“Third Window” and “Single Window” Effects Impede Surgical Success: Analysis of Retrofenestral Otosclerosis Involving the Internal Auditory Canal or Round Window
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Temporal Bone Computed Tomography (TBCT) Protocol
2.3. Imaging Analysis
2.4. Audiological Data Analysis
2.5. Statistical Analysis
3. Results
3.1. TBCT Findings
3.2. Demographic and Audiological Results According to Retrofenestral Subsite
3.3. Correlation between Surgical Failure and Retrofenestral Subsites
4. Discussion
4.1. Importance of Excavating Anatomical Subsites of Otosclerosis relevant to Surgical Outcome
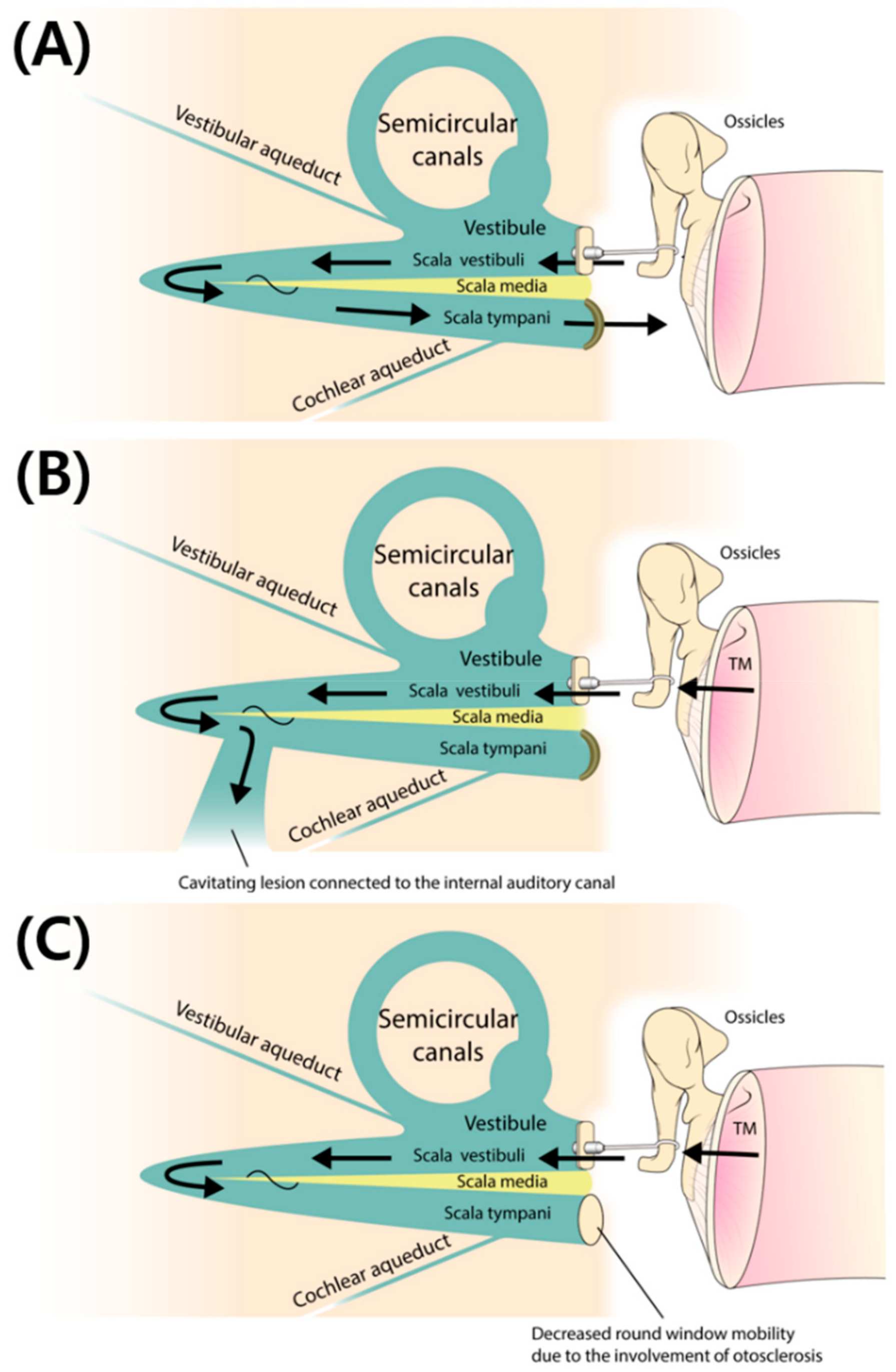
4.2. Cavitating Otosclerosis Involving the IAC: A Third Window Affecting the Surgical Outcome
4.3. Involvement of the Round Window (RW) in Subjects with Retrofenestral Otosclerosis: An Indicator of the “Single Window” Effect
4.4. Limitations of This Study and Proposals for Future Investigations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Richard, C.; Linthicum, F.H., Jr. An unexpected third window in a case of advanced cavitating otosclerosis. Otol. Neurotol. 2012, 33, e47–e48. [Google Scholar] [PubMed]
- Pippin, K.J.; Muelleman, T.J.; Hill, J.; Leever, J.; Staecker, H.; Ledbetter, L.N. Prevalence of internal auditory canal diverticulum and its association with hearing loss and otosclerosis. AJNR Am. J. Neuroradiol. 2017, 38, 2167–2171. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.D.; Faerber, E.N.; Wolfson, R.J.; Marlowe, F.I. Fenestral otosclerosis: Significance of preoperative CT evaluation. Radiology 1984, 151, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.D.; Mandell, D.W.; Berman, S.E.; Wolfson, R.J.; Marlowe, F.I.; Popky, G.L. Cochlear otosclerosis (otospongiosis): CT analysis with audiometric correlation. Radiology 1985, 155, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Valvassori, G.E. Imaging of otosclerosis. Otolaryngol. Clin. N. Am. 1993, 26, 359–371. [Google Scholar] [PubMed]
- Shin, Y.J.; Fraysse, B.; Deguine, O.; Cognard, C.; Charlet, J.P.; Sevely, A. Sensorineural hearing loss and otosclerosis: A clinical and radiologic survey of 437 cases. Acta Otolaryngol. 2001, 121, 200–204. [Google Scholar] [PubMed]
- Dudau, C.; Salim, F.; Jiang, D.; Connor, S.E. Diagnostic efficacy and therapeutic impact of computed tomography in the evaluation of clinically suspected otosclerosis. Eur. Radiol. 2017, 27, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Shea, J.J., Jr. Fenestration of the oval window. AMA Arch. Otolaryngol. 1960, 71, 257–269. [Google Scholar] [CrossRef]
- Shea, J.J., Jr. Fenestration of the oval window. Ann. Otol. Rhinol. Laryngol. 1958, 67, 932–951. [Google Scholar] [CrossRef]
- Lagleyre, S.; Sorrentino, T.; Calmels, M.N.; Shin, Y.J.; Escude, B.; Deguine, O.; Fraysse, B. Reliability of high-resolution CT scan in diagnosis of otosclerosis. Otol. Neurotol. 2009, 30, 1152–1159. [Google Scholar] [CrossRef]
- Naumann, I.C.; Porcellini, B.; Fisch, U. Otosclerosis: Incidence of positive findings on high-resolution computed tomography and their correlation to audiological test data. Ann. Otol. Rhinol. Laryngol. 2005, 114, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Choi, H.G.; Oh, S.H.; Chang, S.O.; Kim, C.S.; Lee, J.H. Unilateral sensorineural hearing loss in children: The importance of temporal bone computed tomography and audiometric follow-up. Otol. Neurotol. 2009, 30, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Wycherly, B.J.; Berkowitz, F.; Noone, A.M.; Kim, H.J. Computed tomography and otosclerosis: A practical method to correlate the sites affected to hearing loss. Ann. Otol. Rhinol. Laryngol. 2010, 119, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Kiyomizu, K.; Tono, T.; Yang, D.; Haruta, A.; Kodama, T.; Komune, S. Correlation of CT analysis and audiometry in Japanese otosclerosis. Auris Nasus Larynx 2004, 31, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Marx, M.; Lagleyre, S.; Escude, B.; Demeslay, J.; Elhadi, T.; Deguine, O.; Fraysse, B. Correlations between CT scan findings and hearing thresholds in otosclerosis. Acta Otolaryngol. 2011, 131, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, M.; Ueda, H.; Uchida, Y.; Sone, M. Factors affecting postoperative outcome in otosclerosis patients: Predictive role of audiological and clinical features. Auris Nasus Larynx 2015, 42, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.J.; Bae, Y.J.; An, G.S.; Lee, K.; Kim, Y.; Lee, S.Y.; Choi, B.Y.; Choi, B.S.; Kim, J.H.; Koo, J.W.; et al. Involvement of the internal auditory canal in subjects with cochlear otosclerosis: A less acknowledged third window that affects surgical outcome. Otol. Neurotol. 2019, 40, e186–e190. [Google Scholar] [CrossRef] [PubMed]
- Veillon, F.; Riehm, S.; Emachescu, B.; Haba, D.; Roedlich, M.N.; Greget, M.; Tongio, J. Imaging of the windows of the temporal bone. Semin. Ultrasound CT MR 2001, 22, 271–280. [Google Scholar] [CrossRef]
- Makarem, A.O.; Linthicum, F.H. Cavitating otosclerosis. Otol. Neurotol. 2008, 29, 730–731. [Google Scholar] [CrossRef]
- Makarem, A.O.; Hoang, T.A.; Lo, W.W.; Linthicum, F.H., Jr.; Fayad, J.N. Cavitating otosclerosis: Clinical, radiologic, and histopathologic correlations. Otol. Neurotol. 2010, 31, 381–384. [Google Scholar] [CrossRef]
- Bou-Assaly, W.; Mukherji, S.; Srinivasan, A. Bilateral cavitary otosclerosis: A rare presentation of otosclerosis and cause of hearing loss. Clin. Imaging 2013, 37, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. Otolaryngol. Head Neck Surg. 1995, 113, 186–187. [Google Scholar] [CrossRef]
- Song, J.J.; An, G.S.; Choi, I.; De Ridder, D.; Kim, S.Y.; Choi, H.S.; Park, J.H.; Choi, B.Y.; Koo, J.W.; Lee, K. Objectification and differential diagnosis of vascular pulsatile tinnitus by transcanal sound recording and spectrotemporal analysis: A preliminary study. Otol. Neurotol. 2016, 37, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Nam, D.W.; Koo, J.W.; De Ridder, D.; Vanneste, S.; Song, J.J. No auditory experience, no tinnitus: Lessons from subjects with congenital- and acquired single-sided deafness. Hear. Res. 2017, 354, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, W.; Jeon, Y.J.; Bae, Y.J.; Jang, J.H.; Koo, J.W.; Song, J.J. Typewriter tinnitus revisited: The typical symptoms and the initial response to carbamazepine are the most reliable diagnostic clues. Sci. Rep. 2017, 7, 10615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, Y.J.; Song, J.J.; Choi, B.S.; Kang, Y.; Kim, J.H.; Koo, J.W. Differentiation between intralabyrinthine schwannoma and contrast-enhancing labyrinthitis on MRI: Quantitative analysis of signal intensity characteristics. Otol. Neurotol. 2018, 39, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Hong, S.K.; Lee, S.Y.; Park, S.J.; Kang, S.I.; An, Y.H.; Jang, J.H.; Kim, J.S.; Koo, J.W. Vestibular manifestations in subjects with enlarged vestibular aqueduct. Otol. Neurotol. 2018, 39, e461–e467. [Google Scholar] [CrossRef]
- Heinze, G.; Schemper, M. A solution to the problem of monotone likelihood in Cox regression. Biometrics 2001, 57, 114–119. [Google Scholar] [CrossRef]
- Min, J.Y.; Chung, W.H.; Lee, W.Y.; Cho, Y.S.; Hong, S.H.; Kim, H.J.; Lee, H.S. Otosclerosis: Incidence of positive findings on temporal bone computed tomography (TBCT) and audiometric correlation in Korean patients. Auris Nasus Larynx 2010, 37, 23–28. [Google Scholar] [CrossRef]
- Ramsay, H.A.; Linthicum, F.H., Jr. Mixed hearing loss in otosclerosis: Indication for long-term follow-up. Am. J. Otol. 1994, 15, 536–539. [Google Scholar]
- Marshall, A.H.; Fanning, N.; Symons, S.; Shipp, D.; Chen, J.M.; Nedzelski, J.M. Cochlear implantation in cochlear otosclerosis. Laryngoscope 2005, 115, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, N.; Bartoli, R.; Lopriore, A.; Fernandez-Vega, S.; Giagnotti, F.; Quaranta, A. Cochlear implantation in otosclerosis. Otol. Neurotol. 2005, 26, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, I.; Bouccara, D.; Ambert-Dahan, E.; Ferrary, E.; Sterkers, O. Cochlear implantation and far-advanced otosclerosis. Adv. Otorhinolaryngol. 2007, 65, 323–327. [Google Scholar] [PubMed]
- Van Loon, M.C.; Merkus, P.; Smit, C.F.; Smits, C.; Witte, B.I.; Hensen, E.F. Stapedotomy in cochlear implant candidates with far advanced otosclerosis: A systematic review of the literature and meta-analysis. Otol. Neurotol. 2014, 35, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Kabbara, B.; Gauche, C.; Calmels, M.N.; Lepage, B.; Escude, B.; Deguine, O.; Fraysse, B.; Marx, M. Decisive criteria between stapedotomy and cochlear implantation in patients with far advanced otosclerosis. Otol. Neurotol. 2015, 36, e73–e78. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.; Nicolas, K.; Ahmad, H.H. Round window otosclerosis: Radiologic classification and clinical correlations. Otol. Neurotol. 2011, 32, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Nadol, J.B., Jr. Histopathology of residual and recurrent conductive hearing loss after stapedectomy. Otol. Neurotol. 2001, 22, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Gopen, Q.; Rosowski, J.J.; Merchant, S.N. Anatomy of the normal human cochlear aqueduct with functional implications. Hear. Res. 1997, 107, 9–22. [Google Scholar] [CrossRef]
- Merchant, S.N.; Ravicz, M.E.; Voss, S.E.; Peake, W.T.; Rosowski, J.J. Toynbee Memorial Lecture 1997. Middle ear mechanics in normal, diseased and reconstructed ears. J. Laryngol. Otol. 1998, 112, 715–731. [Google Scholar] [CrossRef]
- Meranger, A.; David, A.; Beigner, B.M.; Charpiot, A.; Tavernier, L. Audiometric results of stapedotomy surgery for otoscelorsis: Influence of the radiological stage. Otol. Neurotol. 2019, 40, e75–e81. [Google Scholar] [CrossRef]
- Merchant, S.N.; Rosowski, J.J. Conductive hearing loss caused by third-window lesions of the inner ear. Otol. Neurotol. 2008, 29, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]

| Subsite | No. |
|---|---|
| Cavitating lesion | 15 |
| Cochlea | 14 |
| RW | 10 |
| Vestibule | 23 |
| SCC | 8 |
| Confluence between cavitating lesion and cochlear involvement | 6 |
| Demographic and Audiometric Data | Cavitating lesion | Cochlea | RW | SCC | Confluence | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P | Yes | No | P | Yes | No | P | Yes | No | P | Yes | No | P | |
| Age (years) | 46.9 ± 10.3 | 47.3 ± 5.0 | 0.825 | 45.6 ± 9.85 | 49.1 ± 6.5 | 0.224 | 48.7 ± 7.8 | 45.7 ± 9.5 | 0.879 | 49.3 ± 12.8 | 45.8 ± 5.7 | 0.131 | 47.7 ± 6.5 | 45.0 ± 13.8 | 0.708 |
| Sex (n, female/male) | 11/4 | 2/6 | 0.039 * | 9/5 | 4/5 | 0.417 | 8/2 | 5/8 | 0.09 | 6/2 | 7/8 | 0.379 | 6/0 | 7/10 | 0.015 * |
| Pre-stapedotomy AC (dB) | 59.8 ± 18.9 | 55.3 ± 8.8 | 0.925 | 61.8 ± 18.5 | 52.6 ± 9.8 | 0.305 | 64.8 ± 20.3 | 53.2 ± 10.0 | 0.376 | 65.5 ± 24.2 | 54.3 ± 8.0 | 0.466 | 72.9 ± 24.0 | 53.0 ± 8.0 | 0.024 * |
| Post-stapedotomy AC (dB) | 44.4 ± 16.4 | 24.7 ± 7.4 | 0.004 * | 43.5 ± 15.6 | 28.3 ± 14.7 | 0.033 * | 49.9 ± 15.1 | 28.1 ± 10.9 | 0.002 * | 47.2 ± 19.7 | 32.4 ± 12.8 | 0.047 * | 46.5 ± 19.6 | 34.4 ± 15.0 | 0.155 |
| Pre-stapedotomy BC (dB) | 34.2 ± 13.4 | 28.9 ± 8.3 | 0.728 | 34.9 ± 13.4 | 28.3 ± 8.5 | 0.277 | 36.9 ± 14.1 | 28.8 ± 9.0 | 0.284 | 38.8 ± 15.3 | 28.9 ± 8.4 | 0.131 | 44.4 ± 15.7 | 29.1 ± 6.7 | 0.024 * |
| Post-stapedotomy BC (dB) | 31.5 ± 12.5 | 19.2 ± 5.2 | 0.011 * | 31.7 ± 12.4 | 20.1 ± 7.1 | 0.016 * | 34.6 ± 13.3 | 21.5 ± 7.1 | 0.012 * | 36.2 ± 14.5 | 22.4 ± 7.0 | 0.013 * | 36.8 ± 15.4 | 23.8 ± 8.7 | 0.044 * |
| Pre-stapedotomy ABG (dB) | 25.6 ± 8.6 | 26.4 ± 8.9 | 0.681 | 26.9 ± 8.3 | 24.3 ± 9.1 | 0.643 | 27.9 ± 8.0 | 24.3 ± 8.9 | 0.376 | 26.7 ± 9.7 | 25.4 ± 8.2 | 0.975 | 28.5 ± 10.5 | 24.9 ± 7.8 | 0.609 |
| Post-stapedotomy ABG (dB) | 13.0 ± 10.1 | 5.5 ± 4.3 | 0.028 * | 11.8 ± 7.2 | 8.2 ± 11.7 | 0.053 | 15.3 ± 10.1 | 6.5 ± 6.4 | 0.005 * | 11.0 ± 7.0 | 10.0 ± 10.3 | 0.428 | 9.7 ± 6.5 | 10.6 ± 10.1 | 0.812 |
| ABGC (dB) | 12.6 ± 12.3 | 20.9 ± 7.8 | 0.065 | 15.1 ± 8.3 | 16.1 ± 15.8 | 0.516 | 12.6 ± 14.5 | 17.8 ± 8.4 | 0.483 | 15.7 ± 9.0 | 15.4 ± 12.9 | 0.875 | 18.8 ± 7.8 | 14.3 ± 12.5 | 0.392 |
| Subsite | Surgical Success | Surgical Failure | p-Values |
|---|---|---|---|
| Cavitating lesion (n, Yes/No) | 4/7 | 11/1 | 0.009 * |
| Cochlea (n, Yes/No) | 4/7 | 10/2 | 0.036 * |
| RW (n, Yes/No) | 1/10 | 9/3 | 0.003 * |
| SCC (n, Yes/No) | 2/9 | 6/6 | 0.193 |
| Confluence between cavitating lesion and cochlear involvement (n, Yes/No) | 2/9 | 4/8 | 0.365 |
| Subsite | Odds Ratio | 95% CI | p-Values |
|---|---|---|---|
| Cavitating lesion | 12.78 | 1.62–100.7 | 0.016 * |
| Cochlea | 7 | 1.15–42.7 | 0.035 * |
| RW | 19 | 2.32–155.9 | 0.006 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, Y.J.; Shim, Y.J.; Choi, B.S.; Kim, J.-H.; Koo, J.-W.; Song, J.-J. “Third Window” and “Single Window” Effects Impede Surgical Success: Analysis of Retrofenestral Otosclerosis Involving the Internal Auditory Canal or Round Window. J. Clin. Med. 2019, 8, 1182. https://doi.org/10.3390/jcm8081182
Bae YJ, Shim YJ, Choi BS, Kim J-H, Koo J-W, Song J-J. “Third Window” and “Single Window” Effects Impede Surgical Success: Analysis of Retrofenestral Otosclerosis Involving the Internal Auditory Canal or Round Window. Journal of Clinical Medicine. 2019; 8(8):1182. https://doi.org/10.3390/jcm8081182
Chicago/Turabian StyleBae, Yun Jung, Ye Ji Shim, Byung Se Choi, Jae-Hyoung Kim, Ja-Won Koo, and Jae-Jin Song. 2019. "“Third Window” and “Single Window” Effects Impede Surgical Success: Analysis of Retrofenestral Otosclerosis Involving the Internal Auditory Canal or Round Window" Journal of Clinical Medicine 8, no. 8: 1182. https://doi.org/10.3390/jcm8081182
APA StyleBae, Y. J., Shim, Y. J., Choi, B. S., Kim, J.-H., Koo, J.-W., & Song, J.-J. (2019). “Third Window” and “Single Window” Effects Impede Surgical Success: Analysis of Retrofenestral Otosclerosis Involving the Internal Auditory Canal or Round Window. Journal of Clinical Medicine, 8(8), 1182. https://doi.org/10.3390/jcm8081182






