Aseptic Ligatures Induce Marginal Peri-Implant Bone Loss—An 8-Week Trial in Rabbits
Abstract
1. Introduction
2. Experimental Section
2.1. Implants
Surface Roughness
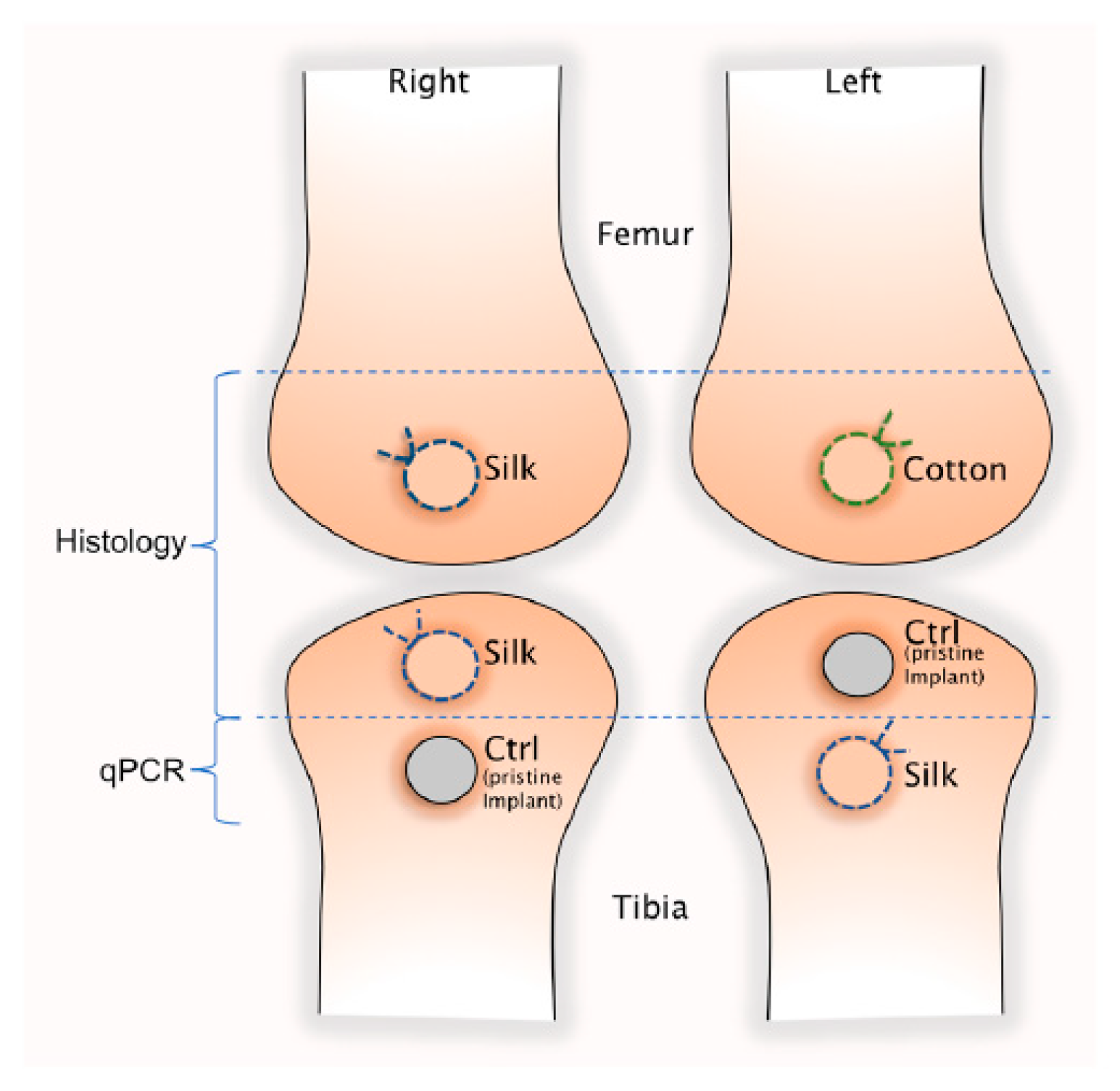
2.2. Animal Model and Surgical Procedure
- (i)
- The soft tissue that adhered to the implant margin was removed by a 6 mm punch after removing the cutis.
- (ii)
- The implants were then unscrewed and the marginal bone trephined out using a 6 mm trephine.
2.3. Histology
2.3.1. Histological Sample Preparation
2.3.2. Histological Analysis: Qualitative and Quantitative
2.4. Gene Expression Analysis—qPCR
2.4.1. mRNA Isolation
2.4.2. Reverse Transcription (RT)
2.4.3. Assays Design and Validation
2.4.4. Real-Time Quantitative Polymerise Chain Reaction (RT-qPCR)
2.4.5. Statistical Analysis
3. Results
3.1. Clinical Appearance at Sacrifice
3.2. Histological Results
3.2.1. Histomorphometrical Results
3.2.2. Qualitative Histological Results
Control Implants (No Ligature Involved)
Test Implants (Ligated with Silk or Cotton)
3.3. Gene Expression Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moraschini, V.; Poubel, L.A.; Ferreira, V.F.; Barboza Edos, S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Doornewaard, R.; Christiaens, V.; De Bruyn, H.; Jacobsson, M.; Cosyn, J.; Vervaeke, S.; Jacquet, W. Long-Term Effect of Surface Roughness and Patients’ Factors on Crestal Bone Loss at Dental Implants. A Systematic Review and Meta-Analysis. Clin. Implant. Dent. Relat. Res. 2017, 19, 372–399. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Buser, D.; Sennerby, L. Crestal bone loss and oral implants. Clin. Implant. Dent. Relat Res. 2012, 14, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Berglundh, T.; Ericsson, I.; Liljenberg, B.; Marinello, C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin. oral implant res. 1992, 3, 9–16. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin. Implant Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [CrossRef]
- Albrektsson, T.; Chrcanovic, B.; Ostman, P.O.; Sennerby, L. Initial and long-term crestal bone responses to modern dental implants. Periodontology 2000 2017, 73, 41–50. [Google Scholar] [CrossRef]
- Frydman, A.; Simonian, K. Review of models for titanium as a foreign body. C.D.A. J. 2014, 42, 829–833. [Google Scholar]
- Jemt, T.; Sunden Pikner, S.; Grondahl, K. Changes of Marginal Bone Level in Patients with “Progressive Bone Loss” at Branemark System(R) Implants: A Radiographic Follow-Up Study over an Average of 9 Years. Clin. Implant Dent. Relat. Res. 2015, 17, 619–628. [Google Scholar] [CrossRef]
- Albrektsson, T.; Becker, W.; Coli, P.; Jemt, T.; Molne, J.; Sennerby, L. Bone loss around oral and orthopedic implants: An immunologically based condition. Clin. Implant Dent. Relat. Res. 2019. [Google Scholar] [CrossRef]
- Harris, W. Vanishing Bone—Conquering a Stealth Disease Caused by Total Hip Replacements; Oxford Press: Oxford, UK, 2018. [Google Scholar]
- Landgraeber, S.; Jäger, M.; Jacobs, J.J.; Hallab, N.J. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediators Inflamm. 2014, 2014, 185150. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jia, T.; Wooley, P.H.; Yang, S.Y. Current research in the pathogenesis of aseptic implant loosening associated with particulate wear debris. Acta. Orthop. Belg. 2013, 79, 1–9. [Google Scholar] [PubMed]
- Abrahamsson, I.; Berglundh, T.; Lindhe, J. Soft tissue response to plaque formation at different implant systems. A comparative study in the dog. Clin. Oral Implants Res. 1998, 9, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Bragger, U.; Walther, D.; Beamer, B.; Kornman, K.S. Ligature-induced peri-implant infection in cynomolgus monkeys. I. Clinical and radiographic findings. Clin. Oral Implants Res. 1993, 4, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Watzak, G.; Zechner, W.; Tangl, S.; Vasak, C.; Donath, K.; Watzek, G. Soft tissue around three different implant types after 1.5 years of functional loading without oral hygiene: A preliminary study in baboons. Clin. Oral Implants Res. 2006, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Reinedahl, D.; Chrcanovic, B.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Ligature-Induced Experimental Peri-Implantitis-A Systematic Review. J. Clin. Med. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Moest, T.; Wrede, J.; Schmitt, C.M.; Stamp, M.; Neukam, F.W.; Schlegel, K.A. The influence of different abutment materials on tissue regeneration after surgical treatment of peri-implantitis—A randomized controlled preclinical study. J. Craniomaxillofac. Surg. 2017, 45, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Carcuac, O.; Abrahamsson, I.; Albouy, J.P.; Linder, E.; Larsson, L.; Berglundh, T. Experimental periodontitis and peri-implantitis in dogs. Clin. Oral Implants Res. 2013, 24, 363–371. [Google Scholar] [CrossRef]
- Rovin, S.; Costich, E.R.; Gordon, H.A. The influence of bacteria and irritation in the initiation of periodontal disease in germfree and conventional rats. J. Periodontal Res. 1966, 1, 193–204. [Google Scholar] [CrossRef]
- Martins, O.; Ramos, J.C.; Baptista, I.P.; Dard, M.M. The dog as a model for peri-implantitis: A review. J. Investig. Surg. 2014, 27, 50–56. [Google Scholar] [CrossRef]
- Baron, M.; Haas, R.; Dortbudak, O.; Watzek, G. Experimentally induced peri-implantitis: A review of different treatment methods described in the literature. Int. J. Oral Maxillofac. Implants 2000, 15, 533–544. [Google Scholar]
- Nguyen Vo, T.N.; Hao, J.; Chou, J.; Oshima, M.; Aoki, K.; Kuroda, S.; Kaboosaya, B.; Kasugai, S. Ligature induced peri-implantitis: Tissue destruction and inflammatory progression in a murine model. Clin. Oral Implants Res. 2017, 28, 129–136. [Google Scholar] [CrossRef]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef]
- Johansson, C.B.; Morberg, P. Importance of ground section thickness for reliable histomorphometrical results. Biomaterials 1995, 16, 91–95. [Google Scholar] [CrossRef]
- Johansson, C.B.; Morberg, P. Cutting directions of bone with biomaterials in situ does influence the outcome of histomorphometrical quantifications. Biomaterials 1995, 16, 1037–1039. [Google Scholar] [CrossRef]
- Wilson, T.G.; Valderrama, P.; Burbano, M.; Blansett, J.; Levine, R.; Kessler, H.; Rodrigues, D.C. Foreign bodies associated with peri-implantitis human biopsies. J. Periodontol. 2015, 86, 9–15. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Feng, Y.; Cheng, H.; Li, D. Macrophage polarization in aseptic bone resorption around dental implants induced by Ti particles in a murine model. J. Periodontal Res. 2019. [Google Scholar] [CrossRef]
- Donath, K.; Laass, M.; Günzl, H.J. The histopathology of different foreign-body reactions in oral soft tissue and bone tissue. Virchows. Arch. A 1992, 420, 131–137. [Google Scholar] [CrossRef]
- Mbalaviele, G.; Novack, D.V.; Schett, G.; Teitelbaum, S.L. Inflammatory osteolysis: A conspiracy against bone. J. Clin. Invest. 2017, 127, 2030–2039. [Google Scholar] [CrossRef]
- Paiva, K.B.S.; Granjeiro, J.M. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Prog Mol. Biol. Transl. Sci. 2017, 148, 203–303. [Google Scholar] [CrossRef]
- Setzen, G.; Williams, E.F., 3rd. Tissue response to suture materials implanted subcutaneously in a rabbit model. Plast. Reconstr. Surg. 1997, 100, 1788–1795. [Google Scholar] [CrossRef]
- Nair, P.N. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef]
- You, T.M.; Choi, B.H.; Zhu, S.J.; Jung, J.H.; Lee, S.H.; Huh, J.Y.; Lee, H.J.; Li, J. Treatment of experimental peri-implantitis using autogenous bone grafts and platelet-enriched fibrin glue in dogs. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 34–37. [Google Scholar] [CrossRef]
- Schou, S.; Holmstrup, P.; Jorgensen, T.; Stoltze, K.; Hjorting-Hansen, E.; Wenzel, A. Autogenous bone graft and ePTFE membrane in the treatment of peri-implantitis. I. Clinical and radiographic observations in cynomolgus monkeys. Clin. Oral Implants Res. 2003, 14, 391–403. [Google Scholar] [CrossRef]
- Yu, X.; Hu, Y.; Freire, M.; Yu, P.; Kawai, T.; Han, X. Role of toll-like receptor 2 in inflammation and alveolar bone loss in experimental peri-implantitis versus periodontitis. J. Periodontal Res. 2018, 53, 98–106. [Google Scholar] [CrossRef]
- Albouy, J.P.; Abrahamsson, I.; Berglundh, T. Spontaneous progression of experimental peri-implantitis at implants with different surface characteristics: An experimental study in dogs. J.Clin. Periodontol. 2012, 39, 182–187. [Google Scholar] [CrossRef]
- Jovanovic, S.A.; Kenney, E.B.; Carranza, F.A., Jr.; Donath, K. The regenerative potential of plaque-induced peri-implant bone defects treated by a submerged membrane technique: An experimental study. Int. J. Oral Maxillofac. Implants 1993, 8, 13–18. [Google Scholar]
- Hayek, R.R.; Araujo, N.S.; Gioso, M.A.; Ferreira, J.; Baptista-Sobrinho, C.A.; Yamada, A.M.; Ribeiro, M.S. Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dogs. J. Periodontol. 2005, 76, 1275–1281. [Google Scholar] [CrossRef]
- Machtei, E.E.; Kim, D.M.; Karimbux, N.; Zigdon-Giladi, H. The use of endothelial progenitor cells combined with barrier membrane for the reconstruction of peri-implant osseous defects: An animal experimental study. J. Clin. Periodontol. 2016, 43, 289–297. [Google Scholar] [CrossRef]
- Abdul-Karim, F.W.; Benevenia, J.; Pathria, M.N.; Makley, J.T. Case report 736: Retained surgical sponge (gossypiboma) with a foreign body reaction and remote and organizing hematoma. Skeletal Radiol. 1992, 21, 466–469. [Google Scholar]
- Puvanesarajah, V.; Fayad, L.M.; Rao, S.S.; McCarthy, E.F.; Morris, C.D. Extremity gossypiboma mimicking sarcoma: Case report and review. Skeletal Radiol. 2019, 48, 629–635. [Google Scholar] [CrossRef]
- Sari, A.; Basterzi, Y.; Karabacak, T.; Tasdelen, B.; Demirkan, F. The potential of microscopic sterile sponge particles to induce foreign body reaction. Int. Wound J. 2006, 3, 363–368. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Nooh, N.; Abdullah, W.A.; Grawish Mel, A.; Ramalingam, S.; Javed, F.; Al-Hezaimi, K. The effects of surgicel and bone wax hemostatic agents on bone healing: An experimental study. Indian J. Orthop. 2014, 48, 319–325. [Google Scholar] [CrossRef]
- Spelzini, F.; Konstantinovic, M.L.; Guelinckx, I.; Verbist, G.; Verbeken, E.; De Ridder, D.; Deprest, J. Tensile strength and host response towards silk and type i polypropylene implants used for augmentation of fascial repair in a rat model. Gynecol. Obstet. Invest. 2007, 63, 155–162. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Osseointegration and foreign body reaction: Titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin. Implant. Dent. Relat. Res. 2018, 20, 82–91. [Google Scholar] [CrossRef]
- Brodbeck, W.G.; Macewan, M.; Colton, E.; Meyerson, H.; Anderson, J.M. Lymphocytes and the foreign body response: Lymphocyte enhancement of macrophage adhesion and fusion. J. Biomed. Mater. Res. A 2005, 74, 222–229. [Google Scholar] [CrossRef]
- Rodriguez, A.; Macewan, S.R.; Meyerson, H.; Kirk, J.T.; Anderson, J.M. The foreign body reaction in T-cell-deficient mice. J. Biomed. Mater. Res. A 2009, 90, 106–113. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Aresta-DaSilva, S.; Tang, K.; Alvarez, D.; Webber, M.J.; Tang, B.C.; Lavin, D.M.; Veiseh, O.; Doloff, J.C.; Bose, S.; et al. Neutrophil Responses to Sterile Implant Materials. PLoS ONE 2015, 10, e0137550. [Google Scholar] [CrossRef]
- Laffey, J.G.; Boylan, J.F.; Cheng, D.C. The systemic inflammatory response to cardiac surgery: Implications for the anesthesiologist. Anesthesiology 2002, 97, 215–252. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Diabetes and oral implant failure: A systematic review. J. Dent. Res. 2014, 93, 859–867. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Reasons for failures of oral implants. J. Oral Rehabil. 2014, 41, 443–476. [Google Scholar] [CrossRef]








| Primer | Forward Sequence | Reverse Sequence | Accession Number | Biological Entity |
|---|---|---|---|---|
| ACTβ | GAGATGCCATGTGACGGAAG | TTACACAAATGCGATGCTGC | NM_001101683.1 | Reference gene |
| ALPL | ACTGTGGACTACCTCTTG | GGTCAGTGATGTTGTTCC | XM_017346489 | Bone mineralization |
| ARG1 | GGATCATTGGAGCCCCTTTCTC | TCAAGCAGACCAGCCTTTCTC | NM_001082108.1 | M2 macrophage |
| C5aR1 | ACGTCAACTGCTGCATCAACC | AGGCTGGGGAGAGACTTGC | NM_017338812.1 | The complement system |
| CD11β | TTCAACCTGGAGACTGAGAACAC | TCAAACTGGACCACGCTCTG | XM_008248697.2 | M1 macrophage |
| CD19 | GGATGTATGTCTGTCGCCGT | AAGCAAAGCCACAACTGGAA | NM_002711879.3 | B lymphocytes |
| CD4 | CAACTGGAAACATGCGAACCA | TTGATGACCAGGGGGAAAGA | NM_008254148.2 | T lymphocytes |
| CD8 | GGCGTCTACTTCTGCATGACC | GAACCGGCACACTCTCTTCT | NM_008254148.2 | T lymphocytes |
| GAPDH | CCGAGACACGATGGTGAAGG | TGTAGACCATGTAGTGGAGGTCA | NM_001082253.1 | Reference gene |
| IL8 | CTTTTTGCCCTGACCATGCC | TCCTTCACAAGCGAGACCAC | NM_001171082.1 | Macrophage |
| LDHA | ACAAGTGCACAAACAAGTGGT | AGAGCCCCTTAAGCATGGTG | NM_001082277 | Reference gene |
| MCP1 | GCTCATAGCAGTCGCCTTCA | CATGAAGATCACAGCTTCTTTGGG | NM_001082294 | Macrophage fusion |
| NCF1 | TTCATCCGCCACATTGCCC | GTCCTGCCACTTCACCAAGA | XM_001082102.1 | Neutrophil |
| OC | AGAGTCTGGCAGAGGCTCAG | TCGCTTCACCACCTCGCT | XM_002715383 | Bone mineralization |
| CTSK | ACTCTGAAGATGCCTACCCCT | TTCAGGGCTTTCTCATTCCCC | NM_001082641 | Bone resorption |
| FGF2 | ATCTACACTGTGGAGCTTGCAG | TCATGCGGTCACACACTTCC | XM_002711077 | Fibroblast |
| IL1β | TCCTTGGTGTTGTCTGGCAC | GGCCACAGGTATCTTGTCGTT | NM_001082201 | M1 macrophage |
| IL6 | GAGGAAAGAGATGTGTGACCAT | AGCATCCGTCTTCTTCTATCAG | NM_001082064 | M1 macrophage |
| VEGFα | CTTGGGTGCATTGGAGCCTT | CTTCACCACTTCGTGGGGTTTA | XM_017345155 | Endothelial cells |
| TNFα | CTCTTTCCTGCTCGTGGCTG | GGAGGTTGTTTGGGGACTCTT | NM_001082263 | M1 Macrophage |
| TRAP | CTGGGTTTGCAGGAGTTG | TTGAAGAGCAGCGACAGA | NM_001081988 | Bone resorption |
| Target Gene | Relative Expression in Test versus Control | 95% CI Low | 95% CI High | p-Value |
|---|---|---|---|---|
| NCF1 | 4.9 | 1.9 | 12.4 | 0.008 |
| CD11β | 2.8 | 1.5 | 5.4 | 0.016 |
| CD4 | 2.3 | 1.7 | 3.1 | 0.016 |
| VEGFα | 1.6 | 1.0 | 2.5 | 0.05 |
| TNFα | 1.7 | 0.9 | 2.9 | 0.08 |
| ARG1 | 2.4 | 0.9 | 6.5 | 0.11 |
| IL8 | 0.5 | 0.2 | 1.2 | 0.11 |
| FGF2 | 0.8 | 0.5 | 1.2 | 0.11 |
| CD8 | 3.9 | 0.5 | 27.4 | 0.22 |
| CD19 | 2.1 | 0.5 | 12.3 | 0.22 |
| OC | 1.7 | 0.7 | 4.2 | 0.25 |
| C5aR1 | 1.3 | 0.9 | 2.0 | 0.31 |
| TRAP | 1.7 | 0.5 | 5.4 | 0.37 |
| IL1β | 0.7 | 0.2 | 2.5 | 0.58 |
| IL6 | 0.8 | 0.3 | 2.5 | 0.58 |
| ALPL | 1.2 | 0.5 | 3.1 | 0.64 |
| MCP1 | 0.9 | 0.3 | 2.8 | 0.84 |
| CTSK | 1.0 | 0.7 | 1.5 | 0.84 |
| Target Gene | Relative Expression in Test versus Control | 95% CI Low | 95% CI High | p-Value |
|---|---|---|---|---|
| IL6 | 0.1 | 0.0 | 0.9 | 0.04 |
| CTSK | 0.7 | 0.5 | 1.0 | 0.04 |
| OC | 0.6 | 0.4 | 1.1 | 0.07 |
| NCF1 | 3.5 | 0.8 | 15.2 | 0.07 |
| MCP1 | 2.2 | 0.8 | 5.9 | 0.08 |
| CD19 | 2.7 | 0.6 | 12.3 | 0.13 |
| CD11β | 2.6 | 0.6 | 12.2 | 0.14 |
| TNFα | 0.4 | 0.1 | 2.3 | 0.19 |
| FGF2 | 0.6 | 0.2 | 1.6 | 0.19 |
| IL1β | 2.1 | 0.5 | 8.8 | 0.19 |
| IL8 | 0.5 | 0.1 | 2.4 | 0.26 |
| C5aR1 | 1.9 | 0.4 | 9.3 | 0.28 |
| CD4 | 2.6 | 0.3 | 25.0 | 0.28 |
| CD8 | 1.4 | 0.4 | 5.2 | 0.42 |
| TRAP | 2.2 | 0.1 | 40.0 | 0.46 |
| VEGFα | 1.8 | 0.2 | 20.4 | 0.49 |
| ALPL | 0.8 | 0.2 | 4.4 | 0.76 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinedahl, D.; Galli, S.; Albrektsson, T.; Tengvall, P.; Johansson, C.B.; Hammarström Johansson, P.; Wennerberg, A. Aseptic Ligatures Induce Marginal Peri-Implant Bone Loss—An 8-Week Trial in Rabbits. J. Clin. Med. 2019, 8, 1248. https://doi.org/10.3390/jcm8081248
Reinedahl D, Galli S, Albrektsson T, Tengvall P, Johansson CB, Hammarström Johansson P, Wennerberg A. Aseptic Ligatures Induce Marginal Peri-Implant Bone Loss—An 8-Week Trial in Rabbits. Journal of Clinical Medicine. 2019; 8(8):1248. https://doi.org/10.3390/jcm8081248
Chicago/Turabian StyleReinedahl, David, Silvia Galli, Tomas Albrektsson, Pentti Tengvall, Carina B. Johansson, Petra Hammarström Johansson, and Ann Wennerberg. 2019. "Aseptic Ligatures Induce Marginal Peri-Implant Bone Loss—An 8-Week Trial in Rabbits" Journal of Clinical Medicine 8, no. 8: 1248. https://doi.org/10.3390/jcm8081248
APA StyleReinedahl, D., Galli, S., Albrektsson, T., Tengvall, P., Johansson, C. B., Hammarström Johansson, P., & Wennerberg, A. (2019). Aseptic Ligatures Induce Marginal Peri-Implant Bone Loss—An 8-Week Trial in Rabbits. Journal of Clinical Medicine, 8(8), 1248. https://doi.org/10.3390/jcm8081248






