawa Y, Fujii G, Tomono S, Miyamoto S, Hamoya T, Takahashi M, Narita T, Komiya M, Kobayashi M, Higami Y, et al. The Radical Scavenger NZ-419 Suppresses Intestinal Polyp Development in Apc-Mutant Mice. Journal of Clinical Medicine. 2020; 9(1):270. https://doi.org/10.3390/jcm9010270
Chicago/Turabian StyleKurokawa, Yurie, Gen Fujii, Susumu Tomono, Shingo Miyamoto, Takahiro Hamoya, Maiko Takahashi, Takumi Narita, Masami Komiya, Masaki Kobayashi, Yoshikazu Higami, and et al. 2020. "The Radical Scavenger NZ-419 Suppresses Intestinal Polyp Development in Apc-Mutant Mice" Journal of Clinical Medicine 9, no. 1: 270. https://doi.org/10.3390/jcm9010270
APA StyleKurokawa, Y., Fujii, G., Tomono, S., Miyamoto, S., Hamoya, T., Takahashi, M., Narita, T., Komiya, M., Kobayashi, M., Higami, Y., & Mutoh, M. (2020). The Radical Scavenger NZ-419 Suppresses Intestinal Polyp Development in Apc-Mutant Mice. Journal of Clinical Medicine, 9(1), 270. https://doi.org/10.3390/jcm9010270






