Quality Assurance of Non-Invasive Prenatal Screening (NIPS) for Fetal Aneuploidy Using Positive Predictive Values as Outcome Measures
Abstract
1. Introduction
2. Materials and Methods
3. Results
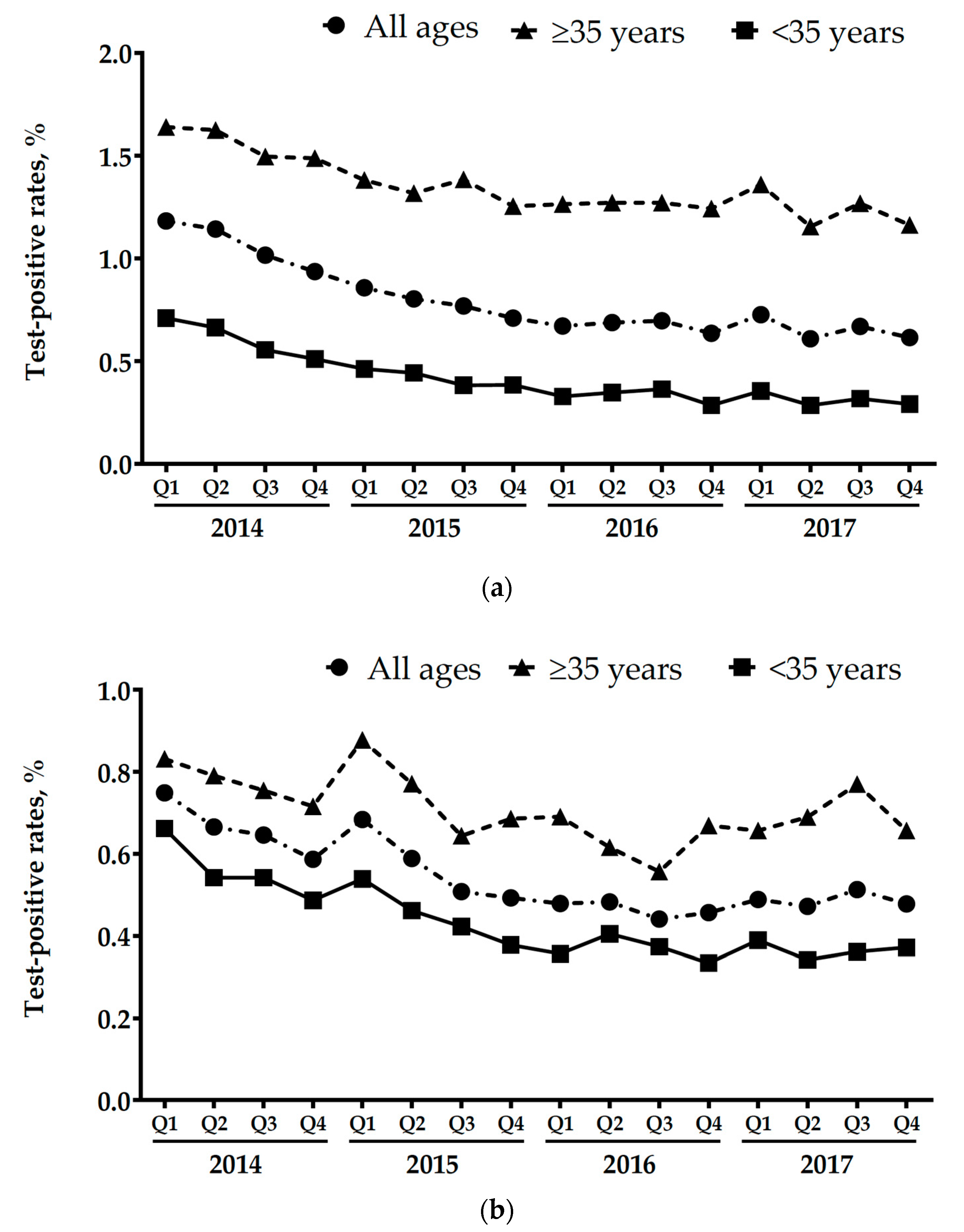
3.1. Changes in The Referral Population and Positive Test Rates
3.2. Overall Test Performance
3.3. Non-Inferiority Analysis
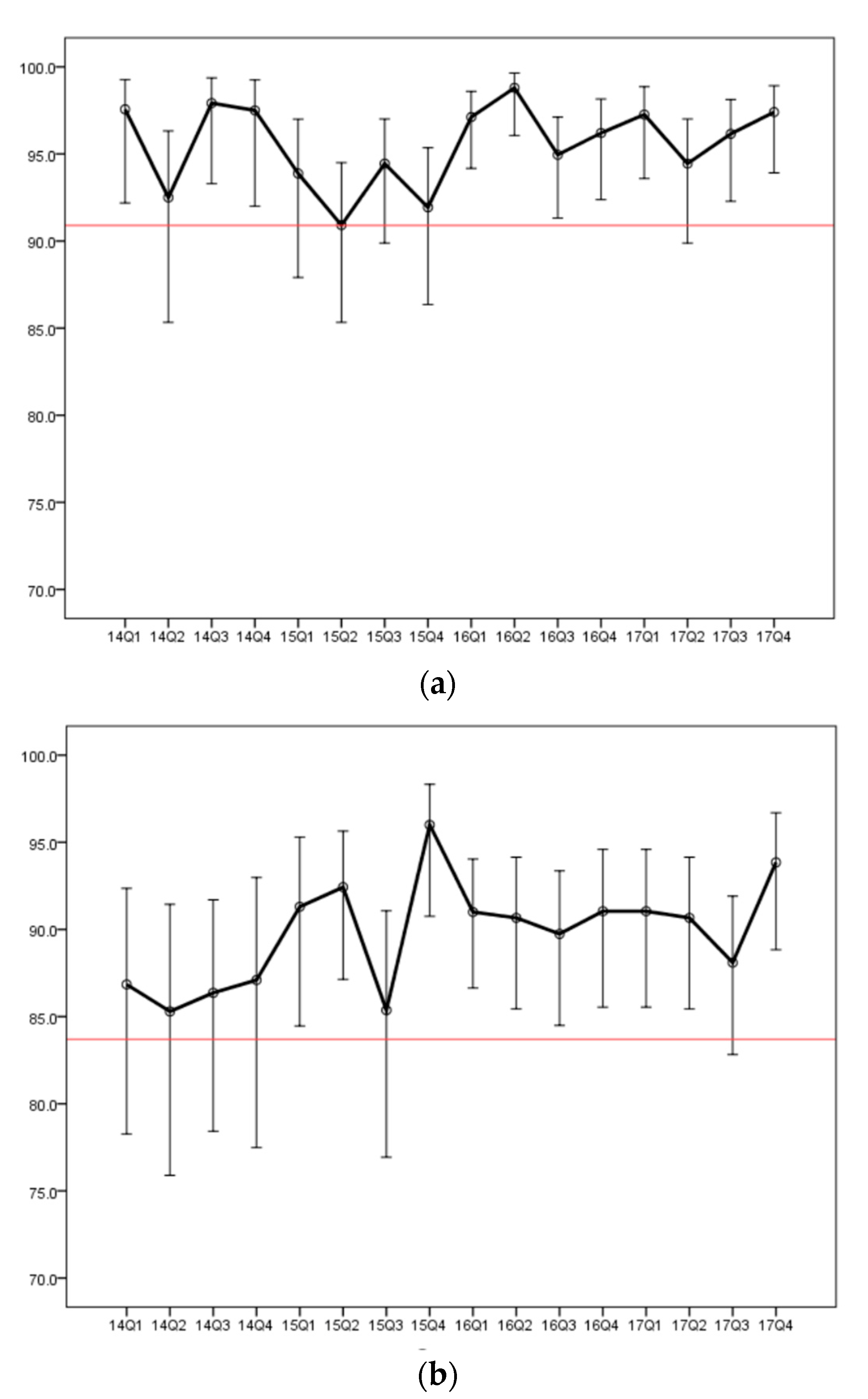
3.4. Trends in Positive Predictive Values
3.5. False-Negative Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cuckle, H.; Benn, P.; Pergament, E. Cell-free DNA screening for fetal aneuploidy as a clinical service. Clin. Biochem. 2015, 48, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.M.; Accurti, V.; Santacruz, B.; Plana, M.N.; Nicolaides, K.H. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet. Gynecol. 2017, 50, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Santorum, M.; Wright, D.; Syngelaki, A.; Karagioti, N.; Nicolaides, K.H. Accuracy of first-trimester combined test in screening for trisomies 21, 18 and 13. Ultrasound Obstet. Gynecol. 2017, 49, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Standards for Certification, Laboratory Requirements; Federal Register: Baltimore, MD, USA, 2007.
- College of American Pathologists. Laboratory General Checklist. 2018. Available online: http://www.cap.org (accessed on 5 May 2019).
- Skotko, B.G.; Allyse, M.A.; Bajaj, K.; Best, R.G.; Klugman, S.; Leach, M.; Meredith, S.; Michie, M.; Gregg, A.R. Adherence of cell-free DNA noninvasive prenatal screens to ACMG recommendations. Genet. Med. 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, B.; Hill, M.; Gemelos, G.; Demko, Z.; Banjevic, M.; Baner, J.; Ryan, A.; Sigurjonsson, S.; Chopra, N.; Dodd, M.; et al. Noninvasive prenatal aneuploidy testing of chromosomes 13, 18, 21, X, and Y, using targeted sequencing of polymorphic loci. Prenat. Diagn. 2012, 32, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Pergament, E.; Cuckle, H.; Zimmermann, B.; Banjevic, M.; Sigurjonsson, S.; Ryan, A.; Hall, M.P.; Dodd, M.; Lacroute, P.; Stosic, M.; et al. Single-nucleotide polymorphism-based noninvasive prenatal screening in a high-risk and low-risk cohort. Obstet. Gynecol. 2014, 124, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.; Hunkapiller, N.; Banjevic, M.; Vankayalapati, N.; Fong, N.; Jinnett, K.N.; Demko, Z.; Zimmermann, B.; Sigurjonsson, S.; Gross, S.J.; et al. Validation of an Enhanced Version of a Single-Nucleotide Polymorphism-Based Noninvasive Prenatal Test for Detection of Fetal Aneuploidies. Fetal Diagn. Ther. 2016, 40, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Ravi, H.; McNeill, G.; Goel, S.; Meltzer, S.D.; Hunkapiller, N.; Ryan, A.; Levy, B.; Demko, Z.P. Validation of a SNP-based non-invasive prenatal test to detect the fetal 22q11.2 deletion in maternal plasma samples. PLoS ONE 2018, 13, e0193476. [Google Scholar] [CrossRef] [PubMed]
- Wapner, R.J.; Babiarz, J.E.; Levy, B.; Stosic, M.; Zimmermann, B.; Sigurjonsson, S.; Wayham, N.; Ryan, A.; Banjevic, M.; Lacroute, P.; et al. Expanding the scope of noninvasive prenatal testing: detection of fetal microdeletion syndromes. Am. J. Obstet. Gynecol. 2015, 212, 332 e1–332 e9. [Google Scholar] [CrossRef]
- Martin, K.; Iyengar, S.; Kalyan, A.; Lan, C.; Simon, A.L.; Stosic, M.; Kobara, K.; Ravi, H.; Truong, T.; Ryan, A.; et al. Clinical experience with a single-nucleotide polymorphism-based non-invasive prenatal test for five clinically significant microdeletions. Clin. Genet. 2018, 93, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Dar, P.; Curnow, K.J.; Gross, S.J.; Hall, M.P.; Stosic, M.; Demko, Z.; Zimmermann, B.; Hill, M.; Sigurjonsson, S.; Ryan, A.; et al. Clinical experience and follow-up with large scale single-nucleotide polymorphism-based noninvasive prenatal aneuploidy testing. Am. J. Obstet. Gynecol. 2014, 211, 527.e1–527.e17. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.; Nowacki, A.S. Understanding equivalence and noninferiority testing. J. Gen. Intern. Med. 2011, 26, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.H.; Gitlin, S.A.; Patrick, J.L.; Crain, J.L.; Wilson, J.M.; Griffin, D.K. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum. Reprod. Update. 2014, 20, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.E.; Jacobsson, B.; Swamy, G.K.; Laurent, L.C.; Ranzini, A.C.; Brar, H.; Tomlinson, M.W.; Pereira, L.; Spitz, J.L.; Hollemon, D.; et al. Cell-free DNA analysis for noninvasive examination of trisomy. N. Engl. J. Med. 2015, 372, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, Y.; Jiang, F.; Fu, M.; Yuan, Y.; Guo, Y.; Zhu, Z.; Lin, M.; Liu, Q.; Tian, Z.; et al. Non-invasive prenatal testing for trisomies 21, 18 and 13: clinical experience from 146,958 pregnancies. Ultrasound Obstet. Gynecol. 2015, 45, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Futch, T.; Spinosa, J.; Bhatt, S.; de Feo, E.; Rava, R.P.; Sehnert, A.J. Initial clinical laboratory experience in noninvasive prenatal testing for fetal aneuploidy from maternal plasma DNA samples. Prenat. Diagn. 2013, 33, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Snyder, H.L.; Curnow, K.J.; Bhatt, S.; Bianchi, D.W. Follow-up of multiple aneuploidies and single monosomies detected by noninvasive prenatal testing: implications for management and counseling. Prenat. Diagn. 2016, 36, 203–209. [Google Scholar] [CrossRef] [PubMed]
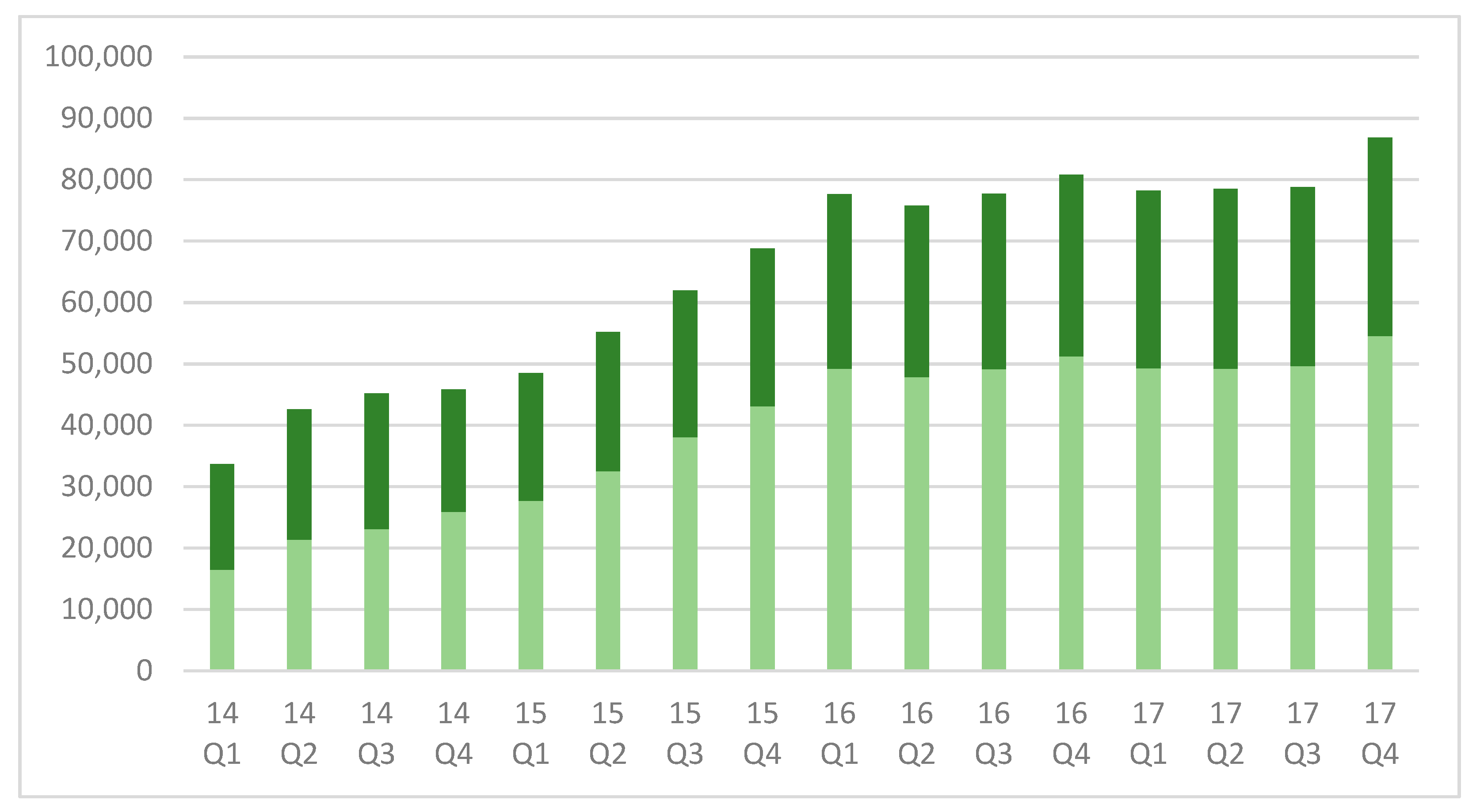
| Test | All Positive (%) | Follow-Up Solicited | Confirmation by Genetics | Confirmation by Genetics, Ultrasound, or Loss | |||||
|---|---|---|---|---|---|---|---|---|---|
| Follow-up Received | Abn Confirmed | PPV% (95% CI) | Follow-up Received | Abn Confirmed | PPV% (95% CI) | ||||
| All referrals (1,035,844) | T21 | 7802 (0.75) | 2347 | 884 | 837 | 94.7 (93.0–96.0) | 1,083 | 1036 | 95.7 (94.3–96.7) |
| T18 | 2205 (0.21) | 845 | 333 | 304 | 91.3 (87.8–93.9) | 476 | 447 | 93.9 (91.4–95.7) | |
| T13 | 1207 (0.12) | 344 | 118 | 80 | 67.8 (58.9–75.6) | 186 | 148 | 79.6 (73.2–84.7) | |
| MX | 2017 (0.19) | 535 | 120 | 93 | 77.5 (69.2–84.1) | 299 | 272 | 91.0 (87.2–93.7) | |
| All | 13,231 (1.28) | 4071 | 1455 | 1314 | 90.3 (88.7–91.7) | 2,044 | 1903 | 93.1 (91.9–94.1) | |
| Referrals from women <35 (628,242) | T21 | 2388 (0.38) | 711 | 271 | 248 | 91.5 (87.6–94.3) | 339 | 316 | 93.2 (90.0–95.4) |
| T18 | 666 (0.11) | 256 | 105 | 92 | 87.6 (80.0–92.6) | 152 | 139 | 91.4 (85.9–94.9) | |
| T13 | 540 (0.09) | 149 | 46 | 27 | 58.7 (44.3–71.7) | 84 | 65 | 77.4 (67.4–85.0) | |
| MX | 1361 (0.22) | 372 | 76 | 59 | 77.6 (58.2–77.4) | 212 | 195 | 92.0 (87.5–94.9) | |
| All | 4955 (0.79) | 1488 | 498 | 426 | 85.5 (82.2–88.4) | 787 | 715 | 90.9 (88.6–92.7) | |
| T21 | 5414 (1.33) | 1636 | 613 | 589 | 96.1 (94.2–97.4) | 744 | 720 | 96.8 (95.3–97.8) | |
| Referrals from women ≥35 (407,602) | T18 | 1539 (0.38) | 589 | 228 | 212 | 93.0 (88.9–95.6) | 324 | 308 | 95.1 (92.1–96.9) |
| T13 | 667 (0.16) | 195 | 72 | 53 | 73.6 (62.4–82.4) | 102 | 83 | 81.4 (72.7–87.7) | |
| MX | 656 (0.16) | 163 | 44 | 34 | 77.3 (63.0–87.2) | 87 | 77 | 88.5 (80.1–93.6) | |
| All | 8,276 (2.03) | 2583 | 957 | 888 | 92.8 (91.0–94.3) | 1257 | 1188 | 94.5 (93.1–95.6) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiNonno, W.; Demko, Z.; Martin, K.; Billings, P.; Egbert, M.; Zneimer, S.; Keen-Kim, D.; Benn, P. Quality Assurance of Non-Invasive Prenatal Screening (NIPS) for Fetal Aneuploidy Using Positive Predictive Values as Outcome Measures. J. Clin. Med. 2019, 8, 1311. https://doi.org/10.3390/jcm8091311
DiNonno W, Demko Z, Martin K, Billings P, Egbert M, Zneimer S, Keen-Kim D, Benn P. Quality Assurance of Non-Invasive Prenatal Screening (NIPS) for Fetal Aneuploidy Using Positive Predictive Values as Outcome Measures. Journal of Clinical Medicine. 2019; 8(9):1311. https://doi.org/10.3390/jcm8091311
Chicago/Turabian StyleDiNonno, Wendy, Zachary Demko, Kimberly Martin, Paul Billings, Melissa Egbert, Susan Zneimer, Dianne Keen-Kim, and Peter Benn. 2019. "Quality Assurance of Non-Invasive Prenatal Screening (NIPS) for Fetal Aneuploidy Using Positive Predictive Values as Outcome Measures" Journal of Clinical Medicine 8, no. 9: 1311. https://doi.org/10.3390/jcm8091311
APA StyleDiNonno, W., Demko, Z., Martin, K., Billings, P., Egbert, M., Zneimer, S., Keen-Kim, D., & Benn, P. (2019). Quality Assurance of Non-Invasive Prenatal Screening (NIPS) for Fetal Aneuploidy Using Positive Predictive Values as Outcome Measures. Journal of Clinical Medicine, 8(9), 1311. https://doi.org/10.3390/jcm8091311





