Ceruloplasmin, NT-proBNP, and Clinical Data as Risk Factors of Death or Heart Transplantation in a 1-Year Follow-Up of Heart Failure Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Assessment
2.2. Biochemical Methods
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Entire Study Population and Subgroups in Relation to Ceruloplasmin Concentration
3.2. Ceruloplasmin, NT-proBNP and Prognosis
3.2.1. Univariate Cox Regression Analysis
3.2.2. Multivariate Cox Regression Analysis
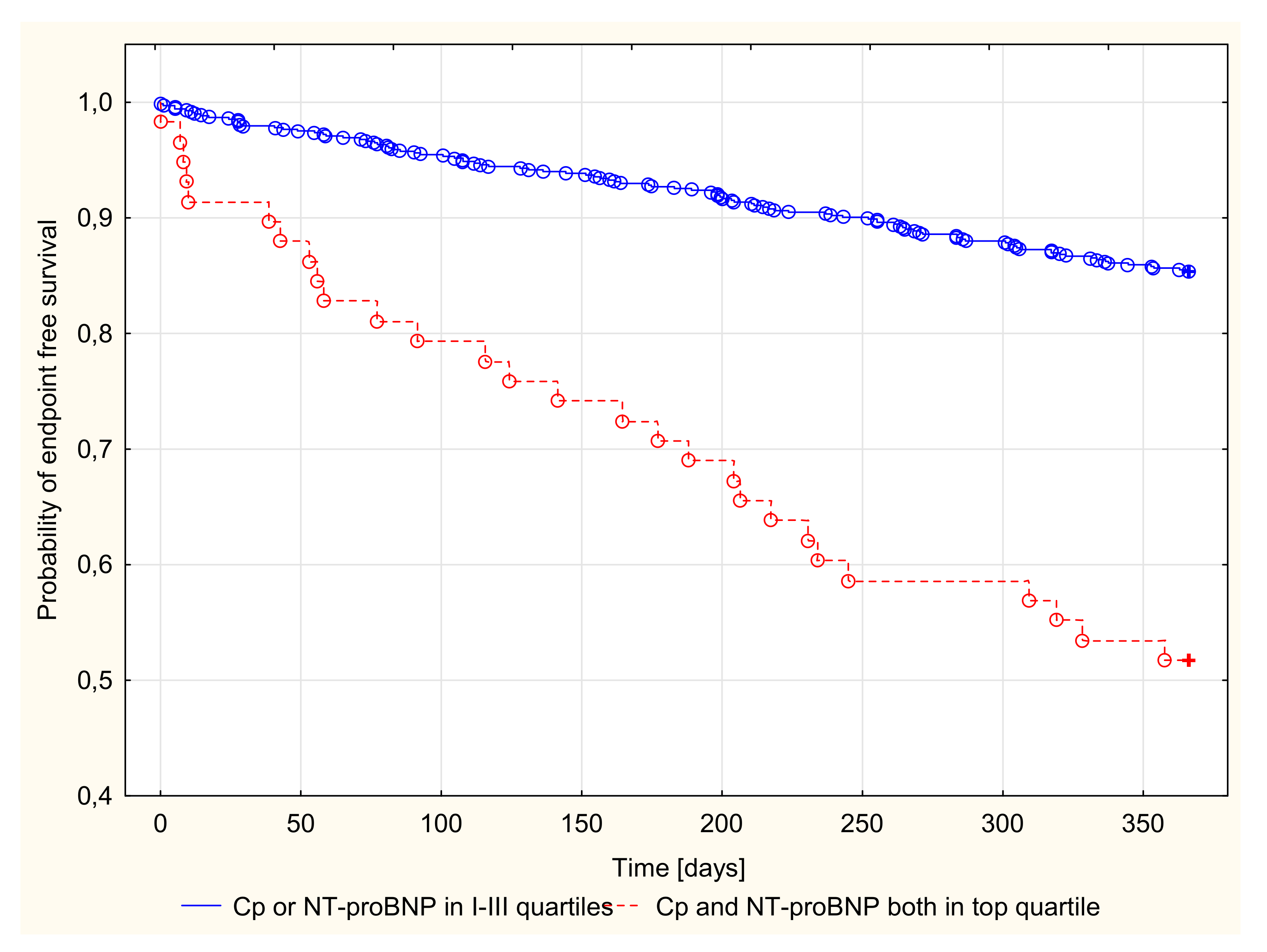
3.2.3. Kaplan–Meier Survival Analysis and Endpoint Odds Ratios
4. Discussion
5. Conclusions
Study Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Seino, Y.; Ogawa, A.; Yamashita, T.; Fukushima, M.; Ogata, K.; Fukumoto, H.; Takan, T. Application of NT-proBNP and BNP measurements in cardiac care: A more discerning marker for the detection and evaluation of heart failure. Eur. J. Heart Fail. 2004, 6, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.; Doehner, W.; Rauchhaus, M.; Sharma, R.; Francis, D.; Knosalla, C.; Davos, C.H.; Cicoira, M.; Shamim, W.; Kemp, M.; et al. Uric Acid and Survival in Chronic Heart Failure Validation and Application in Metabolic, Functional, and Hemodynamic Staging. Circulation 2003, 107, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.H.; Chen, J.W.; Jen, L.H.; Chiang, M.C.; Huang, W.P.; Feng, A.N.; Young, M.S.; Lin, S.J. Independent prognostic value of elevated high-sensitivity C-reactive protein in chronic heart failure. Am. Heart J. 2004, 147, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Neuhold, S.; Huelsmann, M.; Strunk, G.; Stoiser, B.; Struck, J.; Morgenthaler, N.G.; Bergmann, A.; Moertl, D.; Berger, R.; Pacher, R. Comparison of copeptin, B-type natriuretic peptide, and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure: Prediction of death at different stages of the disease. J. Am. Coll. Cardiol. 2008, 52, 266–272. [Google Scholar] [CrossRef]
- Chow, S.L.; Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; de Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L., Jr.; et al. Role of biomarkers for the prevention, assessment, and management of heart failure a scientific statement from the American Heart Association. Circulation 2017, 135, 1054–1091. [Google Scholar] [CrossRef]
- Lai, M.Y.; Kan, W.C.; Huang, Y.T.; Chen, J.; Shiao, C.C. The Predictivity of N-Terminal Pro b-Type Natriuretic Peptide for All-Cause Mortality in Various Follow-Up Periods among Heart Failure Patients. J. Clin. Med. 2019, 13, 357. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Rehman, S.U.; Mohammed, A.A.; Bhardwaj, A.; Barajas, L.; Barajas, J.; Kim, H.N.; Baggish, A.L.; Weiner, R.B.; Chen-Tournoux, A.; et al. Use of amino-terminal pro-B type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J. Am. Coll. Cardiol. 2011, 58, 1881–1889. [Google Scholar] [CrossRef]
- Floris, G.; Medda, R.; Padiglia, A.; Musci, G. The physiopathological significance of caeruloplasmin. Biochem. Pharmacol. 2000, 60, 1735–1741. [Google Scholar] [CrossRef]
- Harris, E.D. A requirement for copper in angiogenesis. Nutr. Rev. 2004, 62, 60–64. [Google Scholar] [CrossRef]
- Hannan, G.N.; McAuslen, B.R. Modulation of synthesis of specific proteins in endothelial cells by copper, cadmium, and disulfiram: An early response to an angiogenic inducer of cell migration. J. Cell. Physiol. 1982, 111, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Maher, J.; Masters, J.; Angelini, G.D.; Jeremy, J.Y. Does oxidative stress change ceruloplasmin from a protective to a vasculopathic factor. Atherosclerosis 2006, 187, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S.; Wang, X.; Ringwood, L.A.; Xu, X.; Yuditskaya, S.; Annavajjhala, V.; Miyajima, H.; Hogg, N.; Harris, Z.L.; Gladwin, M.T. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat. Chem. Biol. 2006, 2, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, H.; Zhou, Y.; Cheng, G.; Xu, G. Ceruloplasmin and the extent of heart failure in ischemic and nonischemic cardiomyopathy patients. Mediat. Inflamm. 2013, 2013, 348145. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Z.; Kaya, B.; Sezen, H.; Bilinc, H.; Asoglu, R.; Yildiz, A.; Taskin, A.; Yalcin, S.; Sezen, Y.; Aksoy, N. Serum ceruloplasmin levels in acute decompensated heart failure. Clin. Ter. 2013, 164, 87–91. [Google Scholar]
- Ahmed, M.S.; Jadhav, A.B.; Hassan, A.; Meng, Q.H. Acute Phase Reactants as Novel Predictors of Cardiovascular Disease. ISRN Inflamm. 2012. [Google Scholar] [CrossRef]
- Studies Investigating Co-Morbidities Aggravating Heart Failure (SICA-HF). 2016. Available online: https://clinicaltrials.gov/ (accessed on 18 November 2019).
- Romuk, E.; Wojciechowska, C.; Jacheć, W.; Nowak, J.; Niedziela, J.; Malinowska-Borowska, J.; Głogowska-Gruszka, A.; Birkner, E.; Rozentryt, P. Comparison of Oxidative Stress Parameters in Heart Failure Patients Depending on Ischaemic or Nonischaemic Aetiology. Oxid. Med. Cell. Longev. 2019, 2019, 13. [Google Scholar] [CrossRef]
- Richterich, R.; Gautier, E.; Stillharth, H.; Rossi, E. Serum ceruloplasmin concentration was determined spectrophotometrically according to Richterich reaction with p-phenyl-diamine. The heterogeneity of caeruloplasmin nd the enzymatic defect in Wilson’s disease. Helv. Paediatr. Acta 1960, 15, 424–436. [Google Scholar]
- Singh, T.K. Serum ceruloplasmin in acute myocardial infarction. Acta Cardiol. 1992, 47, 321–329. [Google Scholar]
- Manttari, M.; Manninen, V.; Huttunen, J.K.; Palosuo, T.; Ehnholm, C.; Heinonen, O.P.; Frick, M.H. Serum ferritin and ceruloplasmin as coronary risk factors. Eur. Heart J. 1994, 15, 1599–1603. [Google Scholar] [CrossRef]
- Atanasiu, R.; Dumoulin, M.J.; Chahine, R.; Mateescu, M.A.; Nadeau, R. Antiarrhythmic effects of ceruloplasmin during reperfusion in the ischemic isolated rat heart. Can. J. Physiol. Pharmacol. 1995, 73, 1253–1261. [Google Scholar] [CrossRef]
- Goda, A.; Lund, L.H.; Mancini, D.M. Comparison across races of peak oxygen consumption and heart failure survival score for selection for cardiac transplantation. Am. J. Cardiol. 2010, 15, 1439–1444. [Google Scholar] [CrossRef]
- Paolillo, S.; Veglia, F.; Salvioni, E.; Corrà, U.; Piepoli, M.; Lagioia, R.; Limongelli, G.; Sinagra, G.; Cattadori, G.; Scardovi, A.B.; et al. Heart failure prognosis over time: How the prognostic role of oxygen consumption and ventilatory efficiency during exercise has changed in the last 20 years. Eur J. Heart Fail. 2019, 21, 208–217. [Google Scholar] [CrossRef]
- Tai, C.; Gan, T.; Zou, L.; Sun, Y.; Zhang, Y.; Chen, W.; Li, J.; Zhang, J.; Xu, Y.; Lu, H.; et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on cardiovascular events in patients with heart failure: A meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2017, 17, 257. [Google Scholar] [CrossRef]
- Rochon, P.A.; Sykora, K.; Bronskill, S.E.; Mamdani, M.; Anderson, G.M.; Gurwitz, J.H.; Gill, S.; Tu, J.V.; Laupacis, A. Use of angiotensin-converting enzyme inhibitor therapy and dose-related outcomes in older adults with new heart failure in the community. J. Gen. Intern. Med. 2004, 19, 676–683. [Google Scholar] [CrossRef][Green Version]
- Al-Khatib, S.M.; Hellkamp, A.; Bardy, G.H.; Hammill, S.; Hall, W.J.; Mark, D.B.; Anstrom, K.J.; Curtis, J.; Al-Khalidi, H.; Curtis, L.H.; et al. Survival of patients receiving a primary prevention implantable cardioverter-defibrillator in clinical practice vs clinical trials. JAMA 2013, 309, 55–62. [Google Scholar] [CrossRef]
- Kociol, R.D.; Horton, J.R.; Fonarow, G.C.; Reyes, E.M.; Shaw, L.K.; O’Connor, C.M.; Felker, G.M.; Hernandez, A.F. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: Data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ. Heart Fail. 2011, 4, 628–636. [Google Scholar] [CrossRef]
- Khanam, S.S.; Son, J.W.; Lee, J.W.; Youn, Y.J.; Yoon, J.; Lee, S.H.; Kim, J.Y.; Ahn, S.G.; Ahn, M.S.; Yoo, B.S. Prognostic value of short-term follow-up BNP in hospitalized patients with heart failure. BMC Cardiovasc. Disord. 2017, 17, 215. [Google Scholar] [CrossRef]
- Cheng, V.; Kazanagra, R.; Garcia, A.; Lenert, L.; Krishnaswamy, P.; Gardetto, N.; Clopton, P.; Maisel, A. A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: A pilot study. J. Am. Coll. Cardiol. 2001, 37, 386–391. [Google Scholar] [CrossRef]
- Bettencourt, P.; Azevedo, A.; Pimenta, J.; Frioes, F.; Ferreira, S.; Ferreira, A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation 2004, 110, 2168–2174. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, 137–161. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Pascual-Figal, D.; Fabregat, J.; Domingo, M.; Planas, F.; Casas, T.; Ordonez-Llanos, J.; Valdes, M.; Cinca, J. Serial NT-proBNP monitoring and outcomes in outpatients with decompensation of heart failure. Int. J. Cardiol. 2007, 120, 338–343. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Ordonez-Llanos, J. Multiple biomarker strategies for risk stratification in heart failure. Clin. Chim. Acta 2015, 443, 120–125. [Google Scholar] [CrossRef]
- Dadu, R.T.; Dodge, R.; Nambi, V.; Virani, S.S.; Hoogeveen, R.C.; Smith, N.L.; Chen, F.; Pankow, J.S.; Guild, C.; Tang, W.H.W.; et al. Ceruloplasmin and heart failure in the Atherosclerosis Risk in Communities study. Circ. Heart Fail. 2013, 6, 936–943. [Google Scholar] [CrossRef]
- Engström, G.; Hedblad, B.; Tydén, P.; Lindgärde, F. Inflammation-sensitive plasma proteins are associated with increased incidence of heart failure: A population-based cohort study. Atherosclerosis 2009, 202, 617–622. [Google Scholar] [CrossRef]
- Bustamante, J.B.; Mateo, M.C.; Fernandez, J.; de Quiros, B.; Manchado, O.O. Zinc, copper and ceruloplasmin in arteriosclerosis. Biomed. Express 1976, 25, 244–245. [Google Scholar]
- Reunanen, A.; Knekt, P.; Aaran, R.K. Serumceruloplasmin level and the risk ofmyocardial infarction and stroke. Am. J. Epidemiol. 1992, 136, 1082–1090. [Google Scholar] [CrossRef]
- Göçmen, A.Y.; Sahin, E.; Semiz, E.; Gümuşlü, S. Is elevated serum ceruloplasmin level associated with increased risk of coronary artery disease? Can. J. Cardiol. 2008, 24, 209–212. [Google Scholar] [CrossRef]
- Sezen, H.; Sezen, Y. How to Change Ceruloplasmin Levels in Heart Disease? Koşuyolu Heart J. 2018, 21, 61–64. [Google Scholar] [CrossRef]
- Cao, D.J.; Hill, J.A. Copper Futures: Ceruloplasmin and Heart Failure. Circ. Res. 2014, 114, 1678–1680. [Google Scholar] [CrossRef]
- Correale, M.; Brunetti, M.D.; de Gennaro, L.; di Biase, M. Acute phase proteins in atherosclerosis (Acute Coronary Syndrome). Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 272–277. [Google Scholar] [CrossRef]
- Cabassi, A.; Binno, S.M.; Tedeschi, S.; Ruzicka, V.; Dancelli, S.; Rocco, R.; Vicini, V.; Coghi, P.; Regolisti, G.; Montanari, A.; et al. Low Serum Ferroxidase I Activity Is Associated With Mortality in Heart Failure and Related to Both Peroxynitrite-Induced Cysteine Oxidation and Tyrosine Nitration of Ceruloplasmin. Circ. Res. 2014, 114, 1723–1732. [Google Scholar] [CrossRef]
- Hammadah, M.; Fan, Y.; Wu, Y.; Hazern, S.L.; Wilson Tang, W.H. Prognostic Value of Elevated Serum Ceruloplasmin Levels in Patients with Heart Failure. J. Card. Fail. 2014, 20, 946–952. [Google Scholar] [CrossRef]
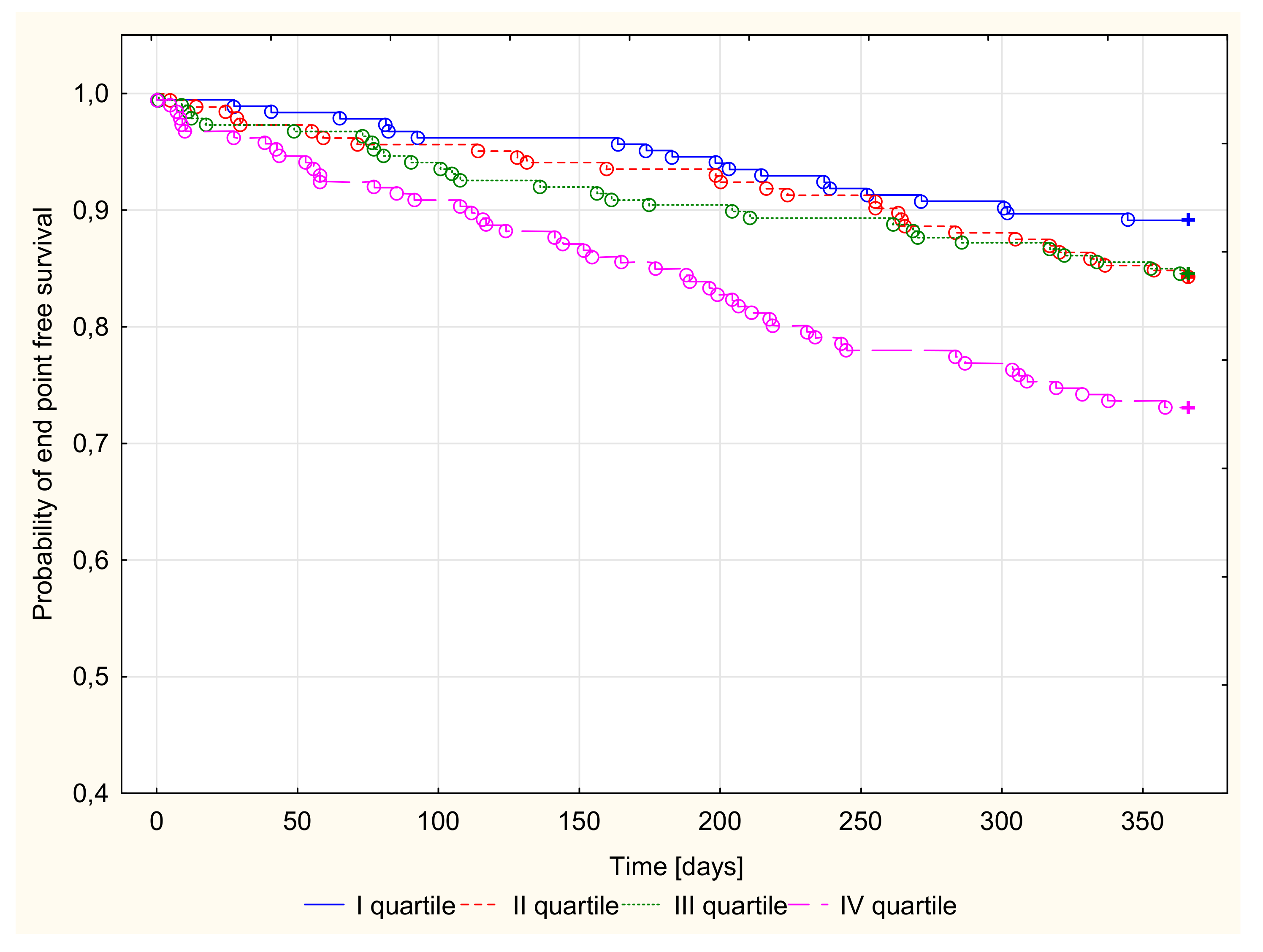
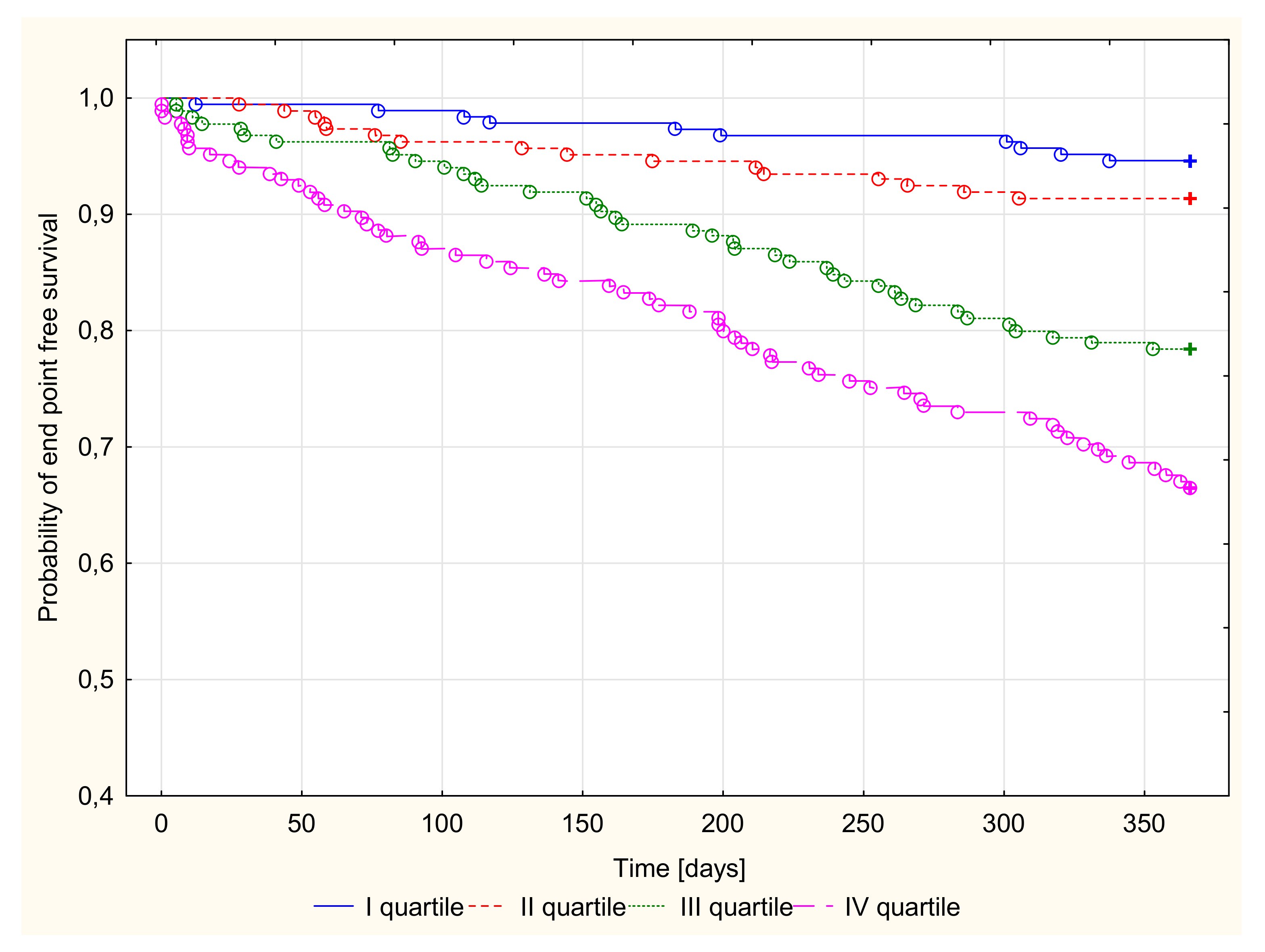
| Ceruloplasmin Quartiles (mg/dL) | All Group | I Quartile 8.0–23.6 | II Quartile 23.7–28.6 | III Quartile 28.7–35.8 | IV Quartile 35.9–81.0 | |
|---|---|---|---|---|---|---|
| Number | N = 741 | N = 184 | N = 184 | N = 187 | N = 186 | |
| Demographic and clinical parameters | ANOVA | |||||
| Deaths (n)/HT (n) All n (%) | 101/27 128 (17.27) | 16/4 20 (10.87) | 24/5 29 (15.76) | 23/6 29 (15.51) | 38/12 50 (26.88) | p < 0.001 |
| Female n (%) | 105 (14.17) | 18 (9.78) | 25 (13.59) | 28 (14.97) | 34 (18.28) | NS |
| Age (years) | 54.00 (48.0–59.0) | 54.00 (48.00–58.00) | 55.00 (49.00–60.00) | 54.00 (48.00–59.00) | 55.00 (49.00–60.00) | NS |
| BMI (kg/m2) | 26.29 (23.50–29.32) | 26.49 (24.04–29.06) | 26.66 (23.58–29.70) | 26.15 (23.36–29.69) | 25.96 (22.50–28.89) | NS |
| Duration of symptoms before inclusion (months) | 33.83 (13.07–69.67) | 29.82 (13.40–58.47) | 33.60 (12.80–69.02) | 31.83 (12.90–68.70) | 43.77 (14.13–79.93) | NS |
| Exercise, capacity, echocardiography | ||||||
| NYHA class III–IV n (%) | 417 (56.28) | 77 (41.85) | 99 (53.80) | 119 (63.64) | 122 (65.59) | p < 0.001 |
| VO2max (mL/min/kg b.w.) | 14.35 (11.70–17.60) | 15.30 (12.30–19.50) | 14.70 (12.00–17.70) | 14.20 (11.40–17.10) | 13.40 (10.75–16.55) | p < 0.001 |
| LVEF (%) | 24.00 (20.00–30.00) | 25.00 (20.50–32.50) | 24.00 (20.00–30.00) | 24.00 22.00–28.00) | 22.00 (19.00–28.00) | p < 0.01 |
| Laboratory parameters | ||||||
| NT-proBNP (pg/mL) /100 | 13.92 (6.44–31.55) | 9.30 (5.00–20.09) | 14.82 (6.64–34.77) | 15.48 (6.55–31.95) | 18.42 (8.97–37.96) | p < 0.001 |
| Ceruloplasmin (mg/dL) | 28.70 (23.70–35.80) | 20.75 (18.20–22.40) | 26.25 (24.90–27.50) | 31.90 (30.00–33.50) | 42.35 (38.10–49.30) | p < 0.001 |
| Hemoglobin (g/dL) | 14.02 (13.05–14.99) | 14.02 (13.05–14.83) | 14.02 (12.89–14.99) | 14.02 (13.22–15.15) | 14.18 (13.05–15.15) | NS |
| Leukocytes (109/L) | 6.94 (5.82–8.27) | 6.83 (5.53–8.26) | 6.77 (5.55–8.27) | 7.23 (5.88–8.65) | 6.92 (6.07–7.84) | 0.060 |
| Blood platelets (109/L) | 185.00 (152.00-223.00) | 183.00 (148.00-218.50) | 185.00 (156.50-220.50) | 197.00 (160.00-238.00) | 174.00 (150.00-218.00) | p < 0.05 |
| Sodium (mmol/L) | 136.00 (134.00–139.00) | 137.00 (135.00–139.00) | 137.00 (134.50–138.00) | 135.00 (133.00–138.00) | 136.00 (134.00–138.00) | p < 0.001 |
| Creatinine clearance (mL/min) | 95.11 (69.98–119.44) | 101.49 (80.86–125.04) | 93.51 (70.86–117.35) | 88.85 (70.07–116.43) | 93.27 (61.00–117.28) | p < 0.01 |
| Uric acid (µmol/L)/10 | 40.90 (33.00–50.60) | 37.85 (33.05–45.00) | 41.10 (32.95–50.15) | 41.50 (33.10–50.80) | 43.25 (32.90–55.60) | p < 0.001 |
| Serum protein (g/L) | 71.00 (67.00–75.00) | 70.00 (66.00–73.50) | 70.00 (66.00–74.00) | 72.00 (67.00–76.00) | 73.00 (69.00–77.00) | p < 0.001 |
| Albumin (g/l) | 42.00 (39.00–44.00) | 42.00 (39.00–44.00) | 41.00 (39.00–43.50) | 41.00 (38.00–44.00) | 43.00 (40.00–45.00) | p < 0.05 |
| Fibrinogen (mg/dL) | 397.00 (338.00–462.00) | 367.00 (320.50–433.50) | 395.50 (340.00–454.50) | 425.00 (367.00–495.00) | 409.50 (343.00–491.00) | p < 0.001 |
| C-reactive protein (mg/dL) | 2.94 (1.34–6.67) | 1.97 (0.91–4.55) | 2.65 (1.27–6.04) | 4.11 (1.82–7.35) | 3.83 (1.86–8.90) | p <0.001 |
| Iron concentration (µmol/L) | 17.10 (12.00–22.20) | 16.91 (13.00–20.25) | 17.10 (11.14–21.40) | 16.90 (11.80–22.40) | 17.60 (12.00–23.92) | NS |
| Bilirubin (µmol/L) | 13.70 (9.70–20.50) | 12.00 (8.45–16.15) | 13.65 (10.00–18.35) | 14.70 (9.30–21.10) | 16.55 (11.00–26.60) | p < 0.001 |
| Aspartate transaminase (IU/L) | 23.0 (19.0–30.9) | 23.0 (18.0–29.0) | 23 (19.0–28.6) | 24 (18.0–310) | 24 (20.0–33.0) | NS |
| Alanine transaminase (IU/L) | 24 (17.0–36.0) | 23 (17.5–35.5) | 24 (17.0–36.0) | 24 (17.0–34.0) | 25 (18.0–38.0) | NS |
| γ-glutamyl transpeptidase (IU/L) | 49 (27.0–100.0) | 39 (24.5–75.5) | 45.5 (27.0–79.0) | 54 (27.0–112.0) | 67.5 (33.0–152.0) | p < 0.001 |
| Alkaline phosphatase (IU/L) | 68.0 (56.0–90.0) | 65.0 (52.0–80.4) | 65.0 (54.0–84.0) | 72.0 (58.0–94.0) | 78.0 (61.0–108.0) | p < 0.001 |
| Fasting glucose (mmol/L) | 5.50 (5.00–6.20) | 5.50 (5.00–6.20) | 5.45 (4.85–6.20) | 5.60 (5.10–6.70) | 5.50 (4.90–6.10) | NS |
| Total Cholesterol (mmol/L) | 4.29 (3.64–5.22) | 4.30 (3.60–5.10) | 4.25 (3.65–5.19) | 4.25 (3.62–5.34) | 4.41 (3.67–5.21) | NS |
| Triglycerides (mmol/L) | 1.20 (0.89–1.73) | 1.17 (0.83–1.73) | 1.22 (0.89–1.93) | 1.23 (0.97–1.69) | 1.20 (0.85–1.74) | NS |
| Cholesterol HDL (mmol/L) | 1.14 (0.94–1.40) | 1.19 (0.98–1.43) | 1.14 (0.92–1.39) | 1.13 (0.94–1.32) | 1.13 (0.88–1.42) | NS |
| Cholesterol LDL (mmol/L) | 2.45 (1.90–3.16) | 2.46 (1.89–3.08) | 2.39 (1.88–3.20) | 2.38 (1.85–3.25) | 2.54 (2.00–3.16) | NS |
| Comorbidities | ||||||
| Non ischemic DCM; n (%) | 280 (37.79) | 58 (31.52) | 77 (41.85) | 78 (41.71) | 67 (36.02) | NS |
| Diabetes; n (%) | 211 (28.48) | 43 (23.37) | 53 (28.80) | 61 (32.62) | 54 (29.03) | NS |
| Arterial hypertension; n (%) | 408 (55.06) | 100 (54.35) | 104 (56.52) | 91 (48.66) | 113 (60.75) | NS |
| Permanent atrial fibrillation; n (%) | 176 (23.75) | 24 (13.04) | 42 (22.83) | 48 (25.67) | 62 (33.33) | p < 0.001 |
| ICD presence; n (%) | 207 (27.94) | 50 (27.17) | 63 (34.24) | 52 (27.81) | 42 (22.58) | NS |
| Smoker; n (%) | 257 (34.68) | 64 (34.78) | 78 (42.39) | 72 (38.50) | 43 (23.12) | p < 0.001 |
| Pharmacotherapy | ||||||
| Beta-blockers; n (%) | 726 (97.98) | 182 (98.91) | 181 (98.37) | 180 (96.26) | 183 (98.39) | NS |
| ACE–inhibitors; n (%) | 641 (86.50) | 166 (90.22) | 161 (87.50) | 159 (85.03) | 155 (83.33) | NS |
| Angiotensin-2 receptor blockers; n (%) | 76 (10.26) | 17 (9.24) | 20 (10.87) | 24 (12.83) | 15 (8.06) | NS |
| ACE–inhibitor or/and * ARB; n (%) | 693 (93.52) | 178 (96.74) | 174 (94,57) | 174 (93,05) | 167 (89,78) | p < 0.05 |
| Loop diuretic; n (%) | 647 (87.31) | 145 (78.80) | 168 (91.30) | 169 (90.37) | 165 (88.71) | p < 0.001 |
| Thiazide diuretics; n (%) | 93 (12.55) | 14 (7.61) | 19 (10.33) | 34 (18.18) | 26 (13.98) | p < 0.05 |
| Aldosterone receptor antagonist; n (%) | 683 (92.19) | 163 (88.65) | 171 (92.93) | 177 (94.65) | 172 (92.47) | NS |
| Statins; n (%) | 487 (65.72) | 128 (69.57) | 127 (69.02) | 124 (66.31) | 108 (58.06) | NS |
| Digitalis; n (%) | 339 (45.75) | 57 (30.98) | 82 (44.57) | 102 54.55 | 98 (52.69) | p < 0.001 |
| Ceruloplasmin/NT-pro-BNP Quartiles (mg/dL) | I–III Quartile | IV–IV Quartiles | ANOVA |
|---|---|---|---|
| Number | N = 683 | N = 58 | |
| Demographic and clinical parameters | |||
| Deaths (n)/HT (n) All n (%) | 79/21 100 (14.64) | 23/5 28 (48.28) | p < 0.001 |
| Female n (%) | 96 (14.06) | 9 (15.52) | NS |
| Age (years) | 54.00 (49.00–59.00) | 56,50 (45,00–61,00) | NS |
| BMI (kg/m2) | 26.44 (23.74–29.41) | 23.55 (19,86–26.76) | p < 0.001 |
| Duration of symptoms before inclusion (months) | 33.53 (12.93–68.70) | 43.12 (14.3–92.20) | NS |
| Exercise, capacity, echocardiography | |||
| NYHA class III-IV n (%) | 369 (54.03) | 48 (82.76) | p < 0.001 |
| VO2max (mL/min/kg b.w.) | 14.50 (11.70–18.00) | 12.30 (9.20–14.50) | p < 0.001 |
| LVEF (%) | 24.00 (20.00-30.00) | 20.00 (17.00-24.00) | p < 0.001 |
| Laboratory parameters | |||
| NT-proBNP (pg/mL) /100 | 12.78 (5.97–25.70) | 52.34 (41.31–78.06) | p < 0.001 |
| Ceruloplasmin (mg/dL) | 28.00 (23.40–33.70) | 46.30 (38.10–54.30) | p < 0.001 |
| Hemoglobin (g/dL) | 14.02 (13.05–14.99) | 13.62 (12.73–15.15) | NS |
| Leukocytes (109/L) | 6.94 (5.79–8.31) | 7.01 (6.17–8.19) | NS |
| Blood platelets (109/L) | 185.00 (152.00–224.00) | 185.00 (152.00–219.00) | NS |
| Sodium (mmol/L) | 137.00 (134.00–139.00) | 134.00 (132.00–137.00) | p < 0.001 |
| Creatinine clearance (mL/min) | 96.93 (73.45–120.64) | 66.41 (50.32–103.34) | p < 0.001 |
| Uric acid (µmol/L)/10 | 40.80 (32.90–49.50) | 44.40 (33.70–69.00) | p < 0.01 |
| Serum protein (g/L) | 71.00 (67.00–75.00) | 71.50 (67.00–77.00) | NS |
| Albumin (g/L) | 42.00 (39.00–44.00) | 40.00 (38.00–44.00) | p < 0.05 |
| Fibrinogen (ug/mL) | 396.00 (337.00–458.00) | 434.00 (360.00–536.00) | p < 0.01 |
| C-reactive protein (mg/dL) | 2.80 (1.27–6.12) | 7.18 (2.67–14.75) | p < 0.001 |
| Iron concentration (µmol/L) | 17.20 (12.10–22.20) | 16.15 (10.50–21.30) | NS |
| Bilirubin (µmol/L) | 13.40 (9.50–19.30) | 22.90 (13.80–32.50) | p < 0.001 |
| Aspartate transaminase (IU/L) | 23.00 (18.00–30.00) | 27.00 (21.00–37.00) | p < 0.01 |
| Alanine transaminase (IU/L) | 24.00 (17.00–36.00) | 25.00 (18.00–41.00) | NS |
| γ-glutamyl transpeptidase (IU/L) | 47.00 (27.00–92.00) | 133.50 (49.00–218.00) | p < 0.001 |
| Alkaline phosphatase (IU/L) | 67.00 (55.00–88.00) | 99.50 (73.00–143.00) | p < 0.001 |
| Fasting glucose (mmol/L) | 5.50 (5.00–6.30) | 5.20 (4.70–5.90) | p < 0.05 |
| Total Cholesterol (mmol/L) | 4.31 (3.66–5.27) | 3.97 (3.33–3.86) | NS |
| Triglycerides (mmol/L) | 1.22 (0.89–1.75) | 1.07 (0.78–1.36) | p < 0.05 |
| Cholesterol HDL (mmol/L) | 1.15 (0.95–1.40) | 1.05 (0.79–1.29) | p < 0.05 |
| Cholesterol LDL (mmol/L) | 2.46 (1.91–3.19) | 2.29 (1.85–3.00) | NS |
| Comorbidities | |||
| Non ischemic DCM; n (%) | 252 (36.90) | 28 (48.27) | NS |
| Diabetes; n (%) | 190 (27.82) | 21 (36.21) | NS |
| Arterial hypertension; n (%) | 382 (56.93) | 20 (34.48) | p < 0.01 |
| Permanent atrial fibrillation; n (%) | 155 (22.69) | 21 (36.21) | p < 0.05 |
| ICD presence; n (%) | 192 (28.11) | 15 (25.86) | NS |
| Smoker; n (%) | 241 (35.29) | 16 27.59) | NS |
| Pharmacotherapy | |||
| Beta-blockers; n (%) | 668 (97,80) | 58 (100,00) | NS |
| ACE–inhibitors; n (%) | 595 (87,12) | 46 (79.310 | NS |
| Angiotensin-2 receptor blockers; n (%) | 71 (10,40) | 5 (79,31) | NS |
| ACE–inhibitors or/and ARB; n (%) | 643 (94.14) | 50 (86,21) | p < 0.05 |
| Loop diuretic; n (%) | 590 (86.38) | 57 (98.28) | p < 0.05 |
| Thiazide diuretics; n (%) | 79 (11.57) | 14 (24.14) | p < 05 |
| Aldosterone receptor antagonist; n (%) | 628 (91,95) | 54 (93,10) | NS |
| Statins; n (%) | 457 (66.91) | 30 (51.72) | p < 0.05 |
| Digitalis; n (%) | 305 (44.66) | 34 (58.62) | NS |
| Univariable Cox Regression | Multivariable Cox Regression | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| General characteristics | ||||||
| BMI ↑ (1 kg/m2) | 0.945 | 0.908–0.985 | p < 0.01 | 0.966 | 0.912–1.022 | NS |
| Duration of symptoms before inclusion ↑ (1month) | 1.004 | 1.000–1.007 | p < 0.05 | 1.000 | 0.996–1.004 | NS |
| NYHA class ↑ (1 class) | 2.936 | 2.280–3.779 | p < 0.001 | 1.099 | 0.759–1.592 | NS |
| VO2max ↓ (1 mL/min/kg b.m.) | 1.198 | 1.142–1.256 | p < 0.001 | 1.113 | 1.048–1.181 | p < 0.001 |
| LVEF ↓ (1 %p) | 1.091 | 1.059–1.122 | p < 0.001 | 1.069 | 1.032–1.106 | p < 0.001 |
| Basic biochemistry | ||||||
| Sodium ↓ (1 mmol/L) | 1.111 | 1.070–1.155 | p < 0.001 | 1.039 | 0.990–1.092 | NS |
| Creatinine clearance ↓ (1 mL/min) | 1.014 | 1.008–1.019 | p < 0.001 | 1.001 | 0.993–1.008 | NS |
| Albumin ↓ (1 g/L) | 1.068 | 1.026–1.114 | p < 0.01 | 1.023 | 0.966–1.083 | NS |
| Cholesterol HDL ↓ (1 mmol/L) | 1.805 | 1.121–2.907 | p < 0.05 | 0.954 | 0.591–1.593 | NS |
| Cp and NT-proBNP ”both in top quartile” (yes/no) | 4.253 | 2.795–6.471 | p < 0.001 | 2.120 | 1.233–3.646 | p < 0.01 |
| Fibrinogen ↑ (1 mg/dL) | 1.003 | 1.001–1.004 | p < 0.001 | 1.001 | 1.000–1.003 | NS |
| Uric acid ↑ (10 µmol/L) | 1.030 | 1.018–1.041 | p < 0.001 | 1.012 | 0.999–1.026 | NS |
| Bilirubin ↑ (1 µmoL/L) | 1.028 | 1.018–1.039 | p < 0.001 | 0.994 | 0.976–1.012 | NS |
| Alkaline phosphatase ↑ (1 U/L) | 1.006 | 1.004–1.009 | p < 0.001 | 1.000 | 0.995–1.006 | NS |
| γ-Glutamyl trans peptidase ↑ (1 U/L) | 1.001 | 1.000–1.002 | p < 0.05 | 1.000 | 0.998–1.002 | NS |
| Comorbidities | ||||||
| Diabetes t.2 (yes/no) | 1.604 | 1.123–2.291 | p < 0.01 | 1.450 | 0.949–2.217 | NS |
| ICD absence (yes/no) | 9.929 | 3.922–20.000 | p < 0.001 | 7.575 | 3.278–17.502 | p < 0.001 |
| Pharmacotherapy | ||||||
| Lack of ACE - I or/and ARB (yes/no) | 3.428 | 2.126–5.256 | p < 0.001 | 2.195 | 1.234–3.906 | p < 0.01 |
| Loop diuretics (yes/no) | 4.895 | 1.809–13.248 | p < 0.01 | 1.735 | 0.525–5.730 | NS |
| Thiazide diuretics (yes/no) | 2.296 | 1.518–3.473 | p < 0.001 | 1.317 | 0.781–2.221 | NS |
| Statins (yes/no) | 0.699 | 0.492–0.993 | p < 0.05 | 1.294 | 0.825–2.032 | NS |
| Digitalis (yes/no) | 1.439 | 1.016–2.036 | p < 0.05 | 0.833 | 0.547–1.267 | NS |
| I-III Quartiles of Cp (mg%) (8.0–35.8) | Top Quartile of Cp (mg%) (35.9–81.0) | I-III Quartiles of NT-proBNP (pg/mL) (122.9–3155.0) | Top Quartile of NTpro-BNP (pg/mL) (3156.0–22378.0) | I-III Quartiles of Cp or NT-proBNP | Cp and NT-proBNP Both in Top Quartile | |
|---|---|---|---|---|---|---|
| End point (+) (n) | 78 | 50 | 66 | 62 | 100 | 28 |
| End point (−) (n) | 477 | 136 | 490 | 123 | 583 | 30 |
| Probability of end point (%) with confidence intervals | 14.054 (11.159–16.941) | 26.881 (20.508–33.251) | 11.871 (9.182–14.558) | 33.513 (26.708–40.312) | 14.641 (11.989–17.291) | 48.276 (35.416–61.136) |
| Odds ratio | 2.248 95%CI (1.503–3.364) p < 0.001 | 3.742 95%CI (2.511–5.578) p < 0.001 | 5.441 95%CI (3.117–9.498) p < 0.001 | |||
| Sensitivity (%) | 26.88 | 33.51 | 48.28 | |||
| Specificity (%) | 77.81 | 79.93 | 95.10 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romuk, E.; Jacheć, W.; Zbrojkiewicz, E.; Mroczek, A.; Niedziela, J.; Gąsior, M.; Rozentryt, P.; Wojciechowska, C. Ceruloplasmin, NT-proBNP, and Clinical Data as Risk Factors of Death or Heart Transplantation in a 1-Year Follow-Up of Heart Failure Patients. J. Clin. Med. 2020, 9, 137. https://doi.org/10.3390/jcm9010137
Romuk E, Jacheć W, Zbrojkiewicz E, Mroczek A, Niedziela J, Gąsior M, Rozentryt P, Wojciechowska C. Ceruloplasmin, NT-proBNP, and Clinical Data as Risk Factors of Death or Heart Transplantation in a 1-Year Follow-Up of Heart Failure Patients. Journal of Clinical Medicine. 2020; 9(1):137. https://doi.org/10.3390/jcm9010137
Chicago/Turabian StyleRomuk, Ewa, Wojciech Jacheć, Ewa Zbrojkiewicz, Alina Mroczek, Jacek Niedziela, Mariusz Gąsior, Piotr Rozentryt, and Celina Wojciechowska. 2020. "Ceruloplasmin, NT-proBNP, and Clinical Data as Risk Factors of Death or Heart Transplantation in a 1-Year Follow-Up of Heart Failure Patients" Journal of Clinical Medicine 9, no. 1: 137. https://doi.org/10.3390/jcm9010137
APA StyleRomuk, E., Jacheć, W., Zbrojkiewicz, E., Mroczek, A., Niedziela, J., Gąsior, M., Rozentryt, P., & Wojciechowska, C. (2020). Ceruloplasmin, NT-proBNP, and Clinical Data as Risk Factors of Death or Heart Transplantation in a 1-Year Follow-Up of Heart Failure Patients. Journal of Clinical Medicine, 9(1), 137. https://doi.org/10.3390/jcm9010137





