Diagnostic Performance of Different Thyroid Imaging Reporting and Data Systems (Kwak-TIRADS, EU-TIRADS and ACR TI-RADS) for Risk Stratification of Small Thyroid Nodules (≤10 mm)
Abstract
:1. Introduction
2. Experimental Section
- TIRADS 3: no suspicious features (risk 1.7%);
- TIRADS 4A: one suspicious feature (risk 3.3%);
- TIRADS 4B: two suspicious features (risk 9.2%);
- TIRADS 4C: three or four suspicious features (risk 44.4–72.4%);
- TIRADS 5: five suspicious features (risk 87.5%).
- EU-TIRADS 2: anechoic or entirely spongiform (benign, risk 0%);
- EU-TIRADS 3: entirely isoechoic or hyperechoic (low risk, risk 2–4%);
- EU-TIRADS 4: mildly hypoechoic (intermediate risk, risk 6–17%);
- EU-TIRADS 5: at least one of the four features of high suspicion (high risk, risk 26–87%).
- TR1: 0 points, benign (aggregate risk level 0.3%);
- TR2: 2 points, not suspicious (aggregate risk level 1.5%);
- TR3: 3 points, mildly suspicious (aggregate risk level 4.8%);
- TR4: 4–6 points, moderately suspicious (aggregate risk level 5.9–12.8%);
- TR5: 7 points or more, highly suspicious (aggregate risk level 20.8–68.4% for 10 points).
2.1. Pathological Examination
2.2. Statistics
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Grady, A.T.; Sosa, J.A.; Tanpitukpongse, T.P.; Choudhury, K.R.; Gupta, R.T.; Hoang, J.K. Radiology reports for incidental thyroid nodules on CT and MRI: High variability across subspecialties. AJNR Am. J. Neuroradiol. 2015, 36, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Farra, J.C.; Picado, O.; Liu, S.; Ouyang, W.; Teo, R.; Franco, A.M.; Lew, J.I. Clinically significant cancer rates in incidentally discovered thyroid nodules by routine imaging. J. Surg. Res. 2017, 219, 341–346. [Google Scholar] [CrossRef]
- Li, Y.; Cui, M.; Azar, N.; Nakamoto, D.; Michael, C.W. Cytological evaluation by fine needle aspiration biopsy of incidental focal increased fluorodeoxyglucose uptake in thyroid on positron emission tomography scan. Diagn. Cytopathol. 2017, 45, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, K.H.; Cho, S.G.; Kim, Y.J.; Lee, H.Y.; Hong, I.K.; Kim, J.H. Incidental thyroid nodules on thoracic contrast-enhanced computed tomography in clinical practice during a 10-year period: Characteristics, clinical outcomes, and factors contributing to further evaluation. Medicine 2017, 96, e6388. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.B.; Perrier, N.D. The incidental thyroid nodule. CA Cancer J. Clin. 2018, 68, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Hao, S.; Gao, B.; Jiang, Y.; Zhang, X.; Zhang, S.; Guo, L.; Yan, J.; Luo, D. Comparing the diagnostic accuracy of RTE and SWE in differentiating malignant thyroid nodules from benign ones: A meta-analysis. Cell. Physiol. Biochem. 2019, 39, 2451–2463. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Han, K.H.; Yoon, J.H.; Moon, H.J.; Son, E.J.; Park, S.H.; Jung, H.K.; Choi, J.S.; Kim, B.M.; Kim, E.K. Thyroid imaging reporting and data system for US features of nodules: A step in establishing better stratification of cancer risk. Radiology 2011, 260, 892–899. [Google Scholar] [CrossRef] [Green Version]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef] [Green Version]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Schmid, K.W.; Synoracki, S.; Dralle, H.; Wittekind, C. Proposal for an extended pTNM classification of thyroid carcinoma: Commentary on deficits of the 8th edition of the TNM classification. Pathologe 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 7th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 58–62. [Google Scholar]
- Dietlein, M.; Eschner, W.; Lassmann, M.; Verburg, F.; Luster, M. Procedure Guideline for Thyroid Scintigraphy (Version 4). 2014. Available online: http://www.nuklearmedizin.de/leistungen/leitlinien/docs/031011l_S1_Schilddruesenszintigraphie_2014-10.pdf (accessed on 2 December 2019).
- Leboulleux, S.; Tuttle, R.M.; Pacini, F.; Schlumberger, M. Papillary thyroid microcarcinoma: Time to shift from surgery to active surveillance? Lancet Diabetes Endocrinol. 2016, 4, 933–942. [Google Scholar] [CrossRef]
- Miyauchi, A. Clinical trials of active surveillance of papillary microcarcinoma of the thyroid. World J. Surg. 2016, 40, 516–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyauchi, A.; Ito, Y.; Oda, H. Insights into the management of papillary microcarcinoma of the thyroid. Thyroid 2018, 28, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Oda, H.; Miyauchi, A.; Ito, Y.; Sasai, H.; Masuoka, H.; Yabuta, T.; Fukushima, M.; Higashiyama, T.; Kihara, M.; Kobayashi, K.; et al. Comparison of the costs of active surveillance and immediate surgery in the management of low-risk papillary microcarcinoma of the thyroid. Endocr. J. 2017, 64, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, E.; Majlis, S.; Rossi, R.; Franco, C.; Niedmann, J.P.; Castro, A.; Dominquez, M. An ultrasound reporting system for thyroid nodules stratifying cancer risk for clinical management. J. Clin. Endocrinol. Metab. 2009, 94, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Castellana, C.; Treglia, G.; Giorgino, F.; Giovanella, L.; Russ, G.; Trimboli, P. Performance of five ultrasound risk stratification systems in selecting thyroid nodules for FNA. A meta-analysis. J. Clin. Endocrinol. Metab. 2019. [Google Scholar] [CrossRef]
- Du, Y.R.; Ji, C.L.; Wu, Y.; Gu, X.G. Combination of ultrasound elastography with TI-RADS in the diagnosis of small thyroid nodules (≤10 mm): A new method to increase the diagnostic performance. Eur. J. Radiol. 2018, 109, 33–40. [Google Scholar] [CrossRef]
- Mendes, G.F.; Garcia, M.R.; Falsarella, P.M.; Rahal, A.; Cavalcante Junior, F.A.; Nery, D.R.; Garcia, R.G. Fine needle aspiration biopsy of thyroid nodule smaller than 1.0 cm: Accuracy of TIRADS classification system in more than 1000 nodules. Br. J. Radiol. 2018, 91, 20170642. [Google Scholar] [CrossRef]
- Ha, S.M.; Kim, J.K.; Baek, J.H. Detection of malignancy among suspicious thyroid nodules ≤1cm on ultrasound with various thyroid image reporting and data systems. Thyroid 2017, 27, 1307–1315. [Google Scholar] [CrossRef]
- Weiss, V.L.; Andreotti, R.F.; Ely, K.A. Use of thyroid imaging, reporting, and data system (TI-RADS) scoring system for the evalution of subcentimeter thyroid nodules. Cancer Cytopathol. 2018, 126, 518–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deveci, M.S.; Deveci, G.; LiVolsi, V.A.; Gupta, P.K.; Baloch, Z.W. Concordance between thyroid nodule size measured by ultrasound and gross pathology examination: Effect on patient management. Diagn. Cytopathol. 2007, 35, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yan, H.; Pang, P.; Fan, X.; Jia, X.; Zang, L.; Luo, Y.; Wang, F.; Yang, G.; Gu, W.; et al. Thyroid nodule size calculated using ultrasound and gross pathology as predictors of cancer: A 23-year retrospective study. Diagn. Cytopathol. 2019, 47, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.Y.; Shin, J.H.; Oh, Y.L.; Son, Y.I. Discrepancies between the ultrasonographic and gross pathological size of papillary thyroid carcinomas. Ultrasonography 2016, 36, 220–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifert, P.; Görges, R.; Zimny, M.; Kreissl, M.C.; Schenke, S. Interobserver agreement and efficacy of consensus reading in Kwak-, EU-, and ACR thyroid imaging recording and data systems and ATA guidelines for the ultrasound risk stratification of thyroid nodules. Endocrine 2019. accepted. [Google Scholar] [CrossRef] [PubMed]
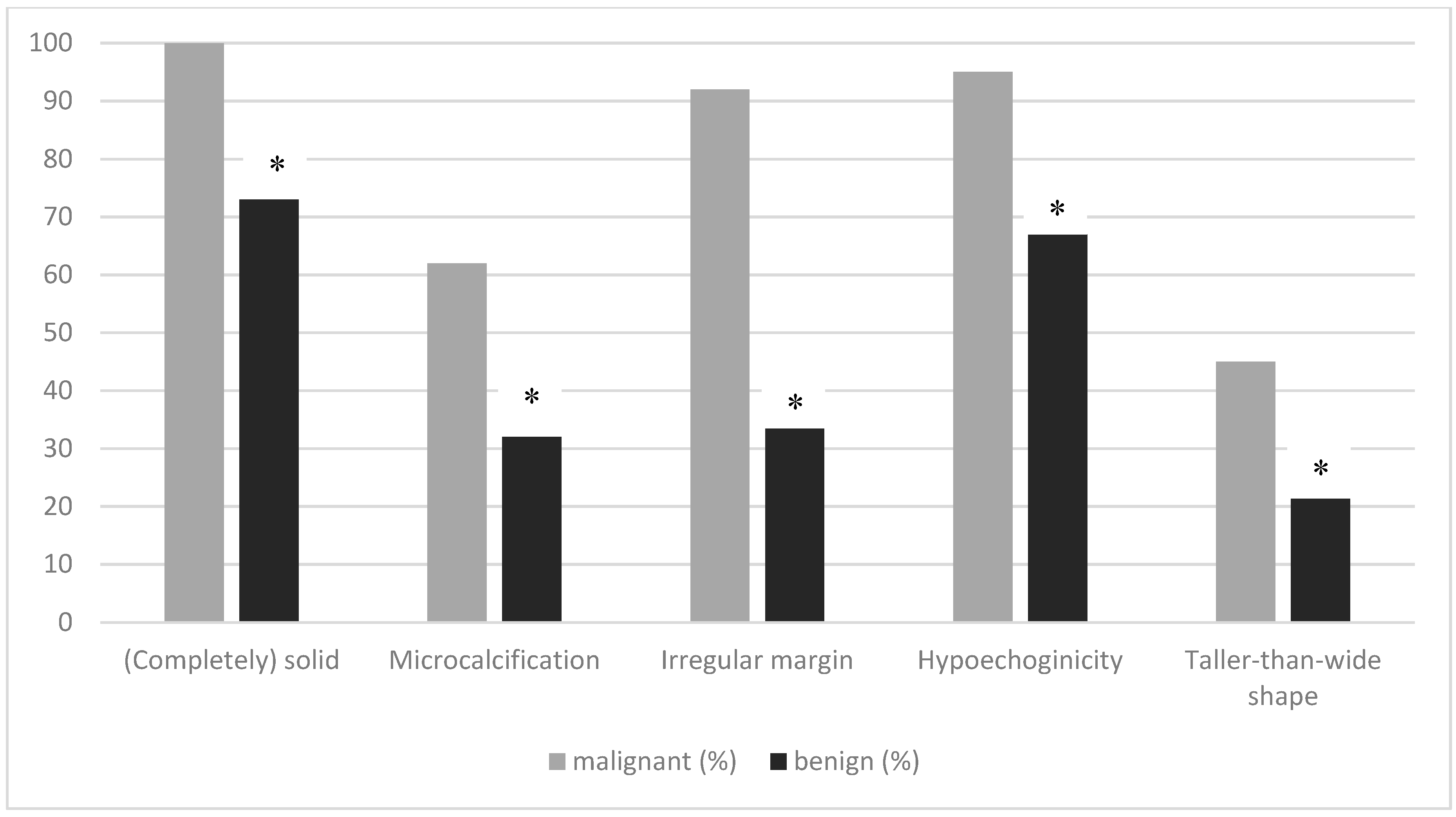
| All TC n = 76 | TC pT1a1 n = 55 | TC pT1a2 n = 9 | TC pT1b n = 12 | |
|---|---|---|---|---|
| pN0 n (%) | 37 (48.7) | 26 (47.2) | 4 (44.5) | 7 (58.3) |
| pN1 n (%) | 15 (19.7) | 9 (16.4) | 3 (33.3) | 3 (25.0) |
| pNX n (%) | 24 (31.6) | 20 (36.4) | 2 (22.2) | 2 (16.7) |
| Malignant Thyroid Nodules (n = 76, %) | Benign Thyroid Nodules (n = 69, %) | Prevalence of Malignancy (%) | |
|---|---|---|---|
| Kwak-TIRADS | |||
| 3 n (%) | 0 (0) | 9 (13.1) | 0 |
| 4A n (%) | 0 (0) | 19 (27.5) | 0 |
| 4B n (%) | 2 (2.6) | 10 (14.5) | 16.7 |
| 4C n (%) | 57 (75.0) | 27 (39.1) | 67.9 |
| 5 n (%) | 17 (22.3) | 4 (5.8) | 81.0 |
| ACR TI-RADS | |||
| TR1 n (%) | 0 (0) | 4 (5.8) | 0 |
| TR2 n (%) | 0 (0) | 8 (11.6) | 0 |
| TR3 n (%) | 0 (0) | 16 (23.2) | 0 |
| TR4 n (%) | 23 (30.3) | 18 (26.1) | 56.1 |
| TR5 n (%) | 53 (69.7) | 23 (33.3) | 69.7 |
| EU-TIRADS | 0 (0) | ||
| 2 n (%) | 0(0) | 0 (0) | 0 |
| 3 n (%) | 1 (1.3) | 23 (33.3) | 4.2 |
| 4 n (%) | 1 (1.3) | 11 (15.9) | 8.3 |
| 5 n (%) | 74 (97.4) | 35 (50.7) | 67.9 |
| Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Accuracy (%) | |
|---|---|---|---|---|---|
| Kwak-TIRADS 4C and 5 | 97.4 | 55.1 | 70.5 | 95 | 77.2 |
| ACR-TI-RADS TR4 and TR5 | 100 | 40.6 | 65 | 100 | 71.7 |
| EU-TIRADS 5 | 97.4 | 49.3 | 67.9 | 94.4 | 74.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schenke, S.; Klett, R.; Seifert, P.; Kreissl, M.C.; Görges, R.; Zimny, M. Diagnostic Performance of Different Thyroid Imaging Reporting and Data Systems (Kwak-TIRADS, EU-TIRADS and ACR TI-RADS) for Risk Stratification of Small Thyroid Nodules (≤10 mm). J. Clin. Med. 2020, 9, 236. https://doi.org/10.3390/jcm9010236
Schenke S, Klett R, Seifert P, Kreissl MC, Görges R, Zimny M. Diagnostic Performance of Different Thyroid Imaging Reporting and Data Systems (Kwak-TIRADS, EU-TIRADS and ACR TI-RADS) for Risk Stratification of Small Thyroid Nodules (≤10 mm). Journal of Clinical Medicine. 2020; 9(1):236. https://doi.org/10.3390/jcm9010236
Chicago/Turabian StyleSchenke, Simone, Rigobert Klett, Philipp Seifert, Michael C. Kreissl, Rainer Görges, and Michael Zimny. 2020. "Diagnostic Performance of Different Thyroid Imaging Reporting and Data Systems (Kwak-TIRADS, EU-TIRADS and ACR TI-RADS) for Risk Stratification of Small Thyroid Nodules (≤10 mm)" Journal of Clinical Medicine 9, no. 1: 236. https://doi.org/10.3390/jcm9010236
APA StyleSchenke, S., Klett, R., Seifert, P., Kreissl, M. C., Görges, R., & Zimny, M. (2020). Diagnostic Performance of Different Thyroid Imaging Reporting and Data Systems (Kwak-TIRADS, EU-TIRADS and ACR TI-RADS) for Risk Stratification of Small Thyroid Nodules (≤10 mm). Journal of Clinical Medicine, 9(1), 236. https://doi.org/10.3390/jcm9010236






