Cytokine Profile in Patients with Aseptic Loosening of Total Hip Replacements and Its Relation to Metal Release and Metal Allergy
Abstract
:1. Introduction
1.1. Background
1.2. Aim
2. Experimental Section
2.1. Patients and Samples
2.2. Cytokine Profile Analysis
2.3. Patch Testing
2.4. ICP-MS (Serum)
2.5. ICP-MS (Tissue and Serum)
2.6. Statistical Analysis
3. Results
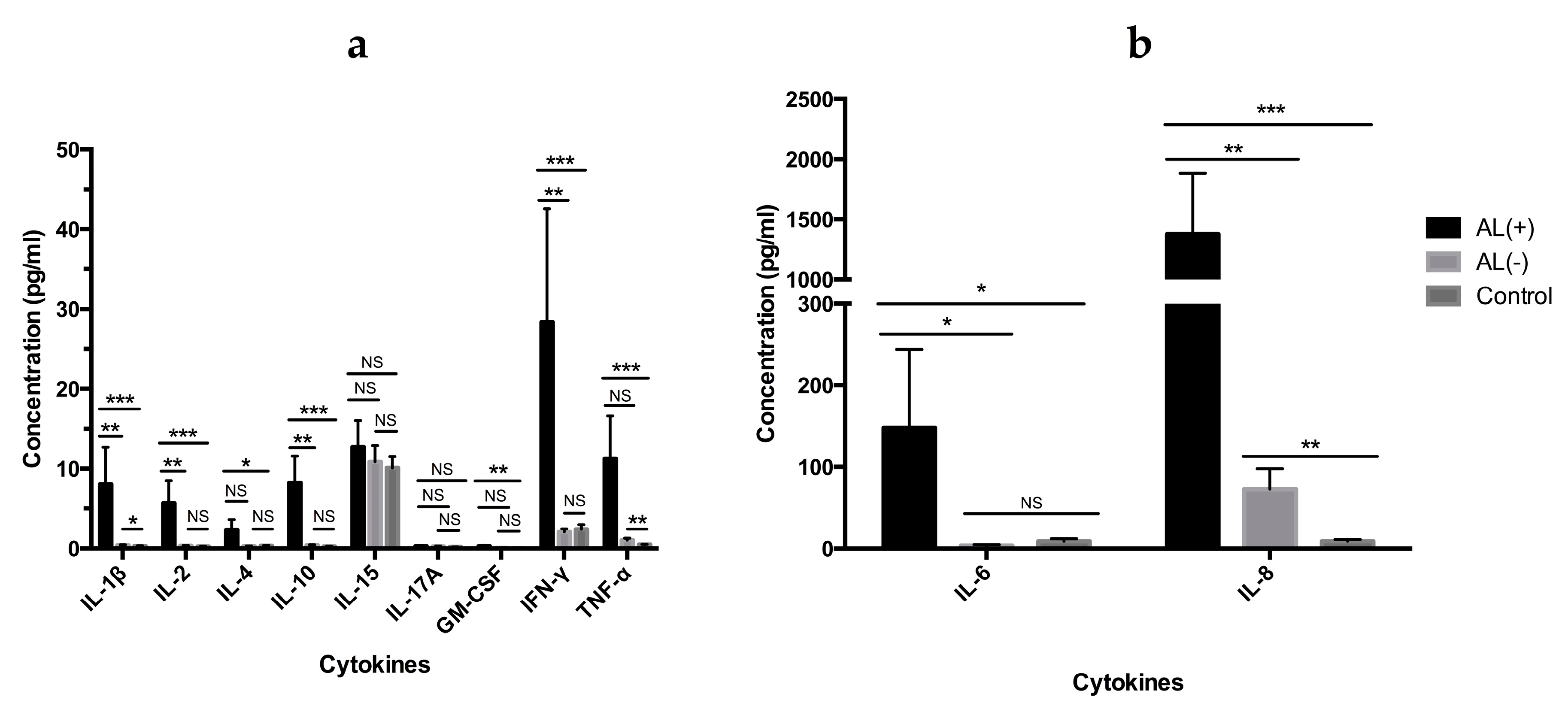
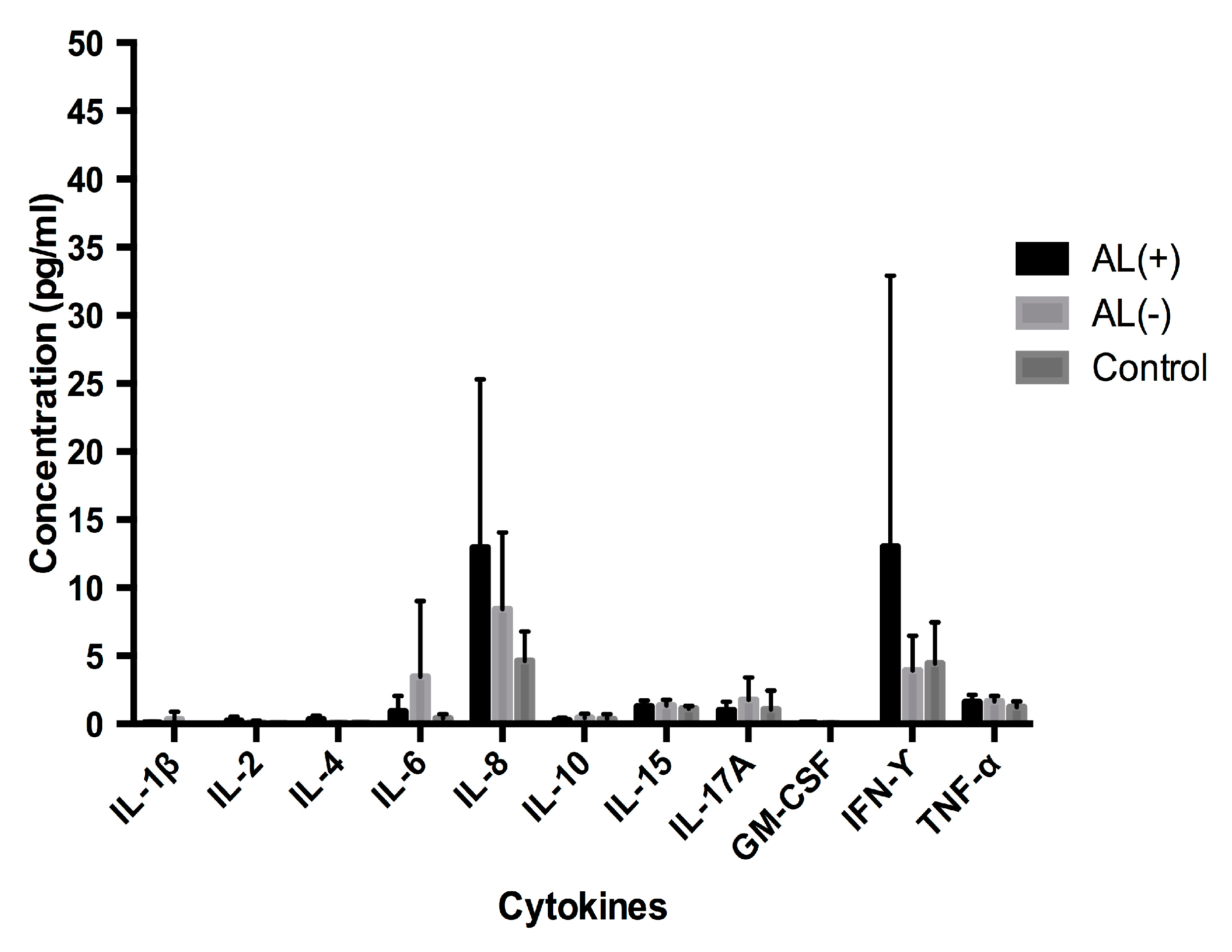
3.1. Cytokine Profile Analysis
3.1.1. Analysis of Cytokine Levels in Periimplant Tissue
3.1.2. Analysis of Cytokine Levels in Serum
3.2. Patch Test
3.3. ICP-MS Analysis
3.3.1. Metal Concentrations in Periimplant Tissue
3.3.2. Metal Concentrations in Serum
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Camuzard, O.; Breuil, V.; Carle, G.F.; Pierrefite-Carle, V. Autophagy Involvement in Aseptic Loosening of Arthroplasty Components. J. Bone Jt. Surg. 2019, 101, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.D.; Seyler, T.M.; Bennett, D.; Delanois, R.E.; Saleh, K.J.; Thongtrangan, I.; Kuskowski, M.; Cheng, E.Y.; Sharkey, P.F.; Parvizi, J.; et al. Total Hip Arthroplasties: What Are the Reasons for Revision? Int. Orthop. 2008, 32, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Cobelli, N.; Scharf, B.; Crisi, G.M.; Hardin, J.; Santambrogio, L. Mediators of the Inflammatory Response to Joint Replacement Devices. Nat. Rev. Rheumatol. 2011, 7, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Raska, M. Particle Disease: Biologic Mechanisms of Periprosthetic Osteolysis in Total Hip Arthroplasty. Innate Immun. 2013, 19, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Holt, G.; Murnaghan, C.; Reilly, J.; Meek, R.M.D.; Features, S. The Biology of Aseptic Osteolysis. Clin. Orthop. Relat. Res. 2007, 460, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Eger, M.; Sterer, N.; Liron, T.; Kohavi, D.; Gabet, Y. Scaling of Titanium Implants Entrains Inflammation-Induced Osteolysis. Sci. Rep. 2017, 7, 39612. [Google Scholar] [CrossRef] [PubMed]
- Dyskova, T.; Gallo, J.; Kriegova, E. The Role of the Chemokine System in Tissue Response to Prosthetic By-Products Leading to Periprosthetic Osteolysis and Aseptic Loosening. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Hallab, N.J.; Jacobs, J.J. Chemokines Associated with Pathologic Responses to Orthopedic Implant Debris. Front. Endocrinol. 2017, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Nich, C.; Takakubo, Y.; Pajarinen, J.; Ainola, M.; Salem, A.; Sillat, T.; Rao, A.J.; Raska, M.; Tamaki, Y.; Takagi, M.; et al. Macrophages-Key Cells in the Response to Wear Debris from Joint Replacements. J. Biomed. Mater. Res. A 2013, 101, 3033–3045. [Google Scholar] [CrossRef]
- Stea, S.; Visentin, M.; Granchi, D.; Ciapetti, G.; Donati, M.; Sudanese, A.; Zanotti, C.; Toni, A. Cytokines and Osteolysis Around Total Hip Prostheses. Cytokine 2000, 12, 1575–1579. [Google Scholar] [CrossRef]
- Wolfe, J.; Goldberg, J.; Harris, H. Production of Cytokines around Loosened Cemented Acetabular Components. J. Bone Jt. Surg. 1993, 75, 663–879. [Google Scholar]
- Fiorillo, L.; Cervino, G.; Herford, A.; Lauritano, F.; D’Amico, C.; Lo Giudice, R.; Laino, L.; Troiano, G.; Crimi, S.; Cicciù, M. Interferon Crevicular Fluid Profile and Correlation with Periodontal Disease and Wound Healing: A Systemic Review of Recent Data. Int. J. Mol. Sci. 2018, 19, 1908. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Tian, L.; Luo, G.; Yu, X. Interferon-Gamma-Mediated Osteoimmunology. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Huie, P.; Song, Y.; Schurman, D.; Maloney, W.; Woolson, S.; Sibley, R. Cellular Profile and Cytokine Production at Prosthetic Interfaces. Study of Tissues Retrieved from Revised Hip and Knee Replacements. J. Bone Joint Surg. Br. 1998, 80, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, Y.; Revell, P.A.; Al-Saffar, N.; Kobayashi, A.; Scott, G.; Freeman, M.A.R. Bone Formation and Bone Resorption in Failed Total Joint Arthroplasties: Histomorphometric Analysis with Histochemical and Immunohistochemical Technique. J. Orthop. Res. 1996, 14, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Büdinger, L.; Hertl, M. Immunologic Mechanisms in Hypersensitivity Reactions to Metal Ions: An Overview. Allergy 2000, 55, 108–115. [Google Scholar] [CrossRef]
- Hallab, N.J.; Mikecz, K.; Vermes, C.; Skipor, A.; Jacobs, J.J. Orthopaedic Implant Related Metal Toxicity in Terms of Human Lymphocyte Reactivity to Metal-Protein Complexes Produced from Cobalt-Base and Titanium-Base Implant Alloy Degradation. Mol. Cell. Biochem. 2001, 222, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Sundfeldt, M.; Carlsson, L.V.; Johansson, C.B.; Thomsen, P.; Gretzer, C. Aseptic Loosening, Not Only a Question of Wear: A Review of Different Theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef]
- Grosse, S.; Haugland, H.K.; Lilleng, P.; Ellison, P.; Hallan, G.; Høl, P.J. Wear Particles and Ions from Cemented and Uncemented Titanium-Based Hip Prostheses-A Histological and Chemical Analysis of Retrieval Material. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 709–717. [Google Scholar] [CrossRef]
- McGrath, L.R.; Shardlow, D.L.; Ingham, E.; Andrews, M.; Ivory, J.; Stone, M.H.; Fisher, J. A Retrieval Study of Capital Hip Prostheses with Titanium Alloy Femoral Stems. J. Bone Jt. Surg. Ser. B 2001, 83, 1195–1201. [Google Scholar] [CrossRef]
- Frigerio, E.; Pigatto, P.D.; Guzzi, G.; Altomare, G. Metal Sensitivity in Patients with Orthopaedic Implants: A Prospective Study. Contact Dermat. 2011, 64, 273–279. [Google Scholar] [CrossRef]
- Hallab, N. Metal Sensitivity in Patients with Orthopedic Implants. J. Clin. Rheumatol. 2001, 7, 215–218. [Google Scholar] [CrossRef]
- Schmidt, M.; Goebeler, M. Immunology of Metal Allergies. JDDG J. Der Dtsch. Dermatol. Ges. 2015, 13, 653–659. [Google Scholar] [CrossRef]
- Summer, B.; Paul, C.; Mazoochian, F.; Rau, C.; Thomsen, M.; Banke, I.; Gollwitzer, H.; Dietrich, K.; Mayer-Wagner, S.; Ruzicka, T.; et al. Nickel (Ni) Allergic Patients with Complications to Ni Containing Joint Replacement Show Preferential IL-17 Type Reactivity to Ni. Contact Dermat. 2010, 63, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Song, Y.; Chun, L.; Huie, P.; Trindade, M.; Smith, R.L.; Goodman, S. The Role of the TH1 and TH2 Immune Responses in Loosening and Osteolysis of Cemented Total Hip Replacements. J. Biomed. Mater. Res. A 2003, 64, 693–697. [Google Scholar] [CrossRef]
- Looney, R.J.; Schwarz, E.M.; Boyd, A.; O’Keefe, R.J. Periprosthetic Osteolysis: An Immunologist’s Update. Curr. Opin. Rheumatol. 2006, 18, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kamme, C.L.L. Aerobic and Anaerobic Bacteria in Deep Infections after Total Hip Arthroplasty: Differential Diagnosis between Infectious and Non-Infectious Loosening. Clin. Orthop. Relat. Res. 1981, 154, 201–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Todd, D.J.; Hasdlev, J.; Metwali, M.; Allen, G.E.; Burrows, D. Day 4 Is Better than Day 3 for a Single Patch Test Reading. Contact Dermat. 1996, 34, 402–404. [Google Scholar] [CrossRef]
- Wilkinson, D.S.; Fregert, S.; Magnusson, B.; Bandmann, H.J.; Calnan, C.D.; Cronin, E.; Hjort, N.; Maibach, H.J.; Malten, K.E.; Meneghini, C.L.; et al. Terminology of Contact Dermatitis. Acta Derm. Venereol. 1970, 50, 287–292. [Google Scholar]
- Krecisz, B.; Kieć-Swierczyńska, M.; Bakowicz-Mitura, K. Allergy to Metals as a Cause of Orthopedic Implant Failure. Int. J. Occup. Med. Environ. Health 2006, 19, 178–180. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Jakobsen, S.S.; Engkilde, K.; Johansen, J.D.; Søballe, K.; Menné, T. The Association between Metal Allergy, Total Knee Arthroplasty, and Revision. Acta Orthop. 2015, 86, 378–383. [Google Scholar] [CrossRef]
- Granchi, D.; Cenni, E.; Giunti, A.; Baldini, N. Metal Hypersensitivity Testing in Patients Undergoing Joint Replacement. J. Bone Jt. Surg. Br. 2012, 94-B, 1126–1134. [Google Scholar] [CrossRef]
- Konttinen, Y.; Xu, J.W.; Pätiälä, H.; Imai, S.; Waris, V.; Li, T.F.; Goodman, S.; Nordsletten, L.; Santavirta, S. Cytokines in Aseptic Loosening of Total Hip Replacement. Curr. Orthop. 1997, 11, 40–47. [Google Scholar] [CrossRef]
- Hirayama, T.; Tamaki, Y.; Takakubo, Y.; Iwazaki, K.; Sasaki, K.; Ogino, T.; Goodman, S.B.; Konttinen, Y.T.; Takagi, M. Toll-like Receptors and Their Adaptors Are Regulated in Macrophages after Phagocytosis of Lipopolysaccharide-Coated Titanium Particles. J. Orthop. Res. 2011, 29, 984–992. [Google Scholar] [CrossRef]
- Shanbhag, A.S.; Kaufman, A.M.; Hayata, K.; Rubash, H.E. Assessing Osteolysis with Use of High-Throughput Protein Chips. J. Bone Jt. Surg. Am. 2007, 89, 1081–1089. [Google Scholar] [CrossRef]
- Baggiolini, M.; Loetscher, P.; Moser, B. Interleukin-8 and the Chemokine Family. Int. J. Immunopharmacol. 1995, 17, 103–108. [Google Scholar] [CrossRef]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 Stimulation of Osteoclastogenesis and Bone Resorption Is a Mechanism for the Increased Osteolysis of Metastatic Bone Disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef]
- Fritz, E.A.; Jacobs, J.J.; Roebuck, A. Chemokine IL-8 Induction by Particulate Wear Debris in Osteoblasts Is Mediated by NF-KB. J. Orthop. Res. 2005, 23, 1249–1257. [Google Scholar] [CrossRef]
- Qin, S.; Larosa, G.; Campbell, J.J.; Smith-heath, H.; Kassam, N.; Zeng, L.; Butcher, E.C.; Mackay, C.R. Expression of Monocyte Chemoattractant Protein-1 and Interleukin-8 Receptors on Subsets of T Cells: Correlation with Transendothelial Chemotactic Potential. Eur. J. Immunol. 1996, 26, 640–647. [Google Scholar] [CrossRef]
- Chan, E.; Cadosch, D.; Gautschi, O.P.; Sprengel, K.; Filgueira, L. Influence of Metal Ions on Human Lymphocytes and the Generation of Titanium-Specific T-Lymphocytes. J. Appl. Biomater. Biomech. 2011, 9, 137–143. [Google Scholar] [CrossRef]
- Hallab, N.J.; Anderson, S.; Stafford, T.; Glant, T.; Jacobs, J.J. Lymphocyte Responses in Patients with Total Hip Arthroplasty. J. Orthop. Res. 2005, 23, 384–391. [Google Scholar] [CrossRef]
- Valladares, R.D.; Nich, C.; Zwingenberger, S.; Li, C.; Swank, K.R.; Gibon, E.; Rao, A.J.; Yao, Z.; Goodman, S.B. Toll-like Receptors-2 and 4 Are Overexpressed in an Experimental Model of Particle-Induced Osteolysis. J. Biomed. Mater. Res. Part A 2014, 102, 3004–3011. [Google Scholar] [CrossRef]
- Lees, J.R. Interferon Gamma in Autoimmunity: A Complicated Player on a Complex Stage. Cytokine 2015, 74, 18–26. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, M.S.; Lee, C.H.; Kim, H.Y.; Chae, S.U.; Kwak, H.B.; Oh, J. Effect of Interferon-γ on the Fusion of Mononuclear Osteoclasts into Bone-Resorbing Osteoclasts. BMB Rep. 2012, 45, 281–286. [Google Scholar] [CrossRef]
- Couper, K.; Blount, D.; Riley, E. IL-10: The Master Regulator of Immunity to Infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Van Roon, J.A.G.; Van Roy, J.L.A.M.; Gmelig-Meyling, F.H.J.; Lafeber, F.P.J.G.; Bijlsma, J.W.J. Prevention and Reversal of Cartilage Degradation in Rheumatoid Arthritis by Interleukin-10 and Interleukin-4. Arthritis Rheum. 1996, 39, 829–835. [Google Scholar] [CrossRef]
- Perretti, M.; Szabó, C.; Thiemermann, C. Effect of Interleukin-4 and Interleukin-10 on Leucocyte Migration and Nitric Oxide Production in the Mouse. Br. J. Pharmacol. 1995, 116, 2251–2257. [Google Scholar] [CrossRef]
- Tarrant, J.M. Blood Cytokines as Biomarkers of In Vivo Toxicity in Preclinical Safety Assessment: Considerations for Their Use. Toxicol. Sci. 2010, 117, 4–16. [Google Scholar] [CrossRef] [Green Version]
- Sarmiento-González, A.; Marchante-Gayón, J.M.; Tejerina-Lobo, J.M.; Paz-Jiménez, J.; Sanz-Medel, A. High-Resolution ICP–MS Determination of Ti, V, Cr, Co, Ni and Mo in Human Blood and Urine of Patients Implanted with a Hip or Knee Prosthesis. Anal. Bioanal. Chem. 2008, 391, 2583–2589. [Google Scholar] [CrossRef]
- Revell, P.A. The Combined Role of Wear Particles, Macrophages and Lymphocytes in the Loosening of Total Joint Prostheses. J. R. Soc. Interface 2008, 5, 1263–1278. [Google Scholar] [CrossRef]
- Pound, B.G. Corrosion Behavior of Metallic Materials in Biomedical Applications. I. Ti and Its Alloys. Corros. Rev. 2014, 32, 1–20. [Google Scholar] [CrossRef]
- Pellier, J.; Geringer, J.; Forest, B. Fretting-Corrosion between 316L SS and PMMA: Influence of Ionic Strength, Protein and Electrochemical Conditions on Material Wear. Application to Orthopaedic Implants. Wear 2011, 271, 1563–1571. [Google Scholar] [CrossRef]
- Merritt, K.; Brown, S.A. Distribution of Cobalt Chromium Wear and Corrosion Products and Biologic Reactions. Clin. Orthop. Relat. Res. 1996, 329, 233–243. [Google Scholar] [CrossRef]
- Hart, A.J.; Sabah, S.A.; Bandi, A.S.; Maggiore, P.; Tarassoli, P.; Sampson, B.; Skinner, J.A. Sensitivity and Specificity of Blood Cobalt and Chromium Metal Ions for Predicting Failure of Metal-on-Metal Hip Replacement. J. Bone Jt. Surg. Br. Vol. 2011, 93-B, 1308–1313. [Google Scholar] [CrossRef]
- Lohmann, C.H.; Meyer, H.; Nuechtern, J.V.; Singh, G.; Schmotzer, H.; Morlock, M.M. Periprosthetic Tissue Metal Content but Not Serum Metal Content Predicts the Type of Tissue Response in Failed Small-Diameter Metal-on-Metal Total Hip Arthroplasties. J. Bone Jt. Surg. 2013, 95, 1561–1568. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Iannello, G.; Santonocito, D.; Risitano, G.; Cicciù, M. Sandblasted and Acid Etched Titanium Dental Implant Surfaces Systematic Review and Confocal Microscopy Evaluation. Materials (Basel) 2019, 12, 1763. [Google Scholar] [CrossRef]
- Howell, J.R. Cemented Hip Arthroplasty: Why I Do It. Orthop. Trauma 2018, 32, 13–19. [Google Scholar] [CrossRef]
- Thomas, S.R.; Shukla, D.; Latham, P.D. Corrosion of Cemented Titanium Femoral Stems. J. Bone Jt. Surg. Br. 2004, 86-B, 974–978. [Google Scholar] [CrossRef]
- Cohen, J. Current Concepts Review. Corrosion of Metal Orthopaedic Implants. J. Bone Jt. Surg. Am. 1998, 80, 1554. [Google Scholar] [CrossRef]
- Cadosch, D.; Sutanto, M.; Chan, E.; Mhawi, A.; Gautschi, O.P.; von Katterfeld, B.; Simmen, H.P.; Filgueira, L. Titanium Uptake, Induction of RANK-L Expression, and Enhanced Proliferation of Human T-Lymphocytes. J. Orthop. Res. 2010, 28, 341–347. [Google Scholar] [CrossRef]
- Dmd, R.T.; Albrektsson, T.; Dds, S.G.; Prgomet, Z.; Tengvall, P.; Dds, A.W. Osseointegration and Foreign Body Reaction: Titanium Implants Activate the Immune System and Suppress Bone Resorption during the First 4 Weeks after Implantation. Clin. Implant Dent. Relat. Res. 2018, 2017, 82–91. [Google Scholar] [CrossRef]
- Cadosch, D.; Chan, E.; Gautschi, O.P.; Meagher, J.; Zellweger, R.; Filgueira, L. Titanium IV Ions Induced Human Osteoclast Differentiation and Enhanced Bone Resorption in Vitro. J. Biomed. Mater. Res. A 2009, 91, 29–36. [Google Scholar] [CrossRef]
- Nuevo-Ordóñez, Y.; Montes-Bayón, M.; Blanco-González, E.; Paz-Aparicio, J.; Raimundez, J.D.; Tejerina, J.M.; Peña, M.A.; Sanz-Medel, A. Titanium Release in Serum of Patients with Different Bone Fixation Implants and Its Interaction with Serum Biomolecules at Physiological Levels. Anal. Bioanal. Chem. 2011, 401, 2747–2754. [Google Scholar] [CrossRef]
- Lazarinis, S.; Mäkelä, K.T.; Eskelinen, A.; Havelin, L.; Hallan, G.; Overgaard, S.; Pedersen, A.B.; Kärrholm, J.; Hailer, N.P. Does Hydroxyapatite Coating of Uncemented Cups Improve Long-Term Survival? An Analysis of 28,605 Primary Total Hip Arthroplasty Procedures from the Nordic Arthroplasty Register Association (NARA). Osteoarthr. Cartil. 2017, 25, 1980–1987. [Google Scholar] [CrossRef]
- Hailer, N.P.; Lazarinis, S.; Mäkelä, K.T.; Eskelinen, A.; Fenstad, A.M.; Hallan, G.; Havelin, L.; Overgaard, S.; Pedersen, A.B.; Mehnert, F.; et al. Hydroxyapatite Coating Does Not Improve Uncemented Stem Survival after Total Hip Arthroplasty! Acta Orthop. 2015, 86, 18–25. [Google Scholar] [CrossRef]
- Fage, S.W.; Muris, J.; Jakobsen, S.S.; Thyssen, J.P. Titanium: A Review on Exposure, Release, Penetration, Allergy, Epidemiology, and Clinical Reactivity. Contact Dermat. 2016, 74, 323–345. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Jensen, P.; Carlsen, B.C.; Engkilde, K.; Menné, T.; Johansen, J.D. The Prevalence of Chromium Allergy in Denmark Is Currently Increasing as a Result of Leather Exposure. Br. J. Dermatol. 2009, 161, 1288–1293. [Google Scholar] [CrossRef]
- Mishra, P.K.; Wu, W.; Rozo, C.; Hallab, N.J.; Benevenia, J.; Gause, W.C. Micrometer-Sized Titanium Particles Can Induce Potent Th2-Type Responses through TLR4-Independent Pathways. J. Immunol. 2011, 187, 6491–6498. [Google Scholar] [CrossRef] [Green Version]
- Eger, M.; Hiram-Bab, S.; Liron, T.; Sterer, N.; Carmi, Y.; Kohavi, D.; Gabet, Y. Mechanism and Prevention of Titanium Particle-Induced Inflammation and Osteolysis. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Thomas, P.; Braathen, L.R.; Dörig, M.; Aubock, J.; Nestle, F.; Werfel, T.; Willert, H.G. Increased Metal Allergy in Patients with Failed Metal-on-Metal Hip Arthroplasty and Peri-Implant T-Lymphocytic Inflammation. Allergy Eur. J. Allergy Clin. Immunol. 2009, 64, 1157–1165. [Google Scholar] [CrossRef]

| Patient # | Type | Femoral | Head | Liner | Acetabular |
|---|---|---|---|---|---|
| 1 AL (+) | MoP | Ti-6Al-4V, ZMR®, uncemented, porous coating | CoCrMo | PE | Ti-6Al-4V, Trilogy®, uncommented, cpTi fiber mesh. |
| 2 AL (+) | MoP | FeCrNiMn, Exeter®, cemented, polished. | CoCrMo | PE | cpTi, Duraloc®, uncemented, porous coating. |
| 3 AL (+) | MoP | CoCrMo, Lubinus®, cemented polished. | CoCrMo | PE | PE, Lubinus®, cemented, all-polycup |
| 4 AL (+) | MoP | FeCrNiM, Exeter®, cemented, polished. | CoCrMo | PE | Ti-6Al-4V, Mallory Head, uncemented, PS. |
| 5 AL (+) | MoP | Ti-6Al-4V, Bi-metric®, uncemented, grit blasted. | CoCrMo | PE | Ti-6Al-4V, Mallory® Head, uncemented, PS. |
| 6 AL (+) | MoM | Ti-6Al-4V, Bi-metric®, uncemented, grit blasted. | CoCrMo | CoCrMo | CoCrMo, ReCap®, uncemented, cpTI PS. |
| 1 AL (−) | MoP | Ti-6Al-7Nb, CLS spotorno®, uncemented, grit blasted. | CoCrMo | PE | Ti-6Al-4V, Trilogy®, uncemented, cpTi fiber mesh. |
| 2 AL (−) | MoP | Ti-6Al-7Nb, CLS spotorno®, uncemented, grit blasted. | CoCrMo | PE | Ti-6Al-4V, Trilogy®, uncemented, cpTi fiber mesh. |
| 3 AL (−) | CoP | Ti-6Al-4V, Biocontact®, uncemented, grit blasted. | Ceramic | PE | Ti-6Al-4V, Plasmacup®, uncemented, plasmapore PS. |
| 4 AL (−) | MoP | FeCrNiMn, Exeter®, cemented, polished. | CoCrMo | PE | cpTi, Pinnacle®, uncemented porocoat, porous coating. |
| 5 AL (−) | MoP | FeCrNiMn, Exeter®, cemented, polished | CoCrMo | PE | Ti-6Al-4V, Trilogy®, uncemented, cpTi fiber mesh. |
| 6 AL (−) | MoP | FeCrNiMn, Exeter®, cemented, polished | CoCrMo | PE | cpTi, Pinnacle®, uncemented porocoat, porous coating. |
| AL (+) (n = 6) | AL (−) (n = 6) | Control (n = 8) | |
|---|---|---|---|
| Reactions | |||
| Metal compound (concentration) | + (+?) | + (+?) | + (+?) |
| Al(III), AlCl3 (0.72%) | 0 (0) | 0 (0) | 0 (1) |
| Ti(IV), TiC4O8 (0.32%) | 0 (0) | 1 (0) | 2 (0) |
| Ti(II), C4K2O9Ti (2.4%) | 0 (0) | 0 (0) | 0 (0) |
| V(III), VCl3 (0.24%) | 1 (2) | 0 (3) | 0 (3) |
| V(III), VCl3 (0.12%) | 1 (0) | 0 (1) | 0 (3) |
| V(III), VCl3 (0.013%) | 0 (0) | 0 (1) | 0 (0) |
| V(III), VCl3 (0.04%) | 0 (0) | 0 (1) | 0 (0) |
| V(IV), VOSO4 (0.36%) | 0 (1) | 0 (1) | 0 (2) |
| V(IV), VOSO4 (0.18%) | 0 (1) | 0 (1) | 0 (0) |
| Cr(VI), K2Cr2O7 (0.054%) | 1 (0) | 0 (0) | 0 (0) |
| Mn(II), MnCl2 (0.24%) | 0 (1) | 1 (2) | 0 (2) |
| Ni(II), NiSO4 (5.0%) | 0 (0) | 0 (0) | 1 (1) |
| Methyl Methacrylate, C5H8O2 (2%) | 0 (0) | 0 (0) | 0 (1) |
| Total reactions | 3 (4) | 2 (8) | 3 (12) |
| Metal | AL (+) n = 6 | AL (−) n = 6 | Control n = 10 | |||
|---|---|---|---|---|---|---|
| Tissue | Serum | Tissue | Serum | Tissue | Serum | |
| Al | 7186 * (1905–29,019) | N/A | 3407 * (845–26,709) | N/A | 1258 (352–2615) | N/A |
| Ti | 1610 * (891–13,328) | 0.65 (0.60–2.95) | 12978 * (588–47,078) | 1.45 (0.60–3.98) | 716.5 (504–1152) | 0.60 (0.60–1.00) |
| V | 210 (128–920) | N/A | 381 (151–573) | N/A | 160 (133–209) | N/A |
| Cr | 3648 (358–21,075) | 0.98 * (0.26–3.4) | 499 (151–6235) | 0.26 (0.26–0.26) | 484 (184–1868) | 0.26 (0.26–0.26) |
| Co | 210 (128–2724) | 0.30 (0.30–1.93) | 167 (118–2549) | 0.30 (0.30–0.74) | 160 (133–209) | 0.30 (0.30–0.30) |
| Ni | 772 * (355–2027) | N/A | 328 (151–1589) | N/A | 212 (162–326) | N/A |
| Implant Alloy | CoCrMo ASTM- (F75) | Orthinox SS ASTM- (F1586) | cpTi ASTM- (F67) | Ti6Al7Nb ASTM- (F1295) | Ti6Al4V ASTM-(F136) |
|---|---|---|---|---|---|
| Element | Composition, wt.% | ||||
| Aluminum (Al) | 0.10 | - | 0.03 | 5.50–6.50 | 5.5–6.50 |
| Carbon (C) | 0.35 | 0.08 | 0.08 | 0.08 | 0.08 |
| Chromium (Cr) | 27–30 | 19.5–22 | - | - | - |
| Cobalt (Co) | Balance | - | - | - | - |
| Copper (Cu) | - | 0.25 | 0.10 | - | - |
| Iron (Fe) | 0.75 | Balance | 0.50 | 0.25 | 0.25 |
| Manganese (Mn) | 1 | 2–4.25 | - | - | - |
| Molybdenum (Mo) | 5–7 | 2–3 | - | - | - |
| Nickel (Ni) | 0.50 | 9.0–11.0 | - | - | - |
| Niobium (Nb) | - | 0.25–0.8 | 0.015 | 6.50–7.50 | - |
| Nitrogen (N) | 0.25 | 0.25–0.5 | 0.15 | 0.05 | 0.05 |
| Oxygen (O) | - | - | 0.40 | 0.20 | 0.13 |
| Tantalum (Ta) | - | - | Balance | 0.50 | - |
| Titanium (Ti) | 0.10 | - | - | Balance | Balance |
| Tungsten (W) | 0.20 | - | - | - | - |
| Vanadium (V) | - | - | - | - | 3.5–4.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christiansen, R.J.; Münch, H.J.; Bonefeld, C.M.; Thyssen, J.P.; Sloth, J.J.; Geisler, C.; Søballe, K.; Jellesen, M.S.; Jakobsen, S.S. Cytokine Profile in Patients with Aseptic Loosening of Total Hip Replacements and Its Relation to Metal Release and Metal Allergy. J. Clin. Med. 2019, 8, 1259. https://doi.org/10.3390/jcm8081259
Christiansen RJ, Münch HJ, Bonefeld CM, Thyssen JP, Sloth JJ, Geisler C, Søballe K, Jellesen MS, Jakobsen SS. Cytokine Profile in Patients with Aseptic Loosening of Total Hip Replacements and Its Relation to Metal Release and Metal Allergy. Journal of Clinical Medicine. 2019; 8(8):1259. https://doi.org/10.3390/jcm8081259
Chicago/Turabian StyleChristiansen, Rune J., Henrik J. Münch, Charlotte M. Bonefeld, Jacob P. Thyssen, Jens J. Sloth, Carsten Geisler, Kjeld Søballe, Morten S. Jellesen, and Stig S. Jakobsen. 2019. "Cytokine Profile in Patients with Aseptic Loosening of Total Hip Replacements and Its Relation to Metal Release and Metal Allergy" Journal of Clinical Medicine 8, no. 8: 1259. https://doi.org/10.3390/jcm8081259
APA StyleChristiansen, R. J., Münch, H. J., Bonefeld, C. M., Thyssen, J. P., Sloth, J. J., Geisler, C., Søballe, K., Jellesen, M. S., & Jakobsen, S. S. (2019). Cytokine Profile in Patients with Aseptic Loosening of Total Hip Replacements and Its Relation to Metal Release and Metal Allergy. Journal of Clinical Medicine, 8(8), 1259. https://doi.org/10.3390/jcm8081259






